Biological Sunglasses in a Deep-Sea Squid: Pigment Migration in the Retina of Gonatus onyx
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Acquisition
2.2. Experimental Design
2.3. Pigment Migration
2.4. Neurotransmitter Expression
2.5. Imaging
2.6. Barcoding
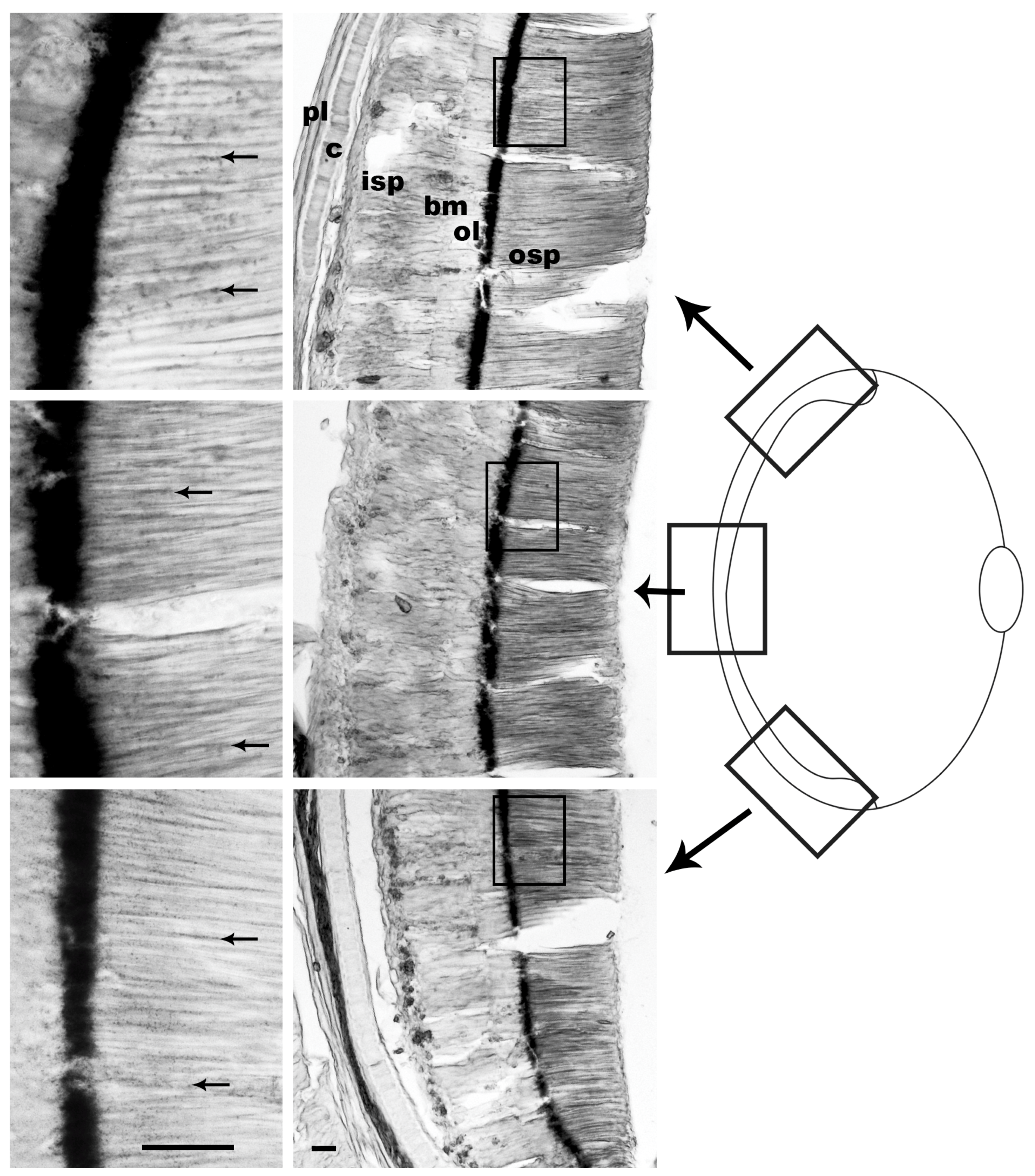
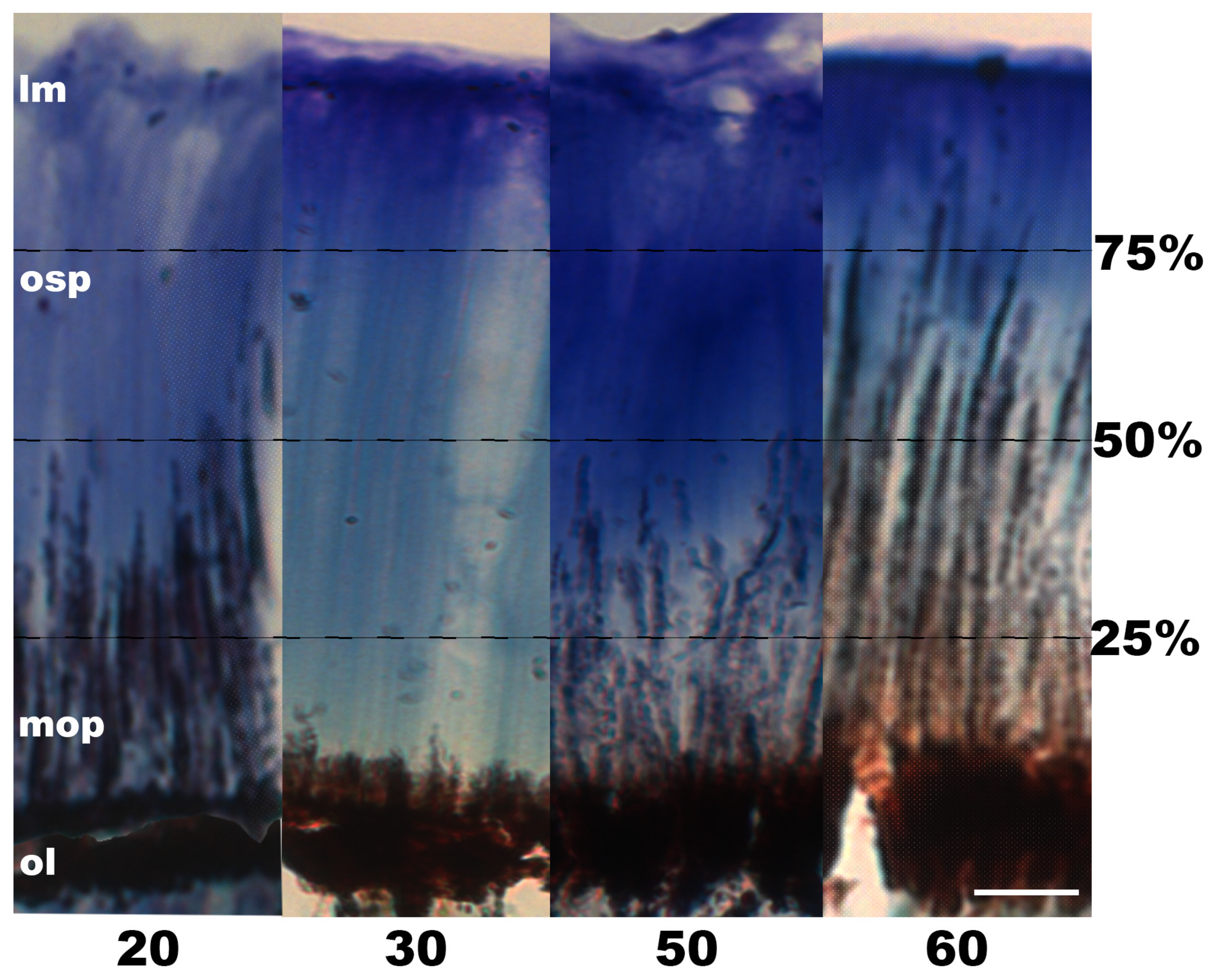
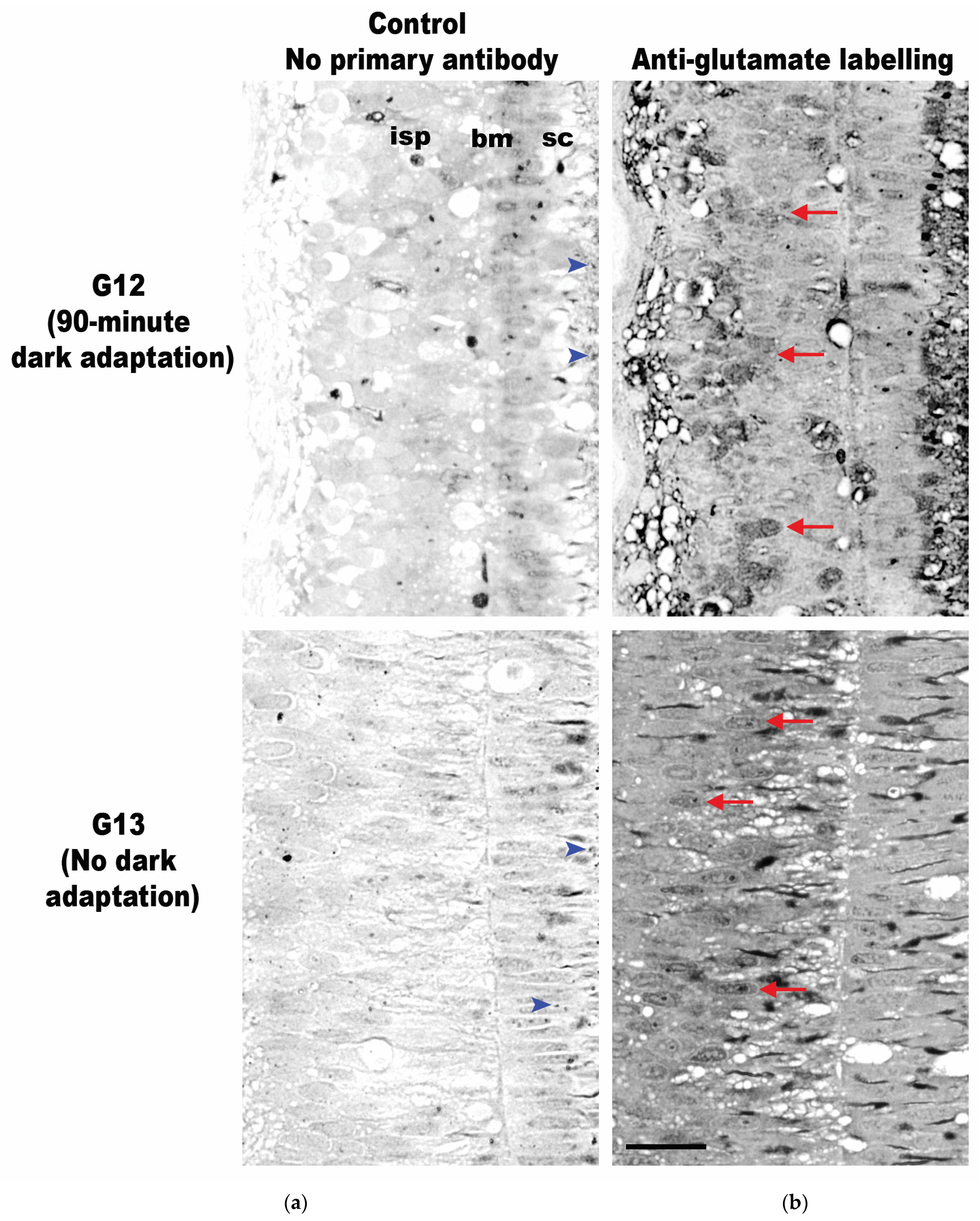
3. Results
3.1. Pigment Migration
3.2. Neurotransmitter Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawitz, B. Über Pigmentverschiebungen Im Cephalopodenauge. Zool. Anz. 1891, 14, 157–158. [Google Scholar]
- Butenandt, A. Wirkstoffe Des Insektenreiches. Naturwissenschaften 1959, 46, 461–471. [Google Scholar] [CrossRef]
- Daw, N.W.; Pearlman, A.L. Pigment Migration and Adaptation in the Eye of the Squid, Loligo pealei. J. Gen. Physiol. 1974, 63, 22–36. [Google Scholar] [CrossRef]
- Hagins, W.A.; Liebman, P.A. Light Induced Pigment Migration in the Squid Retina. Biol. Bull. 1962, 123, 498. [Google Scholar]
- Young, J.Z. Light- and Dark-Adaptation in the Eyes of Some Cephalopods. Proc. Zool. Soc. Lond. 1963, 140, 255–272. [Google Scholar] [CrossRef]
- Gleadall, I.G.; Ohtsu, K.; Gleadall, E.; Tsukahara, Y. Screening-Pigment Migration in the Octopus Retina Includes Control by Dopaminergic Efferents. J. Exp. Biol. 1993, 185, 1–16. [Google Scholar] [CrossRef]
- Wolken, J.J. Retinal Structure: Mollusc Cephalopods: Octopus, Sepia. J. Biophys. Biochem. Cytol. 1958, 4, 835–837. [Google Scholar] [CrossRef]
- Evans, A.B.; Acosta, M.L.; Bolstad, K.S. Retinal Development and Ommin Pigment in the Cranchiid Squid Teuthowenia pellucida (Cephalopoda: Oegopsida). PLoS ONE 2015, 10, 11. [Google Scholar] [CrossRef]
- Kier, C.K.; Chamberlain, S.C. Dual Controls For Screening Pigment Movement In Photoreceptors of The Limulus Lateral Eye: Circadian Efferent Input And Light. Vis. Neurosci. 1990, 4, 237–255. [Google Scholar] [CrossRef]
- Gavriouchkina, D.; Tan, Y.; Ziadi-Künzli, F.; Hasegawa, Y.; Piovani, L.; Zhang, L.; Sugimoto, C.; Luscombe, N.; Marlétaz, F.; Rokhsar, D.S. A Single-Cell Atlas of Bobtail Squid Visual and Nervous System Highlights Molecular Principles of Convergent Evolution. bioRxiv 2022, 2022.05.06.490366. [Google Scholar] [CrossRef]
- D’Aniello, S.; Spinelli, P.; Ferrandino, G.; Peterson, K.; Tsesarskia, M.; Fisher, G.; D’Aniello, A. Cephalopod Vision Involves Dicarboxylic Amino Acids: D-Aspartate, L-Aspartate and L-Glutamate. Biochem. J. 2005, 386, 331–340. [Google Scholar] [CrossRef] [PubMed]
- MBARI’s Deep Sea Guide-Data Artifacts for Gonatus onyx. Available online: http://dsg.mbari.org/dsg/plots/concept/Gonatus%20onyx/Global (accessed on 20 April 2023).
- Hunt, J.C.; Seibel, B.A. Life History of Gonatus onyx (Cephalopoda: Teuthoidea): Ontogenetic Changes in Habitat, Behavior and Physiology. Mar. Biol. 2000, 136, 543–552. [Google Scholar] [CrossRef]
- Seibel, B.A.; Robison, B.H.; Haddock, S.H.D. Post-Spawning Egg Care by a Squid. Nature 2005, 438, 929. [Google Scholar] [CrossRef] [PubMed]
- Marc, R.E.; Liu, W.L.S.; Kalloniatis, M.; Raiguel, S.F.; Van Haesendonck, E. Patterns of Glutamate Immunoreactivity in the Goldfish Retina. J. Neurosci. 1990, 10, 4006–4034. [Google Scholar] [CrossRef] [PubMed]
- Kalloniatis, M.; Loh, C.S.; Acosta, M.L.; Tomisich, G.; Zhu, Y.; Nivison-Smith, L.; Fletcher, E.L.; Chua, J.; Sun, D.; Arunthavasothy, N. Retinal Amino Acid Neurochemistry in Health and Disease. Clin. Exp. Optom. 2013, 96, 310–332. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.L.; Kalloniatis, M. Short- and Long-Term Enzymatic Regulation Secondary to Metabolic Insult in the Rat Retina. J. Neurochem. 2005, 92, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Nivison-Smith, L.; Collin, S.P.; Zhu, Y.; Ready, S.; Acosta, M.L.; Hunt, D.M.; Potter, I.C.; Kalloniatis, M. Retinal Amino Acid Neurochemistry of the Southern Hemisphere Lamprey, Geotria australis. PLoS ONE 2013, 8, e58406. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. Bold: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Braid, H.E.; Bolstad, K.S.R. Cephalopod Biodiversity of the Kermadec Islands: Implications for Conservation and Some Future Taxonomic Priorities. Invertebr. Syst. 2019, 33, 402–425. [Google Scholar] [CrossRef]
- Lo, M.V.C.; Pak, W.L. Light-Induced Pigment Granule Migration in the Retinular Cells of Drosophila melanogaster: Comparison of Wild Type with ERG-Defective Mutants. J. Gen. Physiol. 1981, 77, 155–175. [Google Scholar] [CrossRef]
- Hamdorf, K.; Höglund, G.; Juse, A. Ultra-Violet and Blue Induced Migration of Screening Pigment in the Retina of the Moth Deilephila elpenor. J. Comp. Physiol. A 1986, 159, 353–362. [Google Scholar] [CrossRef]
- White, R.H.; Banister, M.J.; Bennett, R.R. Spectral Sensitivity of Screening Pigment Migration in the Compound Eye of Manduca sexta. J. Comp. Physiol. A 1983, 153, 59–66. [Google Scholar] [CrossRef]
- Arechiga, H.; Banuelos, E.; Frixione, E.; Picones, A.; Rodriguez-Sosa, L. Modulation of Crayfish Retinal Sensitivity by 5-Hydroxytryptamine. J. Exp. Biol. 1990, 150, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Schraermeyer, U. Further Evidence for Synthesis of Screening Pigment Granules Involved in the Photosensory Membrane Turnover of the Crayfish Photoreceptor. Pigment. Cell Res. 1990, 3, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.M.; Howells, A.J.; Pyliotis, N.A. Biology of Eye Pigmentation in Insects. Adv. Insect Phys. 1982, 16, 119–166. [Google Scholar] [CrossRef]
- Figon, F.; Casas, J. Ommochromes in Invertebrates: Biochemistry and Cell Biology. Biol. Rev. 2019, 94, 156–183. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.G.; Beauchamp, D.A.; Schoen, E.R. Visual Prey Detection Responses of Piscivorous Trout and Salmon: Effects of Light, Turbidity, and Prey Size. Trans. Am. Fish. Soc. 2013, 142, 854–867. [Google Scholar] [CrossRef]
- Seibel, B.A.; Hochberg, F.G.; Carlini, D.B. Life History of Gonatus onyx (Cephalopoda: Teuthoidea): Deep-Sea Spawning and Post-Spawning Egg Care. Mar. Biol. 2000, 137, 519–526. [Google Scholar] [CrossRef]
- Okutani, T.; Nakamura, I.; Seki, K. An Unusual Egg-Brooding Behavior of an Oceanic Squid in the Okhotsk Sea. Jpn. J. Malacol. 1995, 54, 237–239. [Google Scholar]
- Mangold, K. Reproduction. In Cephalopod Life Cycles; Boyle, P.R., Ed.; Academic Press: London, UK, 1983; Volume 2, pp. 157–200. [Google Scholar]
- Young, R.E. Brooding in a Bathypelagic Octopus. Pac. Sci. 1972, 26, 400–404. [Google Scholar]
- Muñoz, J.L.P.; Patiño, M.A.L.; Hermosilla, C.; Conde-Sieira, M.; Soengas, J.L.; Rocha, F.; Míguez, J.M. Melatonin in Octopus (Octopus vulgaris): Tissue Distribution, Daily Changes and Relation with Serotonin and Its Acid Metabolite. J. Comp. Physiol. A 2011, 197, 789–797. [Google Scholar] [CrossRef] [PubMed]

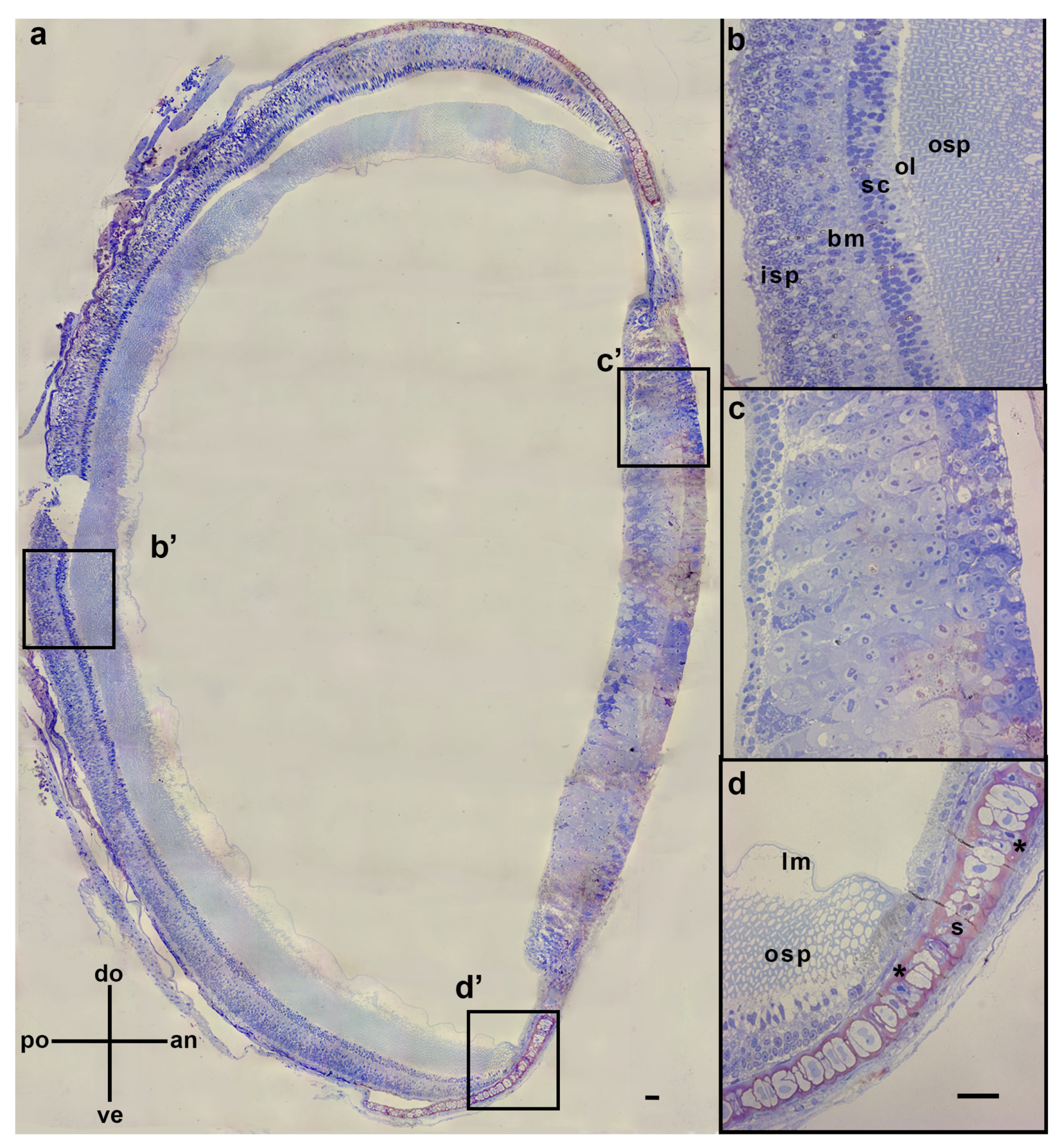
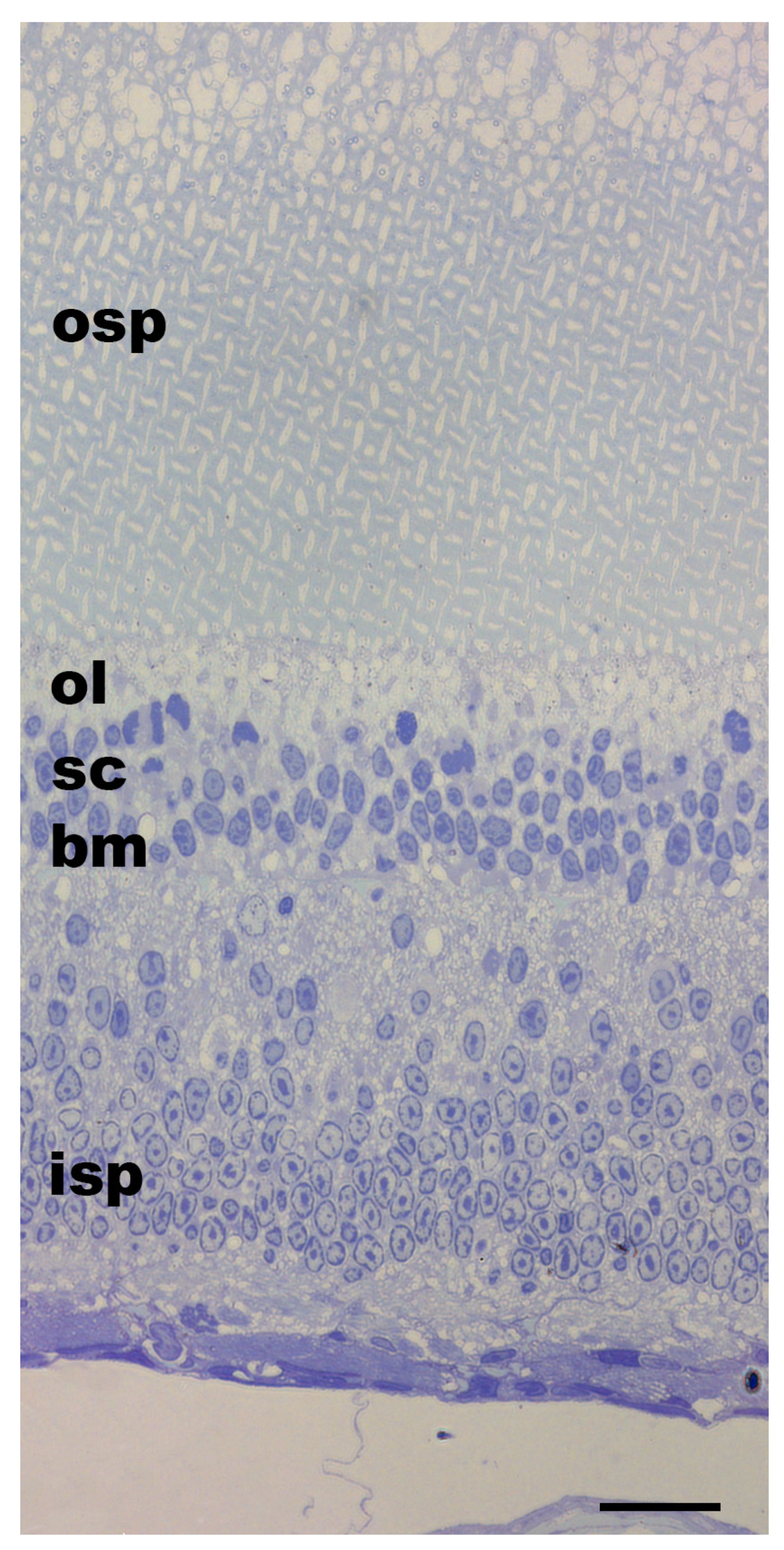
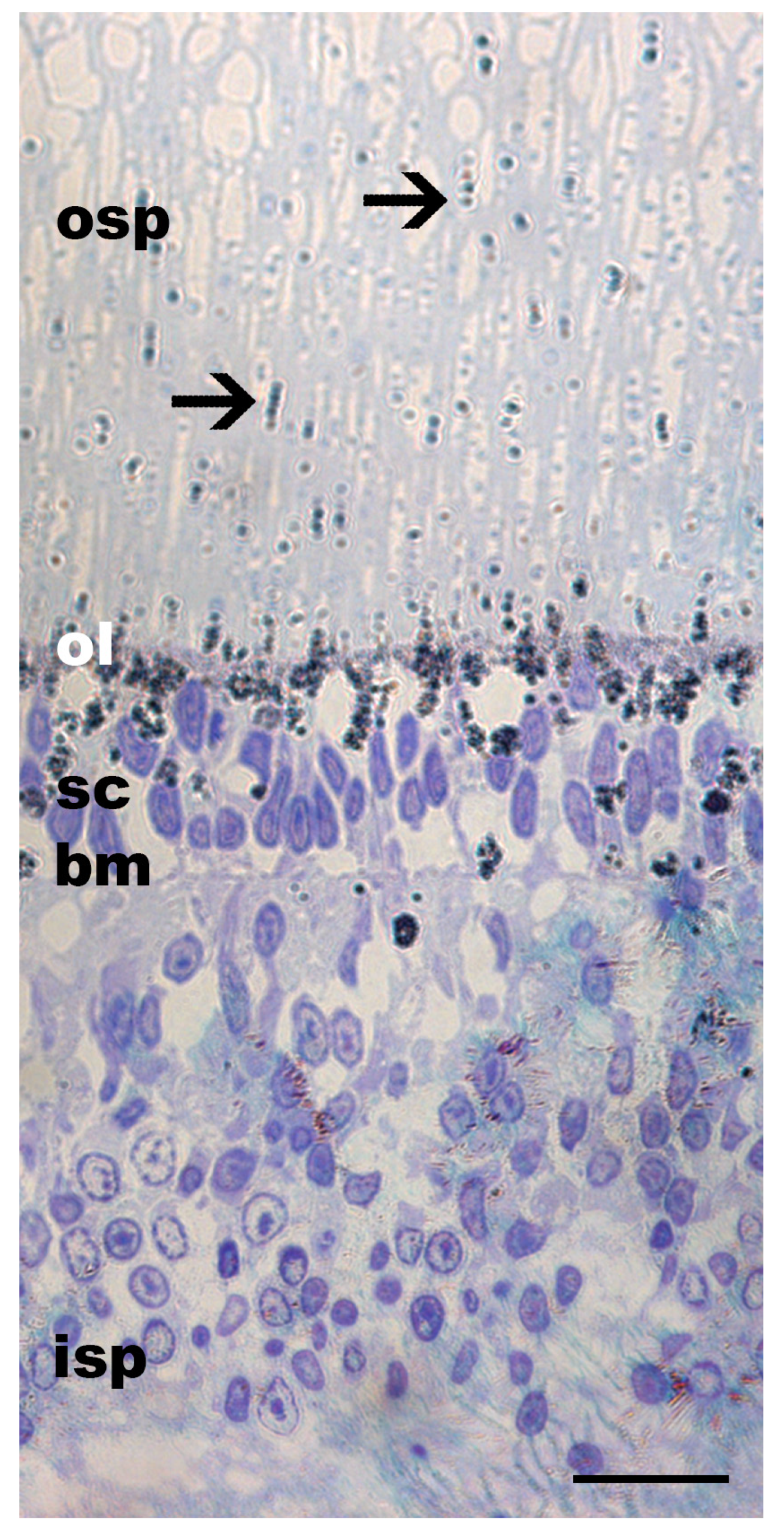
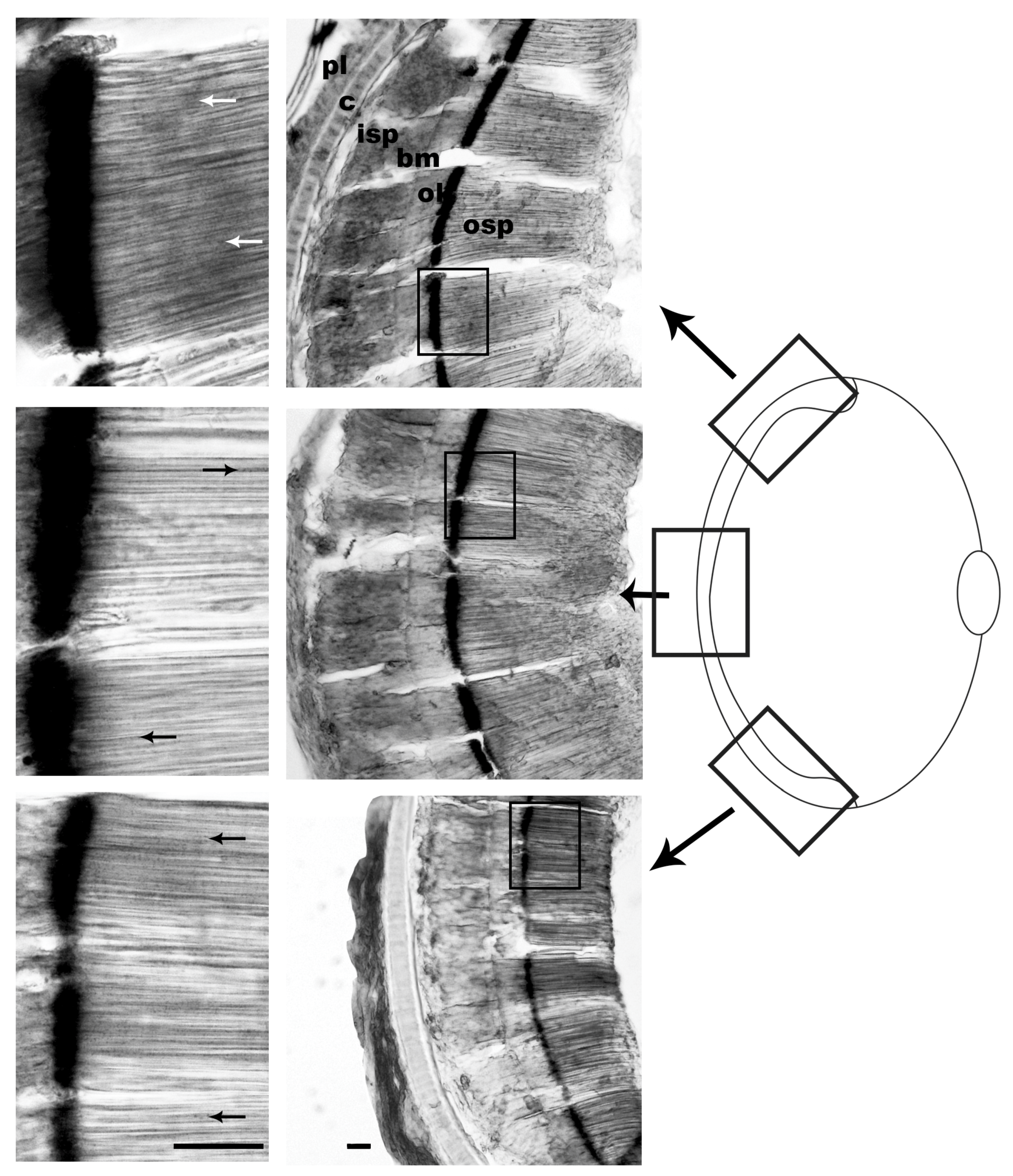

| ID | ML (mm) | Analysis Conducted | Light Exposure (min) | Dark Adaptation (min) |
|---|---|---|---|---|
| G01 | 30 | PM, NC | 90 | 1440 (24 h) |
| G02 | 15 | PM, Group 1 | 20 | 15 |
| G03 * | 15 | PM, Group 1 | 20 | 30 |
| G04 | 15 | PM, Group 1 | 20 | 45 |
| G05 | 15 | PM, Group 1 | 20 | 60 |
| G06 | 15 | PM, Group 1 | 20 | 75 |
| G07 | 10 | PM, Group 2 | 30 | 20 |
| G08 | 10 | PM, Group 2 | 30 | 30 |
| G09 * | 20 | PM, Group 2 | 30 | 40 |
| G10 | 10 | PM, Group 2 | 30 | 50 |
| G11 | 10 | PM, Group 2 | 30 | 60 |
| G12 | 15 | NE | 30 | 90 |
| G13 | 15 | NE | 30 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howard, R.B.; Kniller, J.; Bolstad, K.S.R.; Acosta, M.L. Biological Sunglasses in a Deep-Sea Squid: Pigment Migration in the Retina of Gonatus onyx. Vision 2024, 8, 26. https://doi.org/10.3390/vision8020026
Howard RB, Kniller J, Bolstad KSR, Acosta ML. Biological Sunglasses in a Deep-Sea Squid: Pigment Migration in the Retina of Gonatus onyx. Vision. 2024; 8(2):26. https://doi.org/10.3390/vision8020026
Chicago/Turabian StyleHoward, Ryan B., Jessica Kniller, Kathrin S. R. Bolstad, and Monica L. Acosta. 2024. "Biological Sunglasses in a Deep-Sea Squid: Pigment Migration in the Retina of Gonatus onyx" Vision 8, no. 2: 26. https://doi.org/10.3390/vision8020026
APA StyleHoward, R. B., Kniller, J., Bolstad, K. S. R., & Acosta, M. L. (2024). Biological Sunglasses in a Deep-Sea Squid: Pigment Migration in the Retina of Gonatus onyx. Vision, 8(2), 26. https://doi.org/10.3390/vision8020026





