A Pilot Study to Improve Cognitive Performance and Pupil Responses in Mild Cognitive Impaired Patients Using Gaze-Controlled Gaming
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Statement
2.3. Video Game
2.4. Neuropsychological Pre and Post Assessment Instruments
2.4.1. MoCA
2.4.2. CANTAB
- Rapid visual information processing (RVP) is a measure of sustained attention.
- Paired associates learning (PAL) assesses visual memory and new learning.
- The motor screening task (MST) provides a general assessment of sensorimotor deficits.
- Pattern recognition memory (PRM) is a test of visual pattern recognition memory in a 2-choice forced discrimination paradigm.
- Reaction time (RT) provides assessments of motor and mental response speeds, as well as measures of movement time, reaction time, response accuracy, and impulsivity.
- Spatial working memory (SWM) requires the retention and manipulation of visuospatial information.
- Delayed matching to sample (DMS) assesses both simultaneous visual matching ability and short-term visual recognition memory.
2.4.3. Visual Oddball Paradigm
2.5. Statistical Analysis
3. Results
3.1. Video Gaming
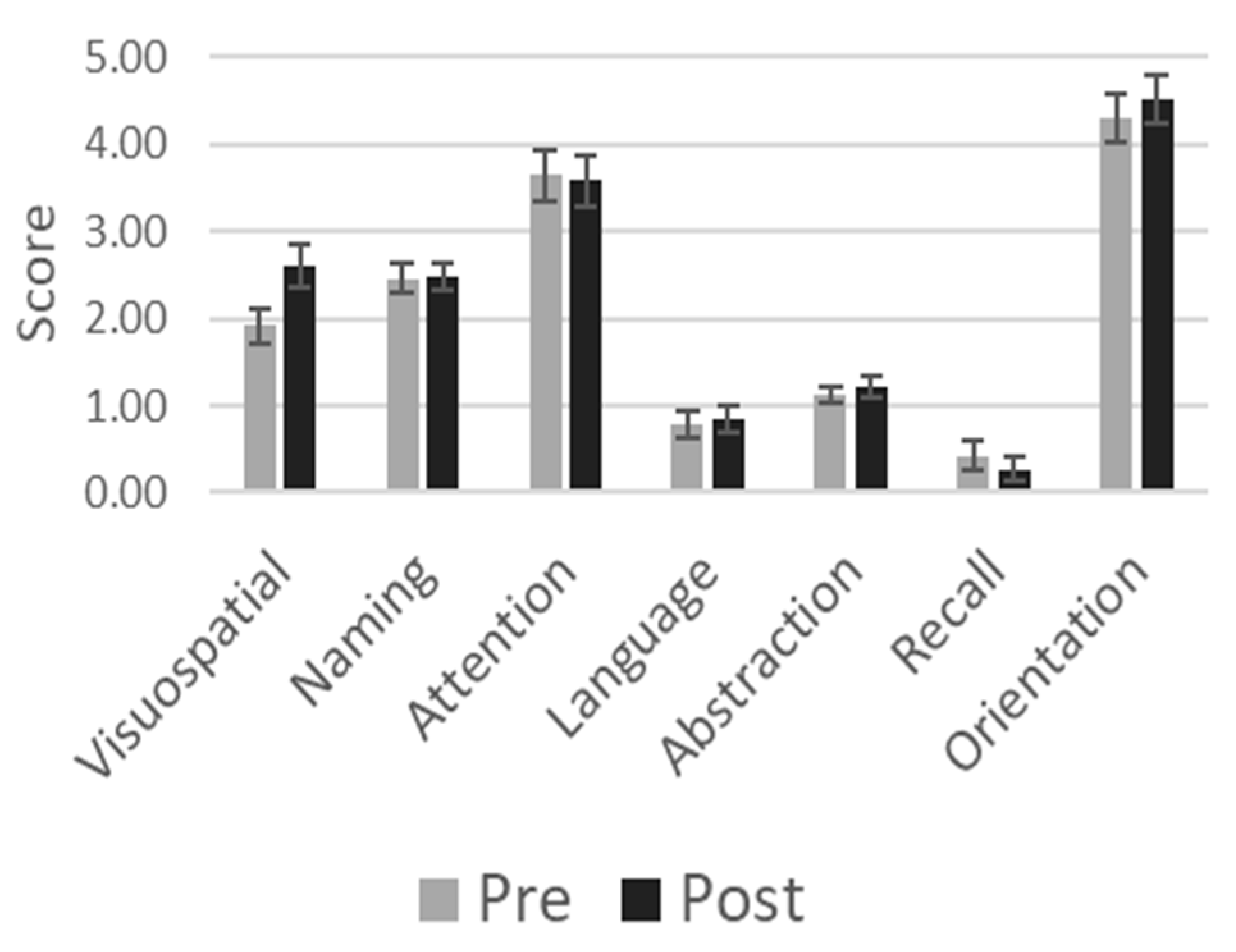
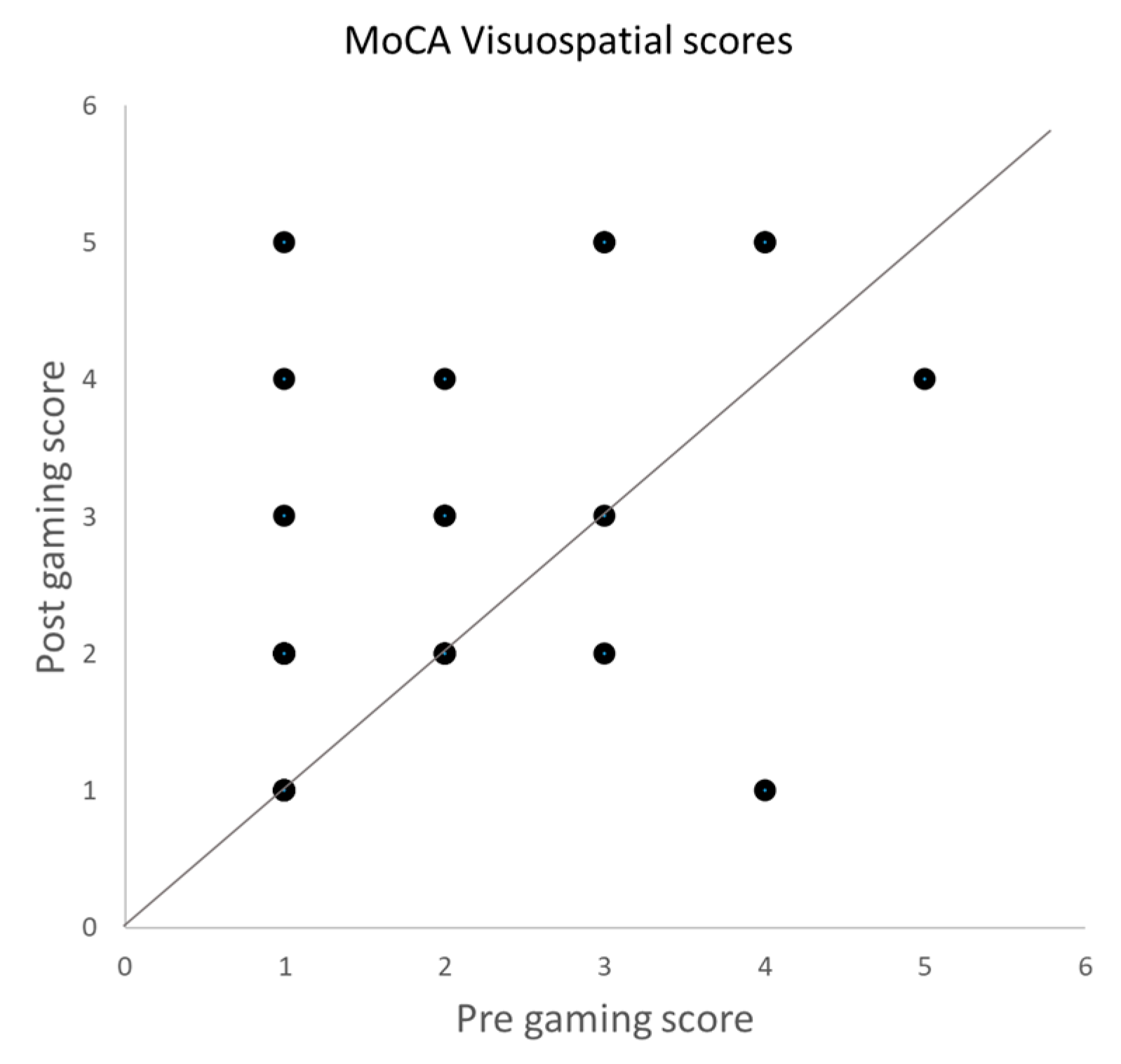
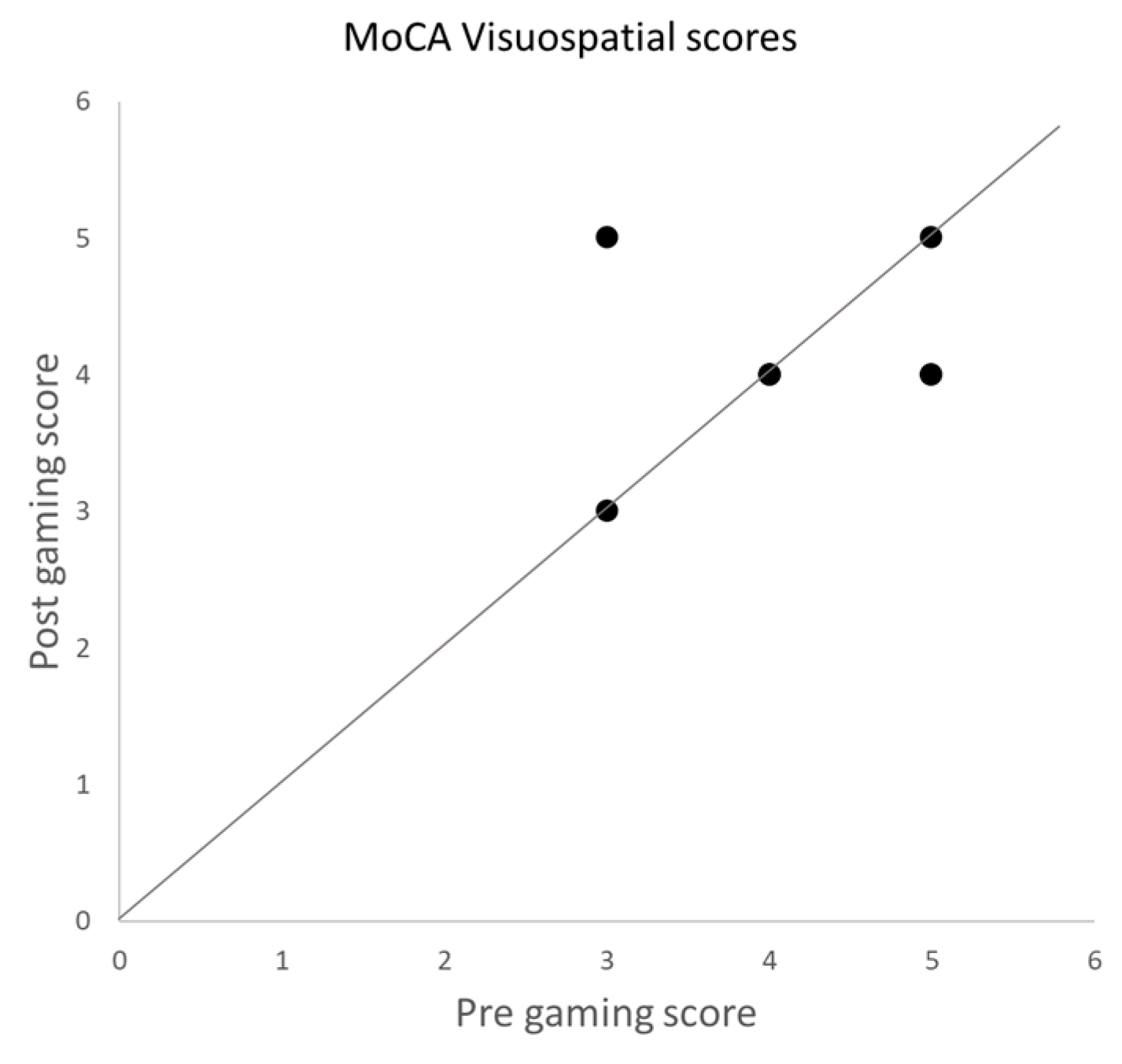
3.2. MoCA
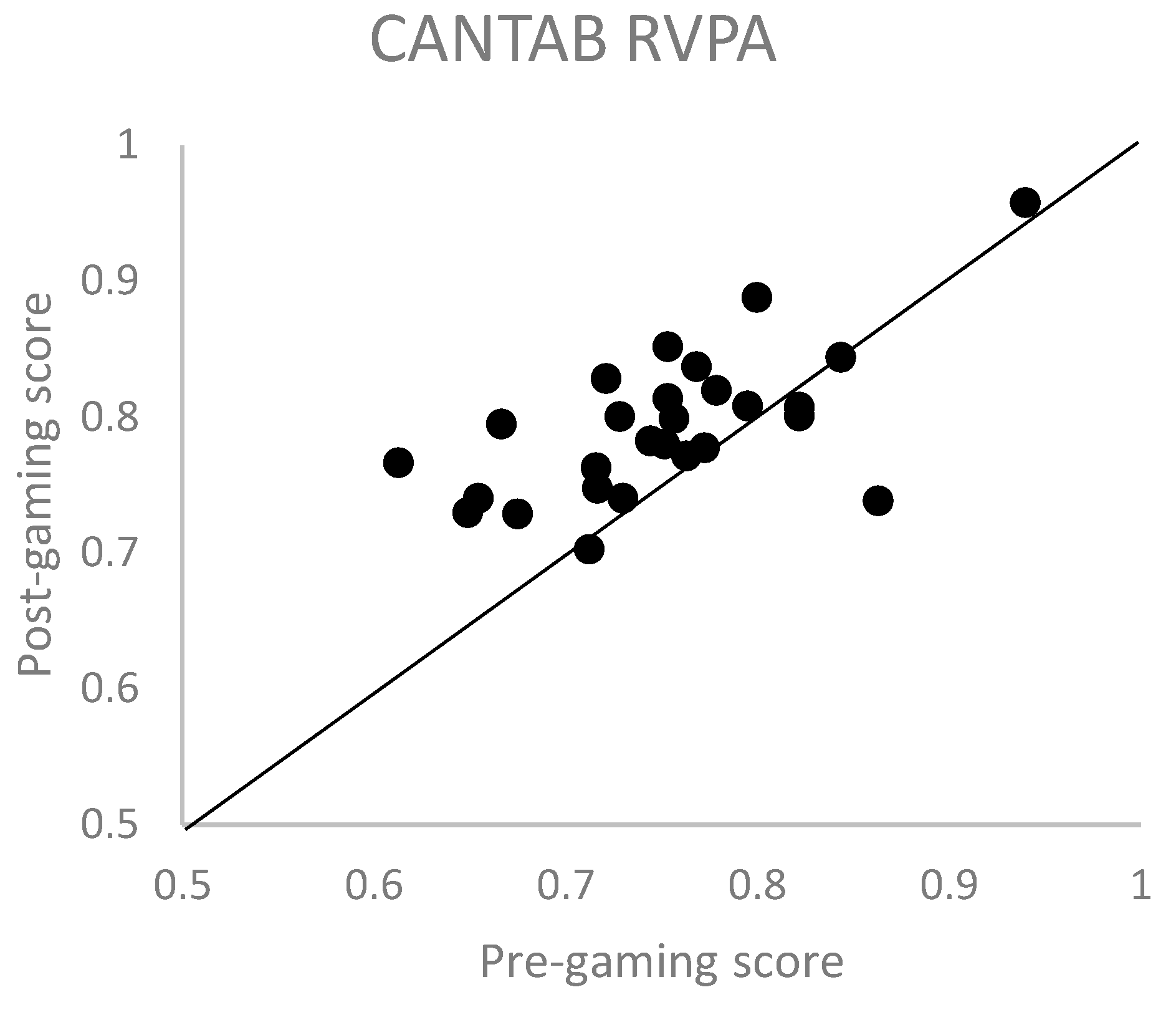
3.3. CANTAB
3.4. Control Group
3.5. Visual Oddball Paradigms and Pupil and Vergence Responses
4. Discussion
4.1. Pupil and Vergence Responses
4.2. Neurobiological Relevance
4.3. Shortcomings
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyle, P.A.; Wilson, R.S.; Aggarwal, N.T.; Tang, Y.; Bennett, D.A. Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology 2006, 67, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Breton, A.; Casey, D.; Arnaoutoglou, N.A. Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: Meta-analysis of diagnostic accuracy studies. Int. J. Geriatr. Psychiatry 2019, 34, 233–242. [Google Scholar] [CrossRef]
- Flicker, C.; Ferris, S.H.; Reisberg, B. Mild cognitive impairment in the elderly Predictors of dementia. Neurology 1991, 41, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Storandt, M.; Miller, J.P.; McKeel, D.W.; Price, J.L.; Rubin, E.H. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 2001, 58, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild Cognitive Impairment. Continuum 2016, 22, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Anguera, J.A.; Boccanfuso, J.; Rintoul, J.L.; Al-Hashimi, O.; Faraji, F.; Janowich, J.; Kong, E.; Larraburo, Y.; Rolle, C.; Johnston, E.; et al. Video game training enhances cognitive control in older adults. Nature 2013, 501, 97–101. [Google Scholar] [CrossRef]
- Basak, C.; Boot, W.R.; Voss, M.W.; Kramer, A.F. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol. Aging 2008, 23, 765. [Google Scholar] [CrossRef] [PubMed]
- Boot, W.R.; Kramer, A.F.; Simons, D.J.; Fabiani, M.; Gratton, G. The effects of video game playing on attention, memory, and executive control. Acta Psychol. 2008, 129, 387–398. [Google Scholar] [CrossRef]
- Green, C.S.; Bavelier, D. Action video game modifies visual selective attention. Nature 2003, 423, 534–537. [Google Scholar] [CrossRef]
- Kueider, A.M.; Parisi, J.M.; Gross, A.L.; Rebok, G.W. Computerized cognitive training with older adults: A systematic review. PLoS ONE 2012, 7, e40588. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Gleich, T.; Lorenz, R.C.; Lindenberger, U.; Gallinat, J. Playing Super Mario induces structural brain plasticity: Gray matter changes resulting from training with a commercial video game. Mol. Psychiatry 2014, 19, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Geng, J.; Yang, R.; Ge, Y.; Hesketh, T. Effectiveness of computerized cognitive training in delaying cognitive function decline in people with Mild Cognitive Impairment: Systematic review and meta-analysis. J. Med. Internet Res. 2022, 24, e38624. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Polat, U.; Makous, W.; Bavelier, D. Enhancing the contrast sensitivity function through action video game training. Nat. Neurosci. 2009, 12, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, S.; Franceschini, S.; Puccio, G.; Mancarella, M.; Gori, S.; Facoetti, A. Action Video Games Enhance Attentional Control and Phonological Decoding in Children with Developmental Dyslexia. Brain Sci. 2021, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Brito, F.; Ribeiro, F.; Aguiar de Sousa, D.; Costa, J.; Caneiras, C.; Carriço, L.; Verdelho, A. Are Video Games Effective to Promote Cognition and Everyday Functional Capacity in Mild Cognitive Impairment/Dementia Patients? A Meta-Analysis of Randomized Controlled Trials. J. Alzheimers Dis. 2021, 84, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Liu, M.F.; Ho, M.H.; Lin, Y.K.; Hsiao, Y.L.; Wang, M.H.; Chang, C.C.; Montayre, J. A Pilot Study of Interactive-Video Games in People with Mild Cognitive Impairment. Int. J. Environ. Res. Public. Health 2022, 19, 3536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.H.; Park, J.H. Does cognition-specific computer training have better clinical outcomes than non-specific computer training? A single-blind, randomized controlled trial. Clin. Rehabil. 2018, 32, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Coyle, H.; Traynor, V.; Solowij, N. Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: Systematic review of the literature. Am. J. Geriatr. Psychiatry 2015, 23, 335–359. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wu, X.; Shu, X.; Hu, H.; Chen, Q.; Peng, L.; Feng, H. Effects of computerised cognitive training on cognitive impairment: A meta-analysis. J. Neurol. 2021, 268, 1680–1688. [Google Scholar] [CrossRef]
- Kletzel, S.L. Effectiveness of Brain Gaming in Older Adults with Cognitive Impairments: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 2281–2288.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huntley, J.; Bhome, R.; Holmes, B.; Cahill, J.; Gould, R.L.; Wang, H.; Yu, X.; Howard, R. Effect of computerised cognitive training on cognitive outcomes in mild cognitive impairment: A systematic review and meta-analysis. BMJ Open 2019, 9, e027062. [Google Scholar] [CrossRef] [PubMed]
- Gates, N.J.; Vernooij, R.W.; Di Nisio, M.; Karim, S.; March, E.; Martínez, G.; Rutjes, A.W. Computerised cognitive training for preventing dementia in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2019, 3, CD012279. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.T.; Mowszowski, L.; Naismith, S.L.; Chadwick, V.L.; Valenzuela, M.; Lampit, A. Computerized Cognitive Training in Older Adults with Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2017, 174, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Guath, M.; Willfors, C.; Björlin Avdic, H.; Nordgren, A.; Kleberg, J. Pupillary response in reward processing in adults with major depressive disorder in remission. J. Int. Neuropsychol. Soc. 2023, 29, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.C.; Sierra-Marcos, A.; Romeo, A.; Hashemi, A.; Leonovych, O.; Bustos Valenzuela, P.; Solé Puig, M.; Supèr, H. Altered Vergence Eye Movements and Pupil Response of Patients with Alzheimer’s Disease and Mild Cognitive Impairment During an Oddball Task. J. Alzheimers Dis. 2021, 82, 421–433. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.; Fouquaet, I.; Boets, B.; Naulaers, G.; Steyaert, J. Autism spectrum disorder and pupillometry: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 120, 479–508. [Google Scholar] [CrossRef]
- Johnson, B.P.; Lum, J.A.; Rinehart, N.J.; Fielding, J. Ocular motor disturbances in autism spectrum disorders: Systematic review and comprehensive meta-analysis. Neurosci. Biobehav. Rev. 2016, 69, 260–279. [Google Scholar] [CrossRef]
- Kapoula, Z.; Yang, Q.; Otero-Millan, J.; Xiao, S.; Macknik, S.L.; Lang, A.; Verny, M.; Martinez-Conde, S. Distinctive Features Microsaccades Alzheimer’s disease and in mild cognitive impairment. Age 2014, 36, 535–543. [Google Scholar] [CrossRef]
- Myles, J.B.; Rossell, S.L.; Phillipou, A.; Thomas, E.; Gurvich, C. Insights to the schizophrenia continuum: A systematic review of saccadic eye movements in schizotypy and biological relatives of schizophrenia patients. Neurosci. Biobehav. Rev. 2017, 72, 278–300. [Google Scholar] [CrossRef]
- Varela Casal, P.; Esposito, F.L.; Morata Martínez, I.; Capdevila, A.; Solé Puig, M.; de la Osa, N.; Ezpeleta, L.; Perera i Lluna, A.; Faraone, S.V.; Ramos-Quiroga, J.A.; et al. Clinical Validation of Eye Vergence as an Objective Marker for Diagnosis of ADHD in Children. J. Atten. Disord. 2019, 23, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Gold, J.I. Pupil Size as a Window on Neural Substrates of Cognition. Trends Cogn. Sci. 2020, 24, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Strauch, C.; Koniakowsky, I.; Huckauf, A. Decision Making and Oddball Effects on Pupil Size: Evidence for a Sequential Process. J. Cogn. 2020, 3, 7. [Google Scholar] [CrossRef]
- Strauch, C.; Wang, C.A.; Einhäuser, W.; Van der Stigchel, S.; Naber, M. Pupillometry as an integrated readout of distinct attentional networks. Trends Neurosci. 2022, 45, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Bonneh, Y.S.; Adini, Y.; Polat, U. Contrast sensitivity revealed by microsaccades. J. Vision. 2015, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Engbert, R.; Kliegl, R. Microsaccades uncover the orientation of covert attention. Vis. Res. 2003, 43, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z.M.; Clark, J.J. Microsaccades as an overt measure of covert attention shifts. Vis. Res. 2002, 42, 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Conde, S.; Macknik, S.L.; Troncoso, X.G.; Dyar, T. Microsaccades counteract visual fading during fixation. Neuron 2006, 49, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Scholes, C.; McGraw, P.V.; Nyström, M.; Roach, N.W. Fixational eye movements predict visual sensitivity. Proc. Biol. Sci. 2015, 282, 20151568. [Google Scholar] [CrossRef]
- Solé Puig, M.; Pérez Zapata, L.; Aznar-Casanova, J.A.; Supèr, H. A role of eye vergence in covert attention. PLoS ONE 2013, 8, e52955. [Google Scholar] [CrossRef]
- García-Baos, A.; D‘Amelio, T.; Oliveira, I.; Collins, P.; Echevarria, C.; Zapata, L.P.; Liddle, E.; Supèr, H. Novel Interactive Eye-Tracking Game for Training Attention in Children with Attention-Deficit/Hyperactivity Disorder. Prim. Care Companion CNS Disord. 2019, 21, 19m02428. [Google Scholar] [CrossRef] [PubMed]
- Egerházi, A.; Berecz, R.; Bartók, E.; Degrell, I. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.A.; Heaps, J.M.; Bolzenius, J.D.; Salminen, L.E.; Baker, L.M.; Scott, S.E.; Paul, R.H. Longitudinal Change in Performance on the Montreal Cognitive Assessment in Older Adults. Clin. Neuropsychol. 2015, 29, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.E.; Fang, H.; Snijders, T.; Allison, D.J.; Zulyniak, M.A.; Chabowski, A.; Parise, G.; Phillips, S.M.; Heisz, J.J. A Multi-Ingredient Nutritional Supplement in Combination with Resistance Exercise and High-Intensity Interval Training Improves Cognitive Function and Increases N-3 Index in Healthy Older Men: A Randomized Controlled Trial. Front. Aging Neurosci. 2019, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.K.S.; Lam, B.Y.H.; Lai, D.W.L.; Bai, X.; Li, J.; Zou, Z.; Chan, C.C.H. Stability of Montreal Cognitive Assessment in Individuals with Mild Cognitive Impairment: Potential Influence of Practice Effect. J. Alzheimers Dis. 2022, 87, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, F.; Salvadori, N.; Eusebi, P.; Lisetti, V.; Luchetti, E.; Calabresi, P.; Parnetti, L. Evidence of practice effect in CANTAB spatial working memory test in a cohort of patients with mild cognitive impairment. Appl. Neuropsychol. Adult 2017, 25, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.M.; Pinho, M.S.; Simões, M.R. Test–retest reliability analysis of the Cambridge Neuropsychological Automated Tests for the assessment of dementia in older people living in retirement homes. Appl. Neuropsychol. Adult 2016, 23, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Lisi, M.; Bonato, M.; Zorzi, M. Pupil dilation reveals top–down attentional load during spatial monitoring. Biol. Psychol. 2015, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Solé Puig, M.; Romeo, A.; Supèr, H. Vergence eye movements during figure-ground perception. Conscious. Cogn. 2021, 92, 103138. [Google Scholar] [CrossRef]
- van der Wel, P.; van Steenbergen, H. Pupil dilation as an index of effort in cognitive control tasks: A review. Psychon. Bull. Rev. 2018, 25, 2005–2015. [Google Scholar] [CrossRef]
- Pajkossy, P.; Szőllősi, Á.; Racsmány, M. Pupil size changes signal hippocampus-related memory functions. Sci. Rep. 2020, 10, 16393. [Google Scholar] [CrossRef] [PubMed]
- Papesh, M.H.; Goldinger, S.D.; Hout, M.C. Memory strength and specificity revealed by pupillometry. Int. J. Psychophys. 2012, 83, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.; Uengoer, M.; Lachnit, H. Pupil dilation indicates the coding of past prediction errors: Evidence for attentional learning theory. Psychophysics 2018, 55, e13020. [Google Scholar] [CrossRef] [PubMed]
- Urai, A.E.; Braun, A.; Donner, T.H. Pupil-linked arousal is driven by decision uncertainty and alters serial choice bias. Nat. Commun. 2017, 8, 14637. [Google Scholar] [CrossRef] [PubMed]
- Van Slooten, J.C.; Jahfari, S.; Knapen, T.; Theeuwes, J. How pupil responses track value-based decision-making during and after reinforcement learning. PLoS Comput. Biol. 2018, 14, e1006632. [Google Scholar] [CrossRef] [PubMed]
- Solé Puig, M.; Marco Pallarés, J.; Perez Zapata, L.; Puigcerver, L.; Cañete Crespillo, J.; Supèr, H. Attentional selection accompanied by eye vergence as revealed by event-related brain potentials. PLoS ONE 2016, 11, e0167646. [Google Scholar] [CrossRef] [PubMed]
- Solé Puig, M.; Romeo, A.; Cañete Crespillo, J.; Supèr, H. Eye vergence responses during a visual memory task. Neuroreport 2016, 28, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Krebs, R.M.; Park, H.R.P.; Bombeke, K.; Boehler, C.N. Modulation of locus coeruleus activity by novel oddball stimuli. Brain Imaging Behav. 2018, 12, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Chavira, A.Q.; García, O.; Arias-Trejo, N. Pupil response and attention skills in Down syndrome. Res. Dev. Disabil. 2017, 70, 40–49. [Google Scholar] [CrossRef]
- Kang, O.E.; Huffer, K.E.; Wheatley, T.P. Pupil Dilation Dynamics Track Attention to High-Level Information. PLoS ONE 2014, 9, e102463. [Google Scholar] [CrossRef]
- Bohlen, M.O.; Warren, S.; May, P.J. A central mesencephalic reticular formation projection to medial rectus motoneurons supplying singly and multiply innervated extraocular muscle fibers. J. Comp. Neurol. 2017, 525, 2000–2018. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Júnior, E.D.; Da Silva, A.V.; Da Silva, K.R.T.; Haemmerle, C.A.S.; Batagello, D.S.; Da Silva, J.M.; Lima, L.B.; Da Silva, R.J.; Diniz, G.B.; Sita, L.V.; et al. The centrally projecting Edinger–Westphal nucleus—I: Efferents in the rat brain. J. Chem. Neuroanat. 2015, 68, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Kozicz, T.; Bittencourt, J.C.; May, P.J.; Reiner, A.; Gamlin, P.D.R.; Palkovits, M.; Horn, A.K.E.; Toledo, C.A.B.; Ryabinin, A.E. The Edinger-westphal nucleus: A historical, structural and functional perspective on a dichotomous terminology. J. Comp. Neurol. 2011, 519, 1413–1434. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Pan, K.; Li, H.; Pang, P.; Guo, Y.; Shu, S.; Cai, Y.; Pei, L.; Liu, D.; et al. Serotonin receptor 2c-expressing cells in the ventral CA1 control attention via innervation of the Edinger–Westphal nucleus. Nat. Neurosci. 2018, 21, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Gamlin, P.D.; Yoon, K. An area for vergence eye movement in primate frontal cortex. Nature 2000, 407, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Jodo, E.; Aston-Jones, G. Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain Res. 1997, 768, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, K.; Rowe, F.J. Role of neural integrators in oculomotor systems: A systematic narrative literature review. Acta Ophthalmol. 2018, 96, e111–e118. [Google Scholar] [CrossRef]
- Kelberman, M.; Keilholz, S.; Weinshenker, D. What’s That (Blue) Spot on my MRI? Multimodal Neuroimaging of the Locus Coeruleus in Neurodegenerative Disease. Front. Neurosci. 2020, 14, 583421. [Google Scholar] [CrossRef]

| Type | Pre-Treatment Mean (SD) | Post-Treatment Mean (SD) | Participants N | Test Statistics | ||
|---|---|---|---|---|---|---|
| Ties | Z | p | ||||
| RVPML | 887.00(340.36) | 787.89(205.26) | 29 | 0 | 1.6866 | 0.0458 |
| RVPLSD | 409.24(138.68) | 337.10(145.22) | 29 | 0 | 2.1842 | 0.0145 |
| RVPA | 0.75(0.07) | 0.79(0.05) | 27 | 2 | −3.4476 | <0.001 |
| RVPTH | 16.59(10.29) | 22.89(15.58) | 27 | 2 | −2.1403 | 0.0162 |
| RVPTFA | 35.81(36.73) | 65.30(99.65) | 25 | 3 | −0.4858 | 0.3136 |
| RVPPH | 0.31(0.19) | 0.42(0.29) | 27 | 2 | −1.9646 | 0.0247 |
| RVPTM | 34.83(13.84) | 30.59(15.63) | 27 | 2 | 1.5505 | 0.0605 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solé Puig, M.; Bustos Valenzuela, P.; Romeo, A.; Supèr, H. A Pilot Study to Improve Cognitive Performance and Pupil Responses in Mild Cognitive Impaired Patients Using Gaze-Controlled Gaming. Vision 2024, 8, 25. https://doi.org/10.3390/vision8020025
Solé Puig M, Bustos Valenzuela P, Romeo A, Supèr H. A Pilot Study to Improve Cognitive Performance and Pupil Responses in Mild Cognitive Impaired Patients Using Gaze-Controlled Gaming. Vision. 2024; 8(2):25. https://doi.org/10.3390/vision8020025
Chicago/Turabian StyleSolé Puig, Maria, Patricia Bustos Valenzuela, August Romeo, and Hans Supèr. 2024. "A Pilot Study to Improve Cognitive Performance and Pupil Responses in Mild Cognitive Impaired Patients Using Gaze-Controlled Gaming" Vision 8, no. 2: 25. https://doi.org/10.3390/vision8020025
APA StyleSolé Puig, M., Bustos Valenzuela, P., Romeo, A., & Supèr, H. (2024). A Pilot Study to Improve Cognitive Performance and Pupil Responses in Mild Cognitive Impaired Patients Using Gaze-Controlled Gaming. Vision, 8(2), 25. https://doi.org/10.3390/vision8020025





