Ocular Biomechanics and Glaucoma
Abstract
1. Introduction
2. The Ocular Response Analyzer (ORA)

3. The Corvis ST Dynamic Scheimpflug Analyzer
4. Hysteresis and Glaucoma
4.1. The Influence of Increased Stiffness of Ocular Structures in the Pathobiology of Glaucoma
4.2. Clinical Evidence on the Relationship between Hysteresis and Glaucoma
5. DCR Parameters and Glaucoma
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Roberts, C.J. Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis. J. Cataract. Refract. Surg. 2005, 31, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Fontes, B.M.; Ambrosio, R., Jr.; Jardim, D.; Velarde, G.C.; Nose, W. Corneal biomechanical metrics and anterior segment parameters in mild keratoconus. Ophthalmology 2010, 117, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.; Fontes, B.M.; Lopes, B.; Ramos, I.; Schor, P.; Ambrosio, R., Jr. ORA waveform-derived biomechanical parameters to distinguish normal from keratoconic eyes. Arq. Bras. Oftalmol. 2013, 76, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ventura, B.V.; Machado, A.P.; Ambrosio, R., Jr.; Ribeiro, G.; Araujo, L.N.; Luz, A.; Lyra, J.M. Analysis of waveform-derived ORA parameters in early forms of keratoconus and normal corneas. J. Refract. Surg. 2013, 29, 637–643. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.A.S.; da Silva, R.S.; Ambrósio, R., Jr. Relevância da biomecânica da córnea no glaucoma. Rev. Bras. Oftalmol. 2012, 73, 37–39. [Google Scholar] [CrossRef]
- Catania, F.; Morenghi, E.; Rosetta, P.; Paolo, V.; Vinciguerra, R. Corneal Biomechanics Assessment with Ultra High Speed Scheimpflug Camera in Primary Open Angle Glaucoma Compared with Healthy Subjects: A meta-analysis of the Literature. Curr. Eye Res. 2023, 48, 161–171. [Google Scholar] [CrossRef]
- Qassim, A.; Mullany, S.; Abedi, F.; Marshall, H.; Hassall, M.M.; Kolovos, A.; Knight, L.S.W.; Nguyen, T.; Awadalla, M.S.; Chappell, A.; et al. Corneal Stiffness Parameters Are Predictive of Structural and Functional Progression in Glaucoma Suspect Eyes. Ophthalmology 2021, 128, 993–1004. [Google Scholar] [CrossRef]
- Salvetat, M.L.; Zeppieri, M.; Tosoni, C.; Felletti, M.; Grasso, L.; Brusini, P. Corneal Deformation Parameters Provided by the Corvis-ST Pachy-Tonometer in Healthy Subjects and Glaucoma Patients. J. Glaucoma 2015, 24, 568–574. [Google Scholar] [CrossRef]
- Lanzagorta-Aresti, A.; Perez-Lopez, M.; Palacios-Pozo, E.; Davo-Cabrera, J. Relationship between corneal hysteresis and lamina cribrosa displacement after medical reduction of intraocular pressure. Br. J. Ophthalmol. 2017, 101, 290–294. [Google Scholar] [CrossRef]
- Zeimer, R.C.; Ogura, Y. The relation between glaucomatous damage and optic nerve head mechanical compliance. Arch. Ophthalmol. 1989, 107, 1232–1234. [Google Scholar] [CrossRef]
- Kimball, E.C.; Nguyen, C.; Steinhart, M.R.; Nguyen, T.D.; Pease, M.E.; Oglesby, E.N.; Oveson, B.C.; Quigley, H.A. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp. Eye Res. 2014, 128, 129–140. [Google Scholar] [CrossRef]
- Luce, D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract. Refract. Surg. 2005, 31, 156–162. [Google Scholar] [CrossRef]
- Brandt, J.D. Central corneal thickness, tonometry, and glaucoma risk—A guide for the perplexed. Can. J. Ophthalmol. 2007, 42, 562–566. [Google Scholar] [CrossRef]
- Roberts, C.J. Corneal hysteresis and beyond: Does it involve the sclera? J. Cataract. Refract. Surg. 2021, 47, 427–429. [Google Scholar] [CrossRef]
- Taroni, L.; Bernabei, F.; Pellegrini, M.; Roda, M.; Toschi, P.G.; Mahmoud, A.M.; Schiavi, C.; Giannaccare, G.; Roberts, C.J. Corneal Biomechanical Response Alteration After Scleral Buckling Surgery for Rhegmatogenous Retinal Detachment. Am. J. Ophthalmol. 2020, 217, 49–54. [Google Scholar] [CrossRef]
- Roberts, C.J. Concepts and misconceptions in corneal biomechanics. J. Cataract. Refract. Surg. 2014, 40, 862–869. [Google Scholar] [CrossRef]
- Hager, A.; Schroeder, B.; Sadeghi, M.; Grossherr, M.; Wiegand, W. The influence of corneal hysteresis and corneal resistance factor on the measurement of intraocular pressure. Ophthalmologe 2007, 104, 484–489. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Li, L.; Sun, R.; Zhang, H. Comparison of intraocular pressure measured by ocular response analyzer and Goldmann applanation tonometer after corneal refractive surgery: A systematic review and meta-analysis. BMC Ophthalmol. 2020, 20, 23. [Google Scholar] [CrossRef]
- Nguyen, B.A.; Roberts, C.J.; Reilly, M.A. Biomechanical Impact of the Sclera on Corneal Deformation Response to an Air-Puff: A Finite-Element Study. Front. Bioeng. Biotechnol. 2018, 6, 210. [Google Scholar] [CrossRef]
- Yuhas, P.T.; Roberts, C.J. Clinical Ocular Biomechanics: Where Are We after 20 Years of Progress? Curr. Eye Res. 2023, 48, 89–104. [Google Scholar] [CrossRef]
- Kaushik, S.; Pandav, S.S. Ocular Response Analyzer. J. Curr. Glaucoma. Pract. 2021, 6, 17–19. [Google Scholar] [CrossRef]
- Nguyen, B.A.; Reilly, M.A.; Roberts, C.J. Biomechanical contribution of the sclera to dynamic corneal response in air-puff induced deformation in human donor eyes. Exp. Eye Res. 2020, 191, 107904. [Google Scholar] [CrossRef]
- Metzler, K.M.; Mahmoud, A.M.; Liu, J.; Roberts, C.J. Deformation response of paired donor corneas to an air puff: Intact whole globe versus mounted corneoscleral rim. J. Cataract. Refract. Surg. 2014, 40, 888–896. [Google Scholar] [CrossRef]
- Ambrósio, R., Jr.; Ramos, I.; Luz, A.; Faria, F.C.; Andreas, S.; Krug, M.; Belin, M.W.; Roberts, C.J. Dynamic ultra-high speed Scheimpflug imaging for assessing corneal biomechanical properties. Rev. Bras. Oftalmol. 2013, 72, 99–102. [Google Scholar] [CrossRef]
- Leszczynska, A.; Moehler, K.; Spoerl, E.; Ramm, L.; Herber, R.; Pillunat, L.E.; Terai, N. Measurement of Orbital Biomechanical Properties in Patients with Thyroid Orbitopathy Using the Dynamic Scheimpflug Analyzer (Corvis ST). Curr. Eye Res. 2018, 43, 289–292. [Google Scholar] [CrossRef]
- Roberts, C.J.; Mahmoud, A.M.; Bons, J.P.; Hossain, A.; Elsheikh, A.; Vinciguerra, R.; Vinciguerra, P.; Ambrosio, R., Jr. Introduction of Two Novel Stiffness Parameters and Interpretation of Air Puff-Induced Biomechanical Deformation Parameters With a Dynamic Scheimpflug Analyzer. J. Refract. Surg. 2017, 33, 266–273. [Google Scholar] [CrossRef]
- Brazuna, R.; Salomão, M.; Esporcatte, B.; Macedo, M.; Esporcatte, L.; Colombini, G.N.; Ambrósio, R., Jr. Corneal Biomechanics and Glaucoma Beyond the Bidirectional Impact of Intraocular Pressure and Corneal Deformation Response. Rev. Bras. Oftalmol. 2022, 81, e0036. [Google Scholar] [CrossRef]
- Joda, A.A.; Shervin, M.M.; Kook, D.; Elsheikh, A. Development and validation of a correction equation for Corvis tonometry. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 943–953. [Google Scholar] [CrossRef]
- Vinciguerra, R.; Ambrosio, R., Jr.; Elsheikh, A.; Roberts, C.J.; Lopes, B.; Morenghi, E.; Azzolini, C.; Vinciguerra, P. Detection of Keratoconus With a New Biomechanical Index. J. Refract. Surg. 2016, 32, 803–810. [Google Scholar] [CrossRef]
- Ambrosio, R., Jr.; Lopes, B.T.; Faria-Correia, F.; Salomao, M.Q.; Buhren, J.; Roberts, C.J.; Elsheikh, A.; Vinciguerra, R.; Vinciguerra, P. Integration of Scheimpflug-Based Corneal Tomography and Biomechanical Assessments for Enhancing Ectasia Detection. J. Refract. Surg. 2017, 33, 434–443. [Google Scholar] [CrossRef]
- Sedaghat, M.R.; Momeni-Moghaddam, H.; Ambrosio, R., Jr.; Roberts, C.J.; Yekta, A.A.; Danesh, Z.; Reisdorf, S.; Khabazkhoob, M.; Heidari, H.R.; Sadeghi, J. Long-term Evaluation of Corneal Biomechanical Properties After Corneal Cross-linking for Keratoconus: A 4-Year Longitudinal Study. J. Refract. Surg. 2018, 34, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Eliasy, A.; Chen, K.J.; Vinciguerra, R.; Lopes, B.T.; Abass, A.; Vinciguerra, P.; Ambrosio, R., Jr.; Roberts, C.J.; Elsheikh, A. Determination of Corneal Biomechanical Behavior in-vivo for Healthy Eyes Using CorVis ST Tonometry: Stress-Strain Index. Front. Bioeng. Biotechnol. 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, T.; Matsuura, M.; Fujino, Y.; Murata, H.; Tokumo, K.; Nakakura, S.; Kiuchi, Y.; Asaoka, R. The Relationship Between Corvis ST Tonometry Parameters and Ocular Response Analyzer Corneal Hysteresis. J. Glaucoma 2020, 29, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Pasquale, L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017, 26, R21–R27. [Google Scholar] [CrossRef]
- Wareham, L.K.; Calkins, D.J. The Neurovascular Unit in Glaucomatous Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 452. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; John, S.W.M. Glaucoma as a Metabolic Optic Neuropathy: Making the Case for Nicotinamide Treatment in Glaucoma. J. Glaucoma 2017, 26, 1161–1168. [Google Scholar] [CrossRef]
- Bell, K.; Funke, S.; Grus, F.H. Autoimmunity and glaucoma. Ophthalmologe 2019, 116, 18–27. [Google Scholar] [CrossRef]
- Grant, W.M. Clinical measurements of aqueous outflow. Am. J. Ophthalmol. 1951, 34, 1603–1605. [Google Scholar] [CrossRef]
- Johnson, M.; Shapiro, A.; Ethier, C.R.; Kamm, R.D. Modulation of outflow resistance by the pores of the inner wall endothelium. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1670–1675. [Google Scholar]
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2147–2152. [Google Scholar] [CrossRef]
- Yemanyi, F.; Raghunathan, V. Lysophosphatidic Acid and IL-6 Trans-signaling Interact via YAP/TAZ and STAT3 Signaling Pathways in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
- Ethier, C.R.; Simmons, C.A. Introductory Biomechanics: From Cells to Organisms; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Dhamodaran, K.; Baidouri, H.; Sandoval, L.; Raghunathan, V. Wnt Activation After Inhibition Restores Trabecular Meshwork Cells Toward a Normal Phenotype. Investig. Ophthalmol. Vis. Sci. 2020, 61, 30. [Google Scholar] [CrossRef] [PubMed]
- Yarishkin, O.; Phuong, T.T.T.; Baumann, J.M.; De Ieso, M.L.; Vazquez-Chona, F.; Rudzitis, C.N.; Sundberg, C.; Lakk, M.; Stamer, W.D.; Križaj, D. Piezo1 channels mediate trabecular meshwork mechanotransduction and promote aqueous fluid outflow. J. Physiol. 2021, 599, 571–592. [Google Scholar] [CrossRef]
- Zhu, W.; Hou, F.; Fang, J.; Bahrani Fard, M.R.; Liu, Y.; Ren, S.; Wu, S.; Qi, Y.; Sui, S.; Read, A.T.; et al. The role of Piezo1 in conventional aqueous humor outflow dynamics. iScience 2021, 24, 102042. [Google Scholar] [CrossRef] [PubMed]
- Lakk, M.; Križaj, D. TRPV4-Rho signaling drives cytoskeletal and focal adhesion remodeling in trabecular meshwork cells. Am. J. Physiol. Cell Physiol. 2021, 320, C1013–C1030. [Google Scholar] [CrossRef]
- Madekurozwa, M.; Stamer, W.D.; Reina-Torres, E.; Sherwood, J.M.; Overby, D.R. The ocular pulse decreases aqueous humor outflow resistance by stimulating nitric oxide production. Am. J. Physiol. Cell Physiol. 2021, 320, C652–C665. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.A.; Ju, H.H.; Kim, J.E.; Paik, S.Y.; Rao, P.V. Role of MCP-1 and IL-8 in viral anterior uveitis, and contractility and fibrogenic activity of trabecular meshwork cells. Sci. Rep. 2021, 11, 14950. [Google Scholar] [CrossRef]
- Sigal, I.A.; Flanagan, J.G.; Ethier, C.R. Factors influencing optic nerve head biomechanics. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4189–4199. [Google Scholar] [CrossRef]
- Hua, Y.; Voorhees, A.P.; Jan, N.J.; Wang, B.; Waxman, S.; Schuman, J.S.; Sigal, I.A. Role of radially aligned scleral collagen fibers in optic nerve head biomechanics. Exp. Eye Res. 2020, 199, 108188. [Google Scholar] [CrossRef]
- Safa, B.N.; Wong, C.A.; Ha, J.; Ethier, C.R. Glaucoma and biomechanics. Curr. Opin. Ophthalmol. 2022, 33, 80–90. [Google Scholar] [CrossRef]
- Szeto, J.; Chow, A.; McCrea, L.; Mozzer, A.; Nguyen, T.D.; Quigley, H.A.; Pitha, I. Regional Differences and Physiologic Behaviors in Peripapillary Scleral Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2021, 62, 27. [Google Scholar] [CrossRef]
- Murienne, B.J.; Chen, M.L.; Quigley, H.A.; Nguyen, T.D. The contribution of glycosaminoglycans to the mechanical behaviour of the posterior human sclera. J. R. Soc. Interface 2016, 13, 20160367. [Google Scholar] [CrossRef]
- Morris, H.J.; Tang, J.; Cruz Perez, B.; Pan, X.; Hart, R.T.; Weber, P.A.; Liu, J. Correlation between biomechanical responses of posterior sclera and IOP elevations during micro intraocular volume change. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7215–7222. [Google Scholar] [CrossRef] [PubMed]
- Sigal, I.A.; Yang, H.; Roberts, M.D.; Grimm, J.L.; Burgoyne, C.F.; Demirel, S.; Downs, J.C. IOP-induced lamina cribrosa deformation and scleral canal expansion: Independent or related? Investig. Ophthalmol. Vis. Sci. 2011, 52, 9023–9032. [Google Scholar] [CrossRef] [PubMed]
- Coudrillier, B.; Campbell, I.C.; Read, A.T.; Geraldes, D.M.; Vo, N.T.; Feola, A.; Mulvihill, J.; Albon, J.; Abel, R.L.; Ethier, C.R. Effects of Peripapillary Scleral Stiffening on the Deformation of the Lamina Cribrosa. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Addicks, E.M. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch. Ophthalmol. 1981, 99, 137–143. [Google Scholar] [CrossRef]
- Voorhees, A.P.; Hua, Y.; Brazile, B.L.; Wang, B.; Waxman, S.; Schuman, J.S.; Sigal, I.A. So-Called Lamina Cribrosa Defects May Mitigate IOP-Induced Neural Tissue Insult. Investig. Ophthalmol. Vis. Sci. 2020, 61, 15. [Google Scholar] [CrossRef]
- Eilaghi, A.; Flanagan, J.G.; Simmons, C.A.; Ethier, C.R. Effects of scleral stiffness properties on optic nerve head biomechanics. Ann. Biomed. Eng. 2010, 38, 1586–1592. [Google Scholar] [CrossRef]
- Wong, B.J.; Moghimi, S.; Zangwill, L.M.; Christopher, M.; Belghith, A.; Ekici, E.; Bowd, C.; Fazio, M.A.; Girkin, C.A.; Weinreb, R.N. Relationship of Corneal Hysteresis and Anterior Lamina Cribrosa Displacement in Glaucoma. Am. J. Ophthalmol. 2020, 212, 134–143. [Google Scholar] [CrossRef]
- Congdon, N.G.; Broman, A.T.; Bandeen-Roche, K.; Grover, D.; Quigley, H.A. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am. J. Ophthalmol. 2006, 141, 868–875. [Google Scholar] [CrossRef]
- De Moraes, C.V.; Hill, V.; Tello, C.; Liebmann, J.M.; Ritch, R. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J. Glaucoma 2012, 21, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Susanna, C.N.; Diniz-Filho, A.; Daga, F.B.; Susanna, B.N.; Zhu, F.; Ogata, N.G.; Medeiros, F.A. A Prospective Longitudinal Study to Investigate Corneal Hysteresis as a Risk Factor for Predicting Development of Glaucoma. Am. J. Ophthalmol. 2018, 187, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.R.; Ehrlich, J.R.; Shimmyo, M.; Radcliffe, N.M. The relationship between corneal hysteresis and the magnitude of intraocular pressure reduction with topical prostaglandin therapy. Br. J. Ophthalmol. 2012, 96, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Hirneiss, C.; Sekura, K.; Brandlhuber, U.; Kampik, A.; Kernt, M. Corneal biomechanics predict the outcome of selective laser trabeculoplasty in medically uncontrolled glaucoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 2383–2388. [Google Scholar] [CrossRef]
- Sun, L.; Shen, M.; Wang, J.; Fang, A.; Xu, A.; Fang, H.; Lu, F. Recovery of corneal hysteresis after reduction of intraocular pressure in chronic primary angle-closure glaucoma. Am. J. Ophthalmol. 2009, 147, 1061–1066.e2. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; Beiser, J.A.; Gordon, M.O.; Kass, M.A.; Ocular Hypertension Treatment Study, G. Central corneal thickness and measured IOP response to topical ocular hypotensive medication in the Ocular Hypertension Treatment Study. Am. J. Ophthalmol. 2004, 138, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, A.; Elsheikh, A.; Roberts, C.R.; Zhu, H.; Garway-Heath, D.F. Corneal thickness- and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5337–5347. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; Gordon, M.O.; Beiser, J.A.; Lin, S.C.; Alexander, M.Y.; Kass, M.A.; Ocular Hypertension Treatment Study, G. Changes in central corneal thickness over time: The ocular hypertension treatment study. Ophthalmology 2008, 115, 1550–1556.e1. [Google Scholar] [CrossRef]
- Sullivan-Mee, M.; Katiyar, S.; Pensyl, D.; Halverson, K.D.; Qualls, C. Relative importance of factors affecting corneal hysteresis measurement. Optom. Vis. Sci. 2012, 89, E803–E811. [Google Scholar] [CrossRef]
- Musch, D.C.; Gillespie, B.W.; Niziol, L.M.; Lichter, P.R.; Varma, R.; Group, C.S. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 2011, 118, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Mahdavi, K.; Hoffman, D.; Coleman, A.L.; Liu, G.; Li, G.; Gaasterland, D.; Caprioli, J.; Advanced Glaucoma Intervention, S. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004, 111, 1627–1635. [Google Scholar] [CrossRef]
- Vinciguerra, R.; Rehman, S.; Vallabh, N.A.; Batterbury, M.; Czanner, G.; Choudhary, A.; Cheeseman, R.; Elsheikh, A.; Willoughby, C.E. Corneal biomechanics and biomechanically corrected intraocular pressure in primary open-angle glaucoma, ocular hypertension and controls. Br. J. Ophthalmol. 2020, 104, 121–126. [Google Scholar] [CrossRef]
- Anand, A.; De Moraes, C.G.; Teng, C.C.; Tello, C.; Liebmann, J.M.; Ritch, R. Corneal hysteresis and visual field asymmetry in open angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6514–6518. [Google Scholar] [CrossRef]
- Helmy, H.; Leila, M.; Zaki, A.A. Corneal biomechanics in asymmetrical normal-tension glaucoma. Clin. Ophthalmol. 2016, 10, 503–510. [Google Scholar] [CrossRef]
- Miki, A.; Yasukura, Y.; Weinreb, R.N.; Maeda, N.; Yamada, T.; Koh, S.; Asai, T.; Ikuno, Y.; Nishida, K. Dynamic Scheimpflug Ocular Biomechanical Parameters in Untreated Primary Open Angle Glaucoma Eyes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 19. [Google Scholar] [CrossRef]
- Pradhan, Z.S.; Deshmukh, S.; Dixit, S.; Sreenivasaiah, S.; Shroff, S.; Devi, S.; Webers, C.A.B.; Rao, H.L. A comparison of the corneal biomechanics in pseudoexfoliation glaucoma, primary open-angle glaucoma and healthy controls using Corvis ST. PLoS ONE 2020, 15, e0241296. [Google Scholar] [CrossRef]
- Silva, N.; Ferreira, A.; Baptista, P.M.; Figueiredo, A.; Reis, R.; Sampaio, I.; Beirao, J.; Vinciguerra, R.; Meneres, P.; Meneres, M.J. Corneal Biomechanics for Ocular Hypertension, Primary Open-Angle Glaucoma, and Amyloidotic Glaucoma: A Comparative Study by Corvis ST. Clin. Ophthalmol. 2022, 16, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Schlotzer-Schrehardt, U.; Zenkel, M.; Nusing, R.M. Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1475–1487. [Google Scholar]
- Sharif, N.A.; Kelly, C.R.; Crider, J.Y.; Williams, G.W.; Xu, S.X. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J. Ocul. Pharmacol. Ther. 2003, 19, 501–515. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Zhao, Y.; Cao, S.; Zhu, R.; Huang, W.; Yu, A.; Huang, J.; Wang, Q.; Wang, J.; et al. Experimental Evaluation of Travoprost-Induced Changes in Biomechanical Behavior of Ex-Vivo Rabbit Corneas. Curr. Eye Res. 2019, 44, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Toris, C.B.; Gabelt, B.T.; Lindsey, J.D.; Kaufman, P.L. Effects of prostaglandins on the aqueous humor outflow pathways. Surv. Ophthalmol. 2002, 47 (Suppl. 1), S53–S64. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lindsey, J.D.; Wang, N.; Weinreb, R.N. Increased human scleral permeability with prostaglandin exposure. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1514–1521. [Google Scholar]
- Lindsey, J.D.; Crowston, J.G.; Tran, A.; Morris, C.; Weinreb, R.N. Direct matrix metalloproteinase enhancement of transscleral permeability. Investig. Ophthalmol. Vis. Sci. 2007, 48, 752–755. [Google Scholar] [CrossRef]
- Meda, R.; Wang, Q.; Paoloni, D.; Harasymowycz, P.; Brunette, I. The impact of chronic use of prostaglandin analogues on the biomechanical properties of the cornea in patients with primary open-angle glaucoma. Br. J. Ophthalmol. 2017, 101, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Roberts, C.J.; Mahmoud, A.M.; Jain, S.G. Evaluating the Relationship of Intraocular Pressure and Anterior Chamber Volume with Use of Prostaglandin Analogues. J. Glaucoma 2021, 30, 421–427. [Google Scholar] [CrossRef]
- Aoki, S.; Murata, H.; Matsuura, M.; Fujino, Y.; Nakakura, S.; Nakao, Y.; Kiuchi, Y.; Asaoka, R. The Relationship between the Waveform Parameters from the Ocular Response Analyzer and the Progression of Glaucoma. Ophthalmol. Glaucoma 2018, 1, 123–131. [Google Scholar] [CrossRef]
- Faria-Correia, F.; Ramos, I.; Valbon, B.; Luz, A.; Roberts, C.J.; Ambrosio, R., Jr. Scheimpflug-based tomography and biomechanical assessment in pressure-induced stromal keratopathy. J. Refract. Surg. 2013, 29, 356–358. [Google Scholar] [CrossRef]
- Goldich, Y.; Marcovich, A.L.; Barkana, Y.; Mandel, Y.; Hirsh, A.; Morad, Y.; Avni, I.; Zadok, D. Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: Results after 2 years of follow-up. Cornea 2012, 31, 609–614. [Google Scholar] [CrossRef]
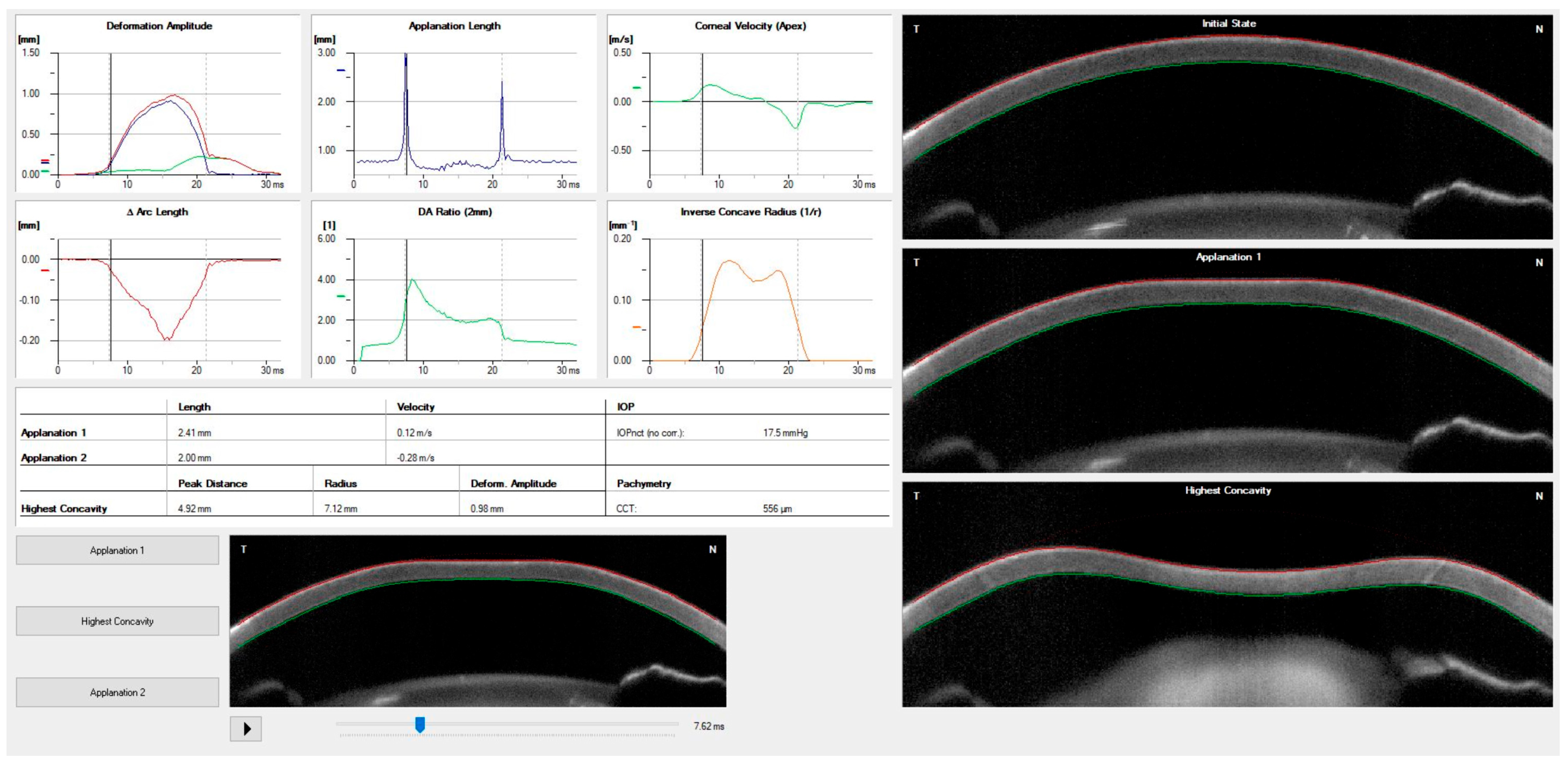
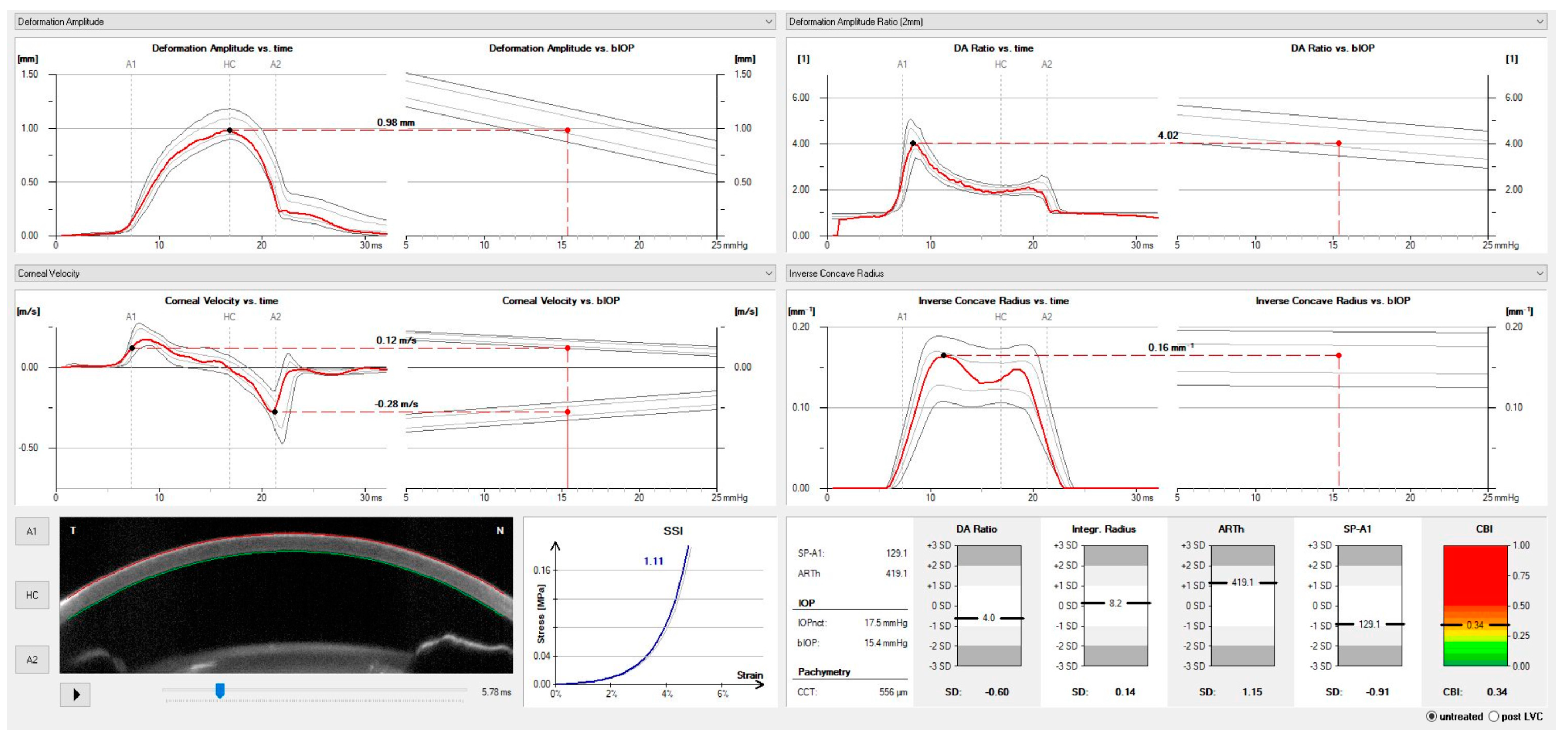
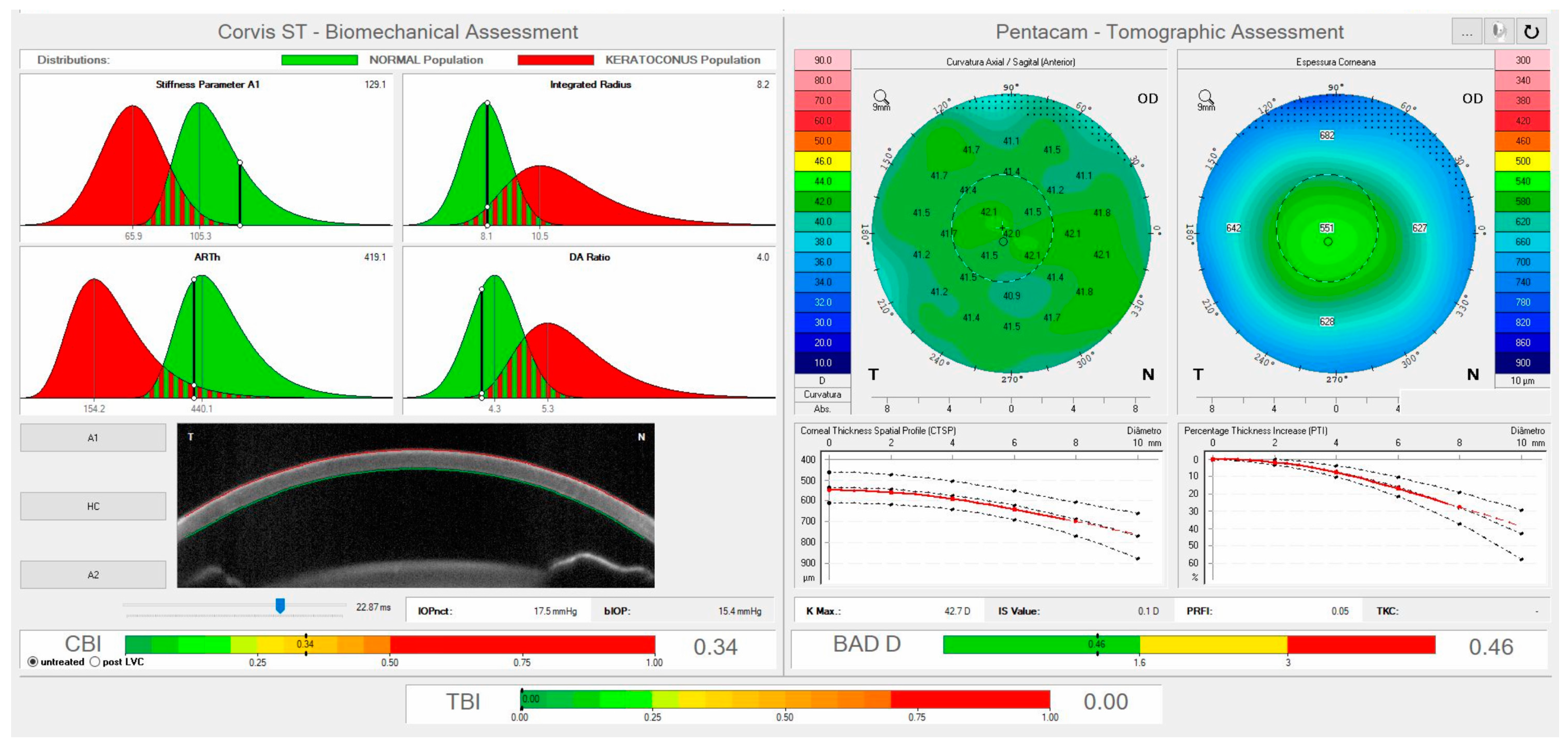
| Corvis ST Parameter | Definition |
|---|---|
| 1st Applanation | The first applanation of the cornea during the air puff (in ms). The length of the applanation at this moment appears in parenthesis (in mm). |
| Highest Concavity | The instant that the cornea assumes its maximum concavity during the air puff (in ms). The length of the distance between the two peaks of the cornea at this moment appears in parenthesis (in mm). |
| 2nd Applanation | The second applanation of the cornea during the air puff (in milliseconds). The length of the applanation at this moment appears in parenthesis (in mm). |
| Maximum Deformation | The amount (in mm) of the maximum corneal deformation during the air puff. |
| Wing Distance | The length of the distance between the two peaks of the cornea at this instant (in mm). |
| Maximum Velocity (in) | The maximum velocity during the ingoing phase (in m/s). |
| Maximum Velocity | The maximum velocity during the outgoing phase (in m/s). |
| Curvature Radius Normal | The cornea in its natural state has a radius of curvature (in mm). |
| Curvature Radius HC | The corneal radius of curvature at the time of maximum concavity during the air puff (in mm). |
| Cornea Thickness | Measurement of the corneal thickness (in mm). |
| IOP | Measurement of the intraocular pressure (in mmHg). |
| bIOP | Biomechanically-corrected IOP. |
| DA ratio Max (Deformation amplitude ratio max. 2 mm) | Ratio between the deformation amplitude at the apex and the average deformation amplitude measured at 2 mm from the center. |
| ARth (Ambrósio’s relational thickness to the horizontal profile) | Describes the thickness profile in the temporal-nasal direction and is defined as corneal thickness from thinnest to pachymetric progression. |
| SP-A1(Stiffness parameter at A1) | Describes corneal stiffness as defined by resultant pressure (Pr) divided by deflection amplitude at A1. |
| SP-HC | Corneal stiffness at the highest concavity point. |
| TBI (Tomographic biomechanical index) | Index that combines tomographic and biomechanical data for keratoconus detection. |
| BGF (Biomechanical Glaucoma factor) | Independent risk indicator for normal tension glaucoma. |
| SSI (Stress-strain index) | Index that indicates the position of the stress-strain curves. Less dependent on corneal thickness and IOP. |
| CBI (Corvis biomechanical index) | Overall biomechanical index for keratoconus detection. |
| Whole eye movement (WEM) | The entire globe’s movement after the cornea passes its limits during the jet air pulse is resisted by the orbital structures. |
| Deformation Amplitude (DA) | The movement of the corneal deformation from the apex to the highest concavity. |
| Deflection amplitude (DeflA) | The difference between the DA and the WEM. |
| HC dArc length | Change in arc length during the highest concavity moment from the initial state, in a defined 7-mm zone. |
| HC deflection length | Length of the flattened cornea at its highest concavity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazuna, R.; Alonso, R.S.; Salomão, M.Q.; Fernandes, B.F.; Ambrósio, R., Jr. Ocular Biomechanics and Glaucoma. Vision 2023, 7, 36. https://doi.org/10.3390/vision7020036
Brazuna R, Alonso RS, Salomão MQ, Fernandes BF, Ambrósio R Jr. Ocular Biomechanics and Glaucoma. Vision. 2023; 7(2):36. https://doi.org/10.3390/vision7020036
Chicago/Turabian StyleBrazuna, Rodrigo, Ruiz S. Alonso, Marcella Q. Salomão, Bruno F. Fernandes, and Renato Ambrósio, Jr. 2023. "Ocular Biomechanics and Glaucoma" Vision 7, no. 2: 36. https://doi.org/10.3390/vision7020036
APA StyleBrazuna, R., Alonso, R. S., Salomão, M. Q., Fernandes, B. F., & Ambrósio, R., Jr. (2023). Ocular Biomechanics and Glaucoma. Vision, 7(2), 36. https://doi.org/10.3390/vision7020036





