Pitch–Luminance Crossmodal Correspondence in the Baby Chick: An Investigation on Predisposed and Learned Processes
Abstract
:1. Introduction
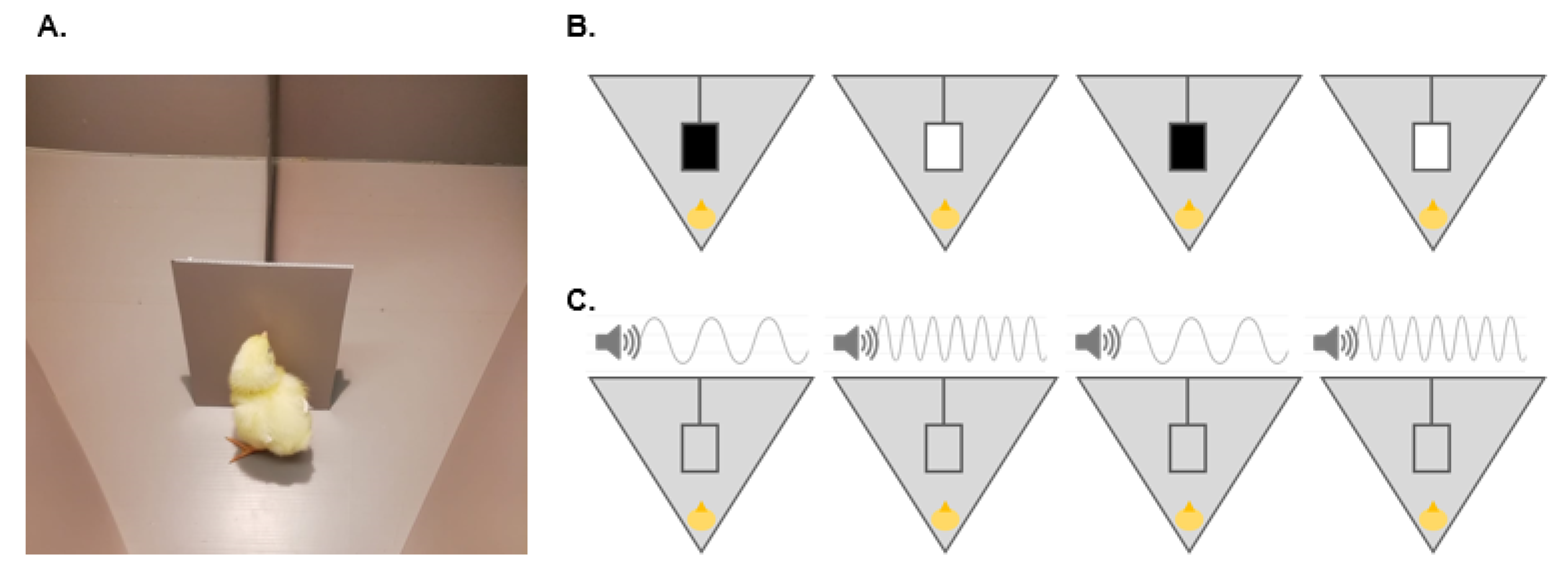
2. Procedures
3. Experiment 1
3.1. Training
3.2. Test
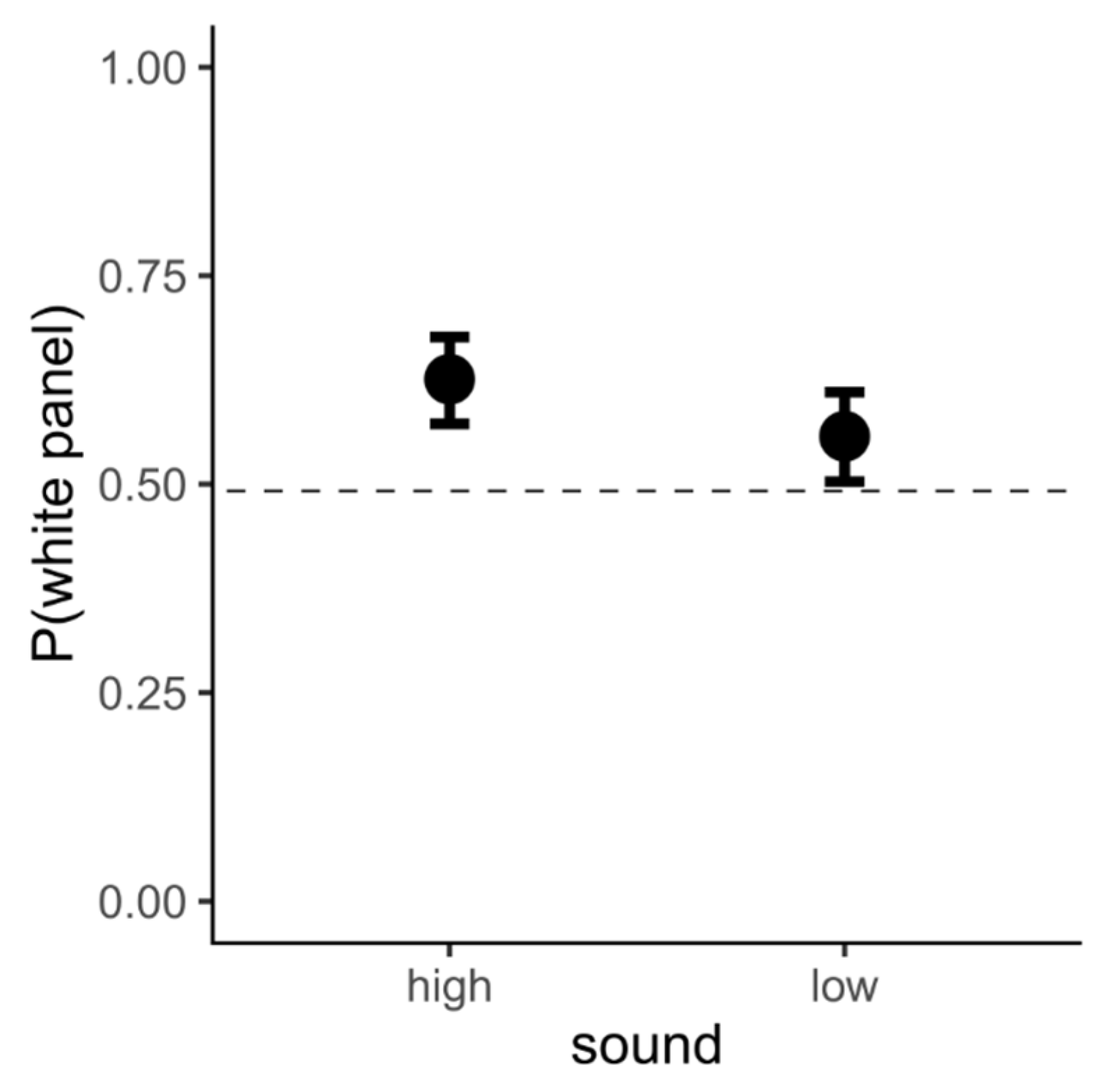
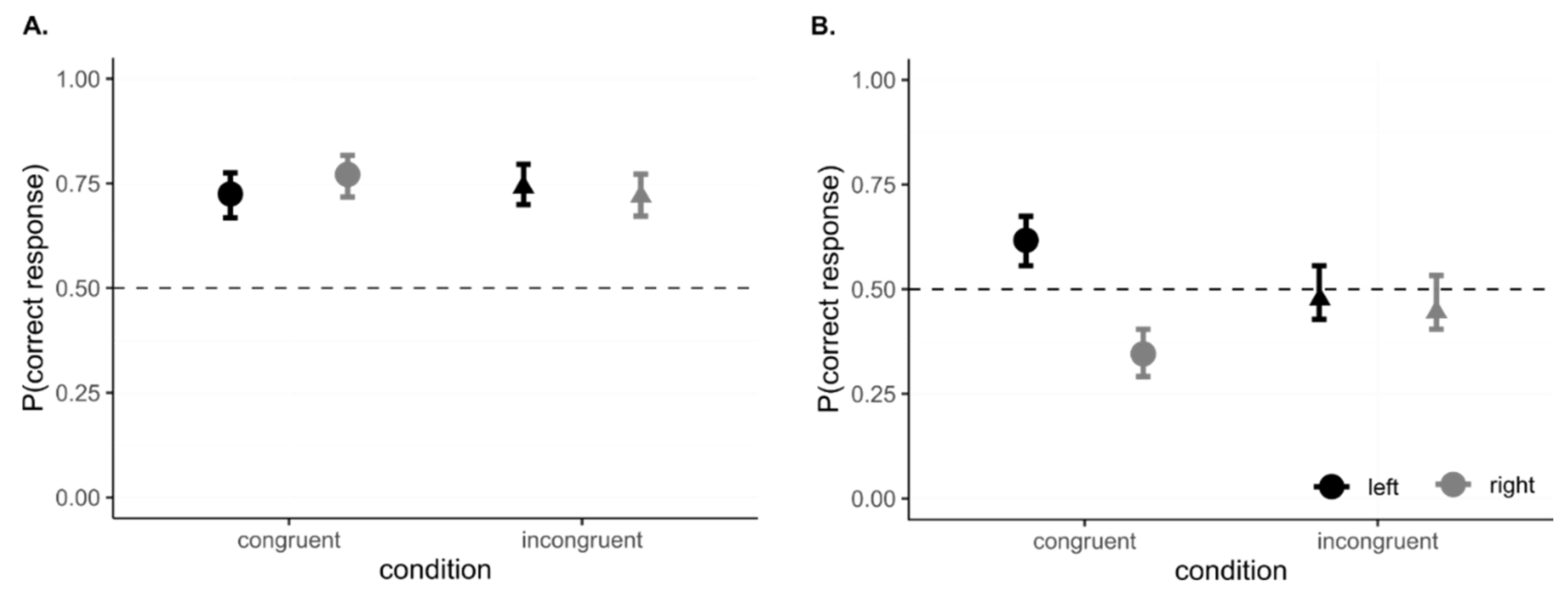
3.3. Data Analysis and Results
3.4. Interim Discussion, Experiment 1
4. Experiment 2
4.1. Training
4.2. Test
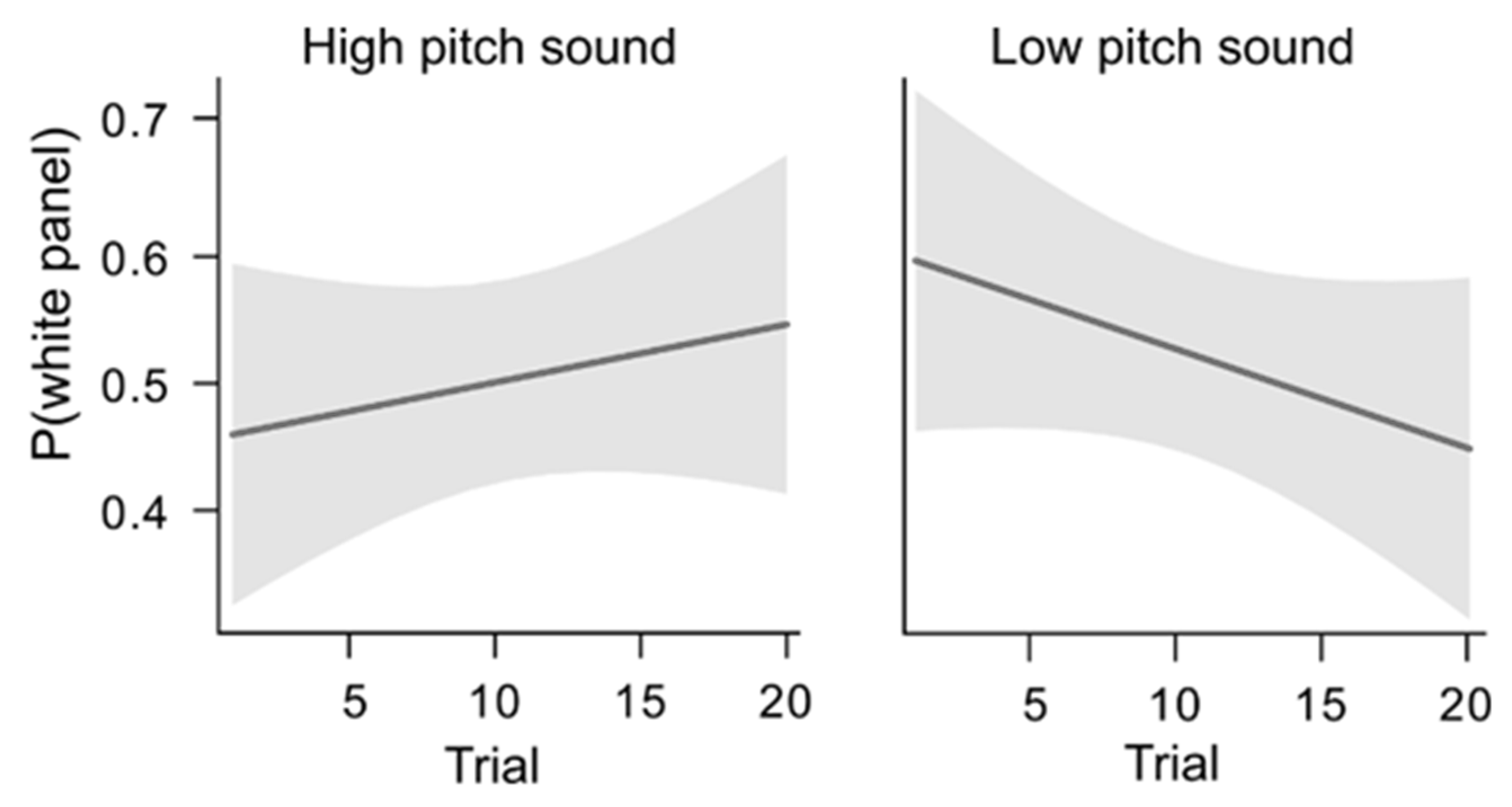
4.3. Data Analysis and Results
4.4. Interim Discussion, Experiment 2
5. Experiment 3
5.1. Subjects
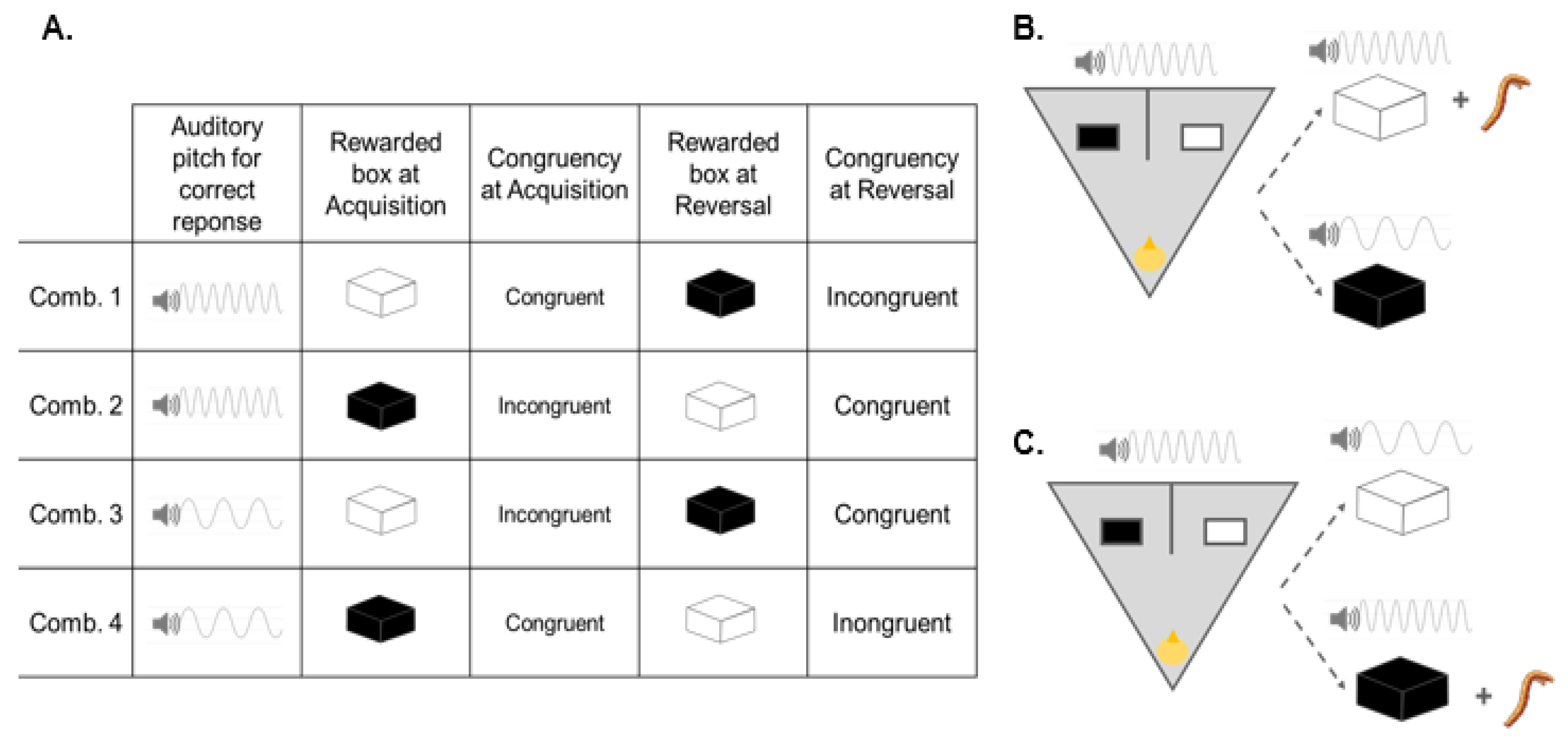
5.2. Training
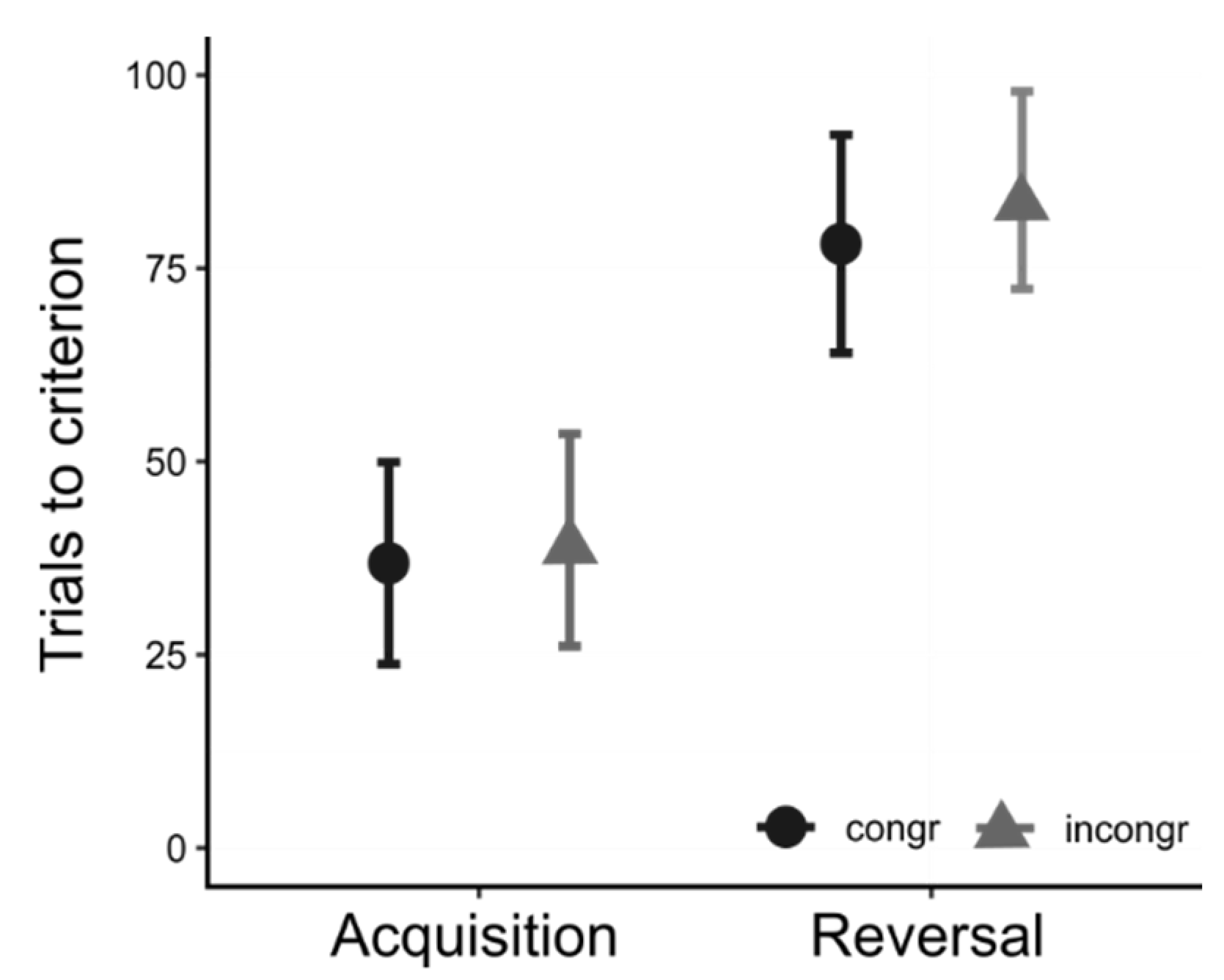
5.3. Acquisition
5.4. Reversal
5.5. Data Analysis and Results
5.6. Interim Discussion, Experiment 3
6. General Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spence, C. Crossmodal Correspondences: A Tutorial Review. Atten. Percept. Psychophys. 2011, 73, 971–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloke, J.M.; Jacklin, D.L.; Winters, B.D. The Neural Bases of Crossmodal Object Recognition in Non-Human Primates and Rodents: A Review. Behav. Brain Res. 2015, 285, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Spence, C.; Deroy, O. Crossmodal Correspondences: Innate or Learned? i-Perception 2012, 3, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Hanson-Vaux, G.; Crisinel, A.-S.; Spence, C. Smelling Shapes: Crossmodal Correspondences between Odors and Shapes. Chem. Senses 2013, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Fumarola, A.; Prpic, V.; Da Pos, O.; Murgia, M.; Umiltà, C.; Agostini, T. Automatic Spatial Association for Luminance. Atten. Percept. Psychophys. 2014, 76, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Gallace, A.; Boschin, E.; Spence, C. On the Taste of “Bouba” and “Kiki”: An Exploration of Word–Food Associations in Neurologically Normal Participants. Cogn. Neurosci. 2011, 2, 34–46. [Google Scholar] [CrossRef]
- Bremner, A.J.; Caparos, S.; Davidoff, J.; de Fockert, J.; Linnell, K.J.; Spence, C. “Bouba” and “Kiki” in Namibia? A Remote Culture Make Similar Shape–Sound Matches, but Different Shape–Taste Matches to Westerners. Cognition 2013, 126, 165–172. [Google Scholar] [CrossRef]
- Morrongiello, B.A.; Fenwick, K.D.; Chance, G. Crossmodal Learning in Newborn Infants: Inferences about Properties of Auditory-Visual Events. Infant Behav. Dev. 1998, 21, 543–553. [Google Scholar] [CrossRef]
- Walker, P.; Bremner, J.G.; Mason, U.; Spring, J.; Mattock, K.; Slater, A.; Johnson, S.P. Preverbal Infants’ Sensitivity to Synaesthetic Cross-Modality Correspondences. Psychol. Sci. 2010, 21, 21–25. [Google Scholar] [CrossRef]
- Ludwig, V.U.; Adachi, I.; Matsuzawa, T. Visuoauditory Mappings between High Luminance and High Pitch Are Shared by Chimpanzees (Pan Troglodytes) and Humans. Proc. Natl. Acad. Sci. USA 2011, 108, 20661–20665. [Google Scholar] [CrossRef] [Green Version]
- Ghazanfar, A.A.; Maier, J.X. Rhesus Monkeys (Macaca Mulatta) Hear Rising Frequency Sounds as Looming. Behav. Neurosci. 2009, 123, 822–827. [Google Scholar] [CrossRef] [Green Version]
- Korzeniowska, A.T.; Root-Gutteridge, H.; Simner, J.; Reby, D. Audio–Visual Crossmodal Correspondences in Domestic Dogs (Canis Familiaris). Biol. Lett. 2019, 15, 20190564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korzeniowska, A.T.; Simner, J.; Root-Gutteridge, H.; Reby, D. High-Pitch Sounds Small for Domestic Dogs: Abstract Crossmodal Correspondences between Auditory Pitch and Visual Size. R. Soc. Open Sci. 2022, 9, 211647. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, M.; Pasculli, M.S.; Regolin, L. Space-Luminance Crossmodal Correspondences in Domestic Chicks. Vis. Res. 2021, 188, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Rugani, R.; Vallortigara, G.; Priftis, K.; Regolin, L. Number-Space Mapping in the Newborn Chick Resembles Humans’ Mental Number Line. Science 2015, 347, 534–536. [Google Scholar] [CrossRef]
- Rugani, R.; de Hevia, M.-D. Number-Space Associations without Language: Evidence from Preverbal Human Infants and Non-Human Animal Species. Psychon. Bull. Rev. 2017, 24, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Bulf, H.; de Hevia, M.D.; Cassia, V.M. Small on the Left, Large on the Right: Numbers Orient Visual Attention onto Space in Preverbal Infants. Dev. Sci. 2016, 19, 394–401. [Google Scholar] [CrossRef] [PubMed]
- McCrink, K.; Opfer, J.E. Development of Spatial-Numerical Associations. Curr. Dir. Psychol. Sci. 2014, 23, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rugani, R.; Vallortigara, G.; Vallini, B.; Regolin, L. Asymmetrical Number-Space Mapping in the Avian Brain. Neurobiol. Learn. Mem. 2011, 95, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Rugani, R.; Vallortigara, G.; Regolin, L. Mapping Number to Space in the Two Hemispheres of the Avian Brain. Neurobiol. Learn. Mem. 2016, 133, 13–18. [Google Scholar] [CrossRef]
- Vallortigara, G.; Cailotto, M.; Zanforlin, M. Sex Differences in Social Reinstatement Motivation of the Domestic Chick (Gallus Gallus) Revealed by Runway Tests with Social and Nonsocial Reinforcement. J. Comp. Psychol. 1990, 104, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Bokkers, E.A.M.; Koene, P. Sex and Type of Feed Effects on Motivation and Ability to Walk for a Food Reward in Fast Growing Broilers. Appl. Anim. Behav. Sci. 2002, 79, 247–261. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Estimated Marginal Means, Aka Least-Squares Means. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 6 March 2022).
- Melara, R.D.; O’Brien, T.P. Interaction between Synesthetically Corresponding Dimensions. J. Exp. Psychol. Gen. 1987, 116, 323–336. [Google Scholar] [CrossRef]
- Gallace, A.; Spence, C. Multisensory Synesthetic Interactions in the Speeded Classification of Visual Size. Percept. Psychophys. 2006, 68, 1191–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.A.; Jarick, M. Multisensory Integration of Speech Signals: The Relationship between Space and Time. Exp. Brain Res. 2006, 174, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Keough, M.; Derrick, D.; Gick, B. Cross-Modal Effects in Speech Perception. Annu. Rev. Linguist. 2019, 5, 49–66. [Google Scholar] [CrossRef]
- Balsam, P.D.; Drew, M.R.; Gallistel, C.R. Time and Associative Learning. Comp. Cogn. Behav. Rev. 2010, 5, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Pearce, J.M.; Bouton, M.E. Theories of Associative Learning in Animals. Ann. Rev. Psychol. 2001, 52, 111–139. [Google Scholar] [CrossRef] [Green Version]
- Loconsole, M.; Perovic, S.; Regolin, L. A Leftward Bias Negatively Correlated with Performance Is Selectively Displayed by Domestic Chicks during Rule Reversal (Not Acquisition). Laterality 2020, 26, 1–18. [Google Scholar] [CrossRef]
- Deng, C.; Rogers, L.J. Differential Contributions of the Two Visual Pathways to Functional Lateralization in Chicks. Behav. Brain Res. 1997, 87, 173–182. [Google Scholar] [CrossRef]
- Dharmaretnam, M.; Rogers, L.J. Hemispheric Specialization and Dual Processing in Strongly versus Weakly Lateralized Chicks. Behav. Brain Res. 2005, 162, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Rogers, L.J.; Bisazza, A. Possible Evolutionary Origins of Cognitive Brain Lateralization. Brain Res. Rev. 1999, 30, 164–175. [Google Scholar] [CrossRef]
- Andrew, R.J. The Nature of Behavioural Lateralization in the Chick. In Neural and Behavioural Plasticity; Oxford University Press: Oxford, UK, 1991; ISBN 978-0-19-852184-6. [Google Scholar]
- McKenzie, R.; Andrew, R.J.; Jones, R.B. Lateralization in Chicks and Hens: New Evidence for Control of Response by the Right Eye System. Neuropsychologia 1998, 36, 51–58. [Google Scholar] [CrossRef]
- Diekamp, B.; Regolin, L.; Güntürkün, O.; Vallortigara, G. A Left-Sided Visuospatial Bias in Birds. Curr. Biol. 2005, 15, R372–R373. [Google Scholar] [CrossRef]
- Diekamp, B.; Prior, H.; Güntürkün, O. Functional Lateralization, Interhemispheric Transfer and Position Bias in Serial Reversal Learning in Pigeons (Columba Livia). Anim. Cogn. 1999, 2, 187–196. [Google Scholar] [CrossRef]
- Anes, M.D.; Kruer, J.L. Investigating Hemispheric Specialization in a Novel Face–Word Stroop Task. Brain Lang. 2004, 89, 136–141. [Google Scholar] [CrossRef]
- Marks, L.E. On Associations of Light and Sound: The Mediation of Brightness, Pitch, and Loudness. Am. J. Psychol. 1974, 87, 173–188. [Google Scholar] [CrossRef]
- Marks, L.E. On Cross-Modal Similarity: Auditory–Visual Interactions in Speeded Discrimination. J. Exp. Psychol. Hum. Percept. Perform. 1987, 13, 384–394. [Google Scholar] [CrossRef]
- Bolhuis, J.J.; Kampen, H.S.V. Auditory Learning and Filial Imprinting in the Chick. Behaviour 1991, 117, 303–319. [Google Scholar] [CrossRef] [Green Version]
- van Horik, J.O.; Langley, E.J.G.; Whiteside, M.A.; Madden, J.R. Differential Participation in Cognitive Tests Is Driven by Personality, Sex, Body Condition and Experience. Behav. Proc. 2017, 134, 22–30. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loconsole, M.; Gasparini, A.; Regolin, L. Pitch–Luminance Crossmodal Correspondence in the Baby Chick: An Investigation on Predisposed and Learned Processes. Vision 2022, 6, 24. https://doi.org/10.3390/vision6020024
Loconsole M, Gasparini A, Regolin L. Pitch–Luminance Crossmodal Correspondence in the Baby Chick: An Investigation on Predisposed and Learned Processes. Vision. 2022; 6(2):24. https://doi.org/10.3390/vision6020024
Chicago/Turabian StyleLoconsole, Maria, Andrea Gasparini, and Lucia Regolin. 2022. "Pitch–Luminance Crossmodal Correspondence in the Baby Chick: An Investigation on Predisposed and Learned Processes" Vision 6, no. 2: 24. https://doi.org/10.3390/vision6020024
APA StyleLoconsole, M., Gasparini, A., & Regolin, L. (2022). Pitch–Luminance Crossmodal Correspondence in the Baby Chick: An Investigation on Predisposed and Learned Processes. Vision, 6(2), 24. https://doi.org/10.3390/vision6020024




