Binocular Viewing Facilitates Size Constancy for Grasping and Manual Estimation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Data Reduction
2.4. Statistical Analysis
3. Results
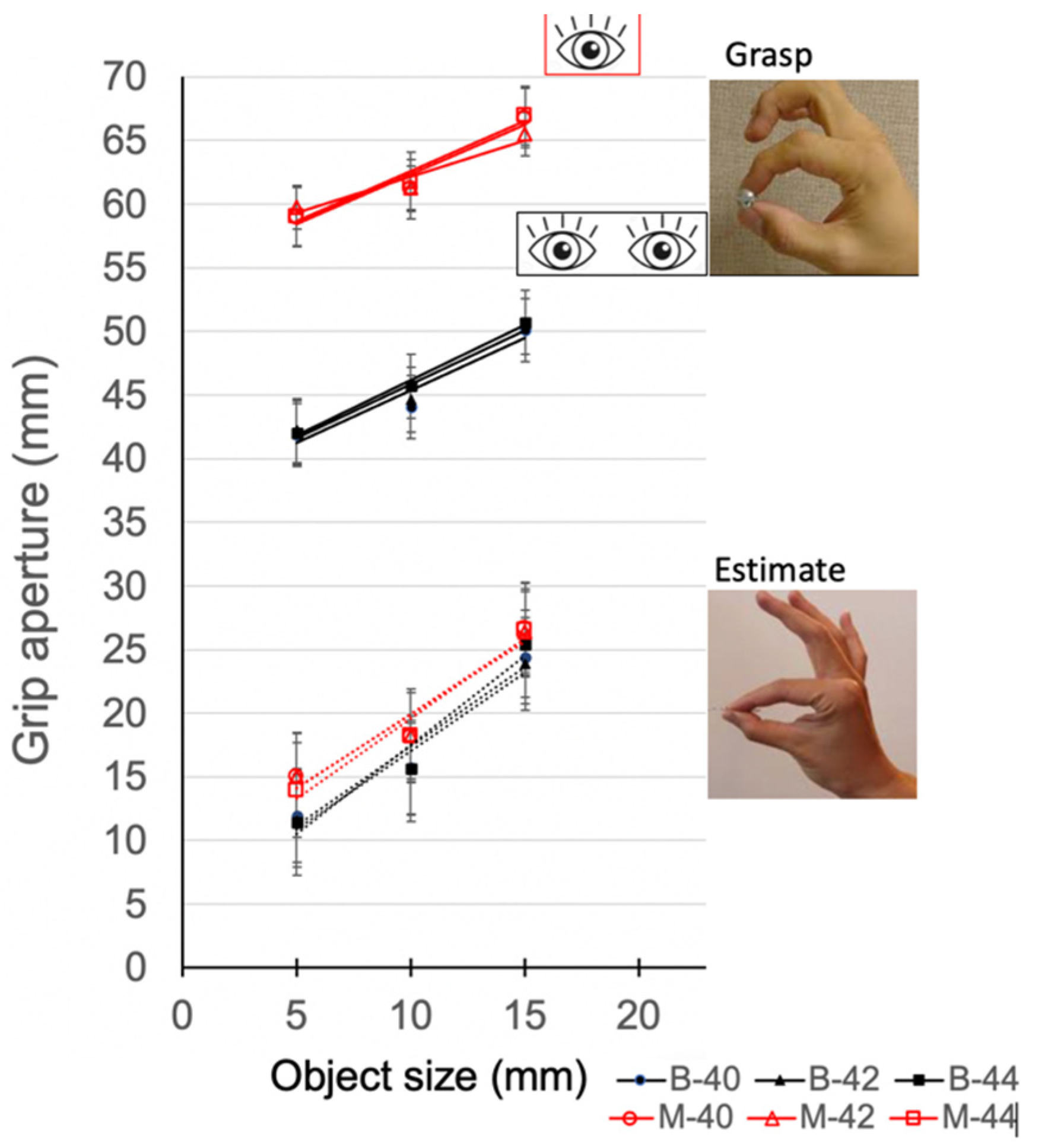
3.1. Mean Grip Aperture
3.2. Precision Grip Aperture
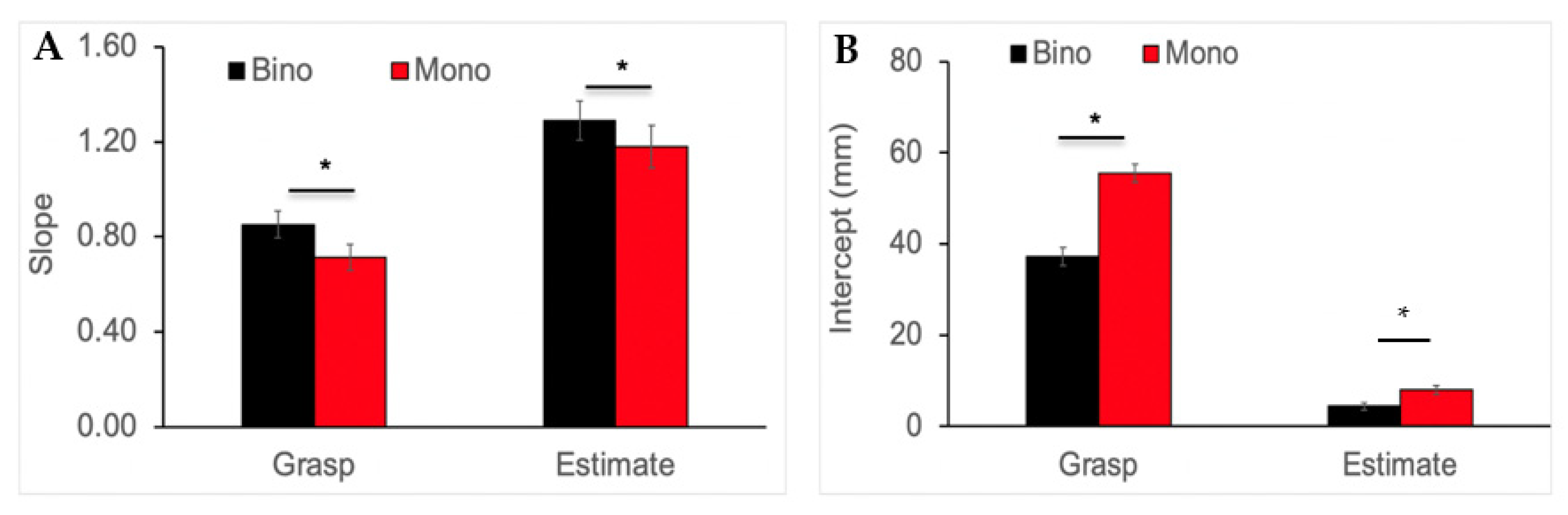
3.3. Slope and Intercept Analysis for Grip Aperture
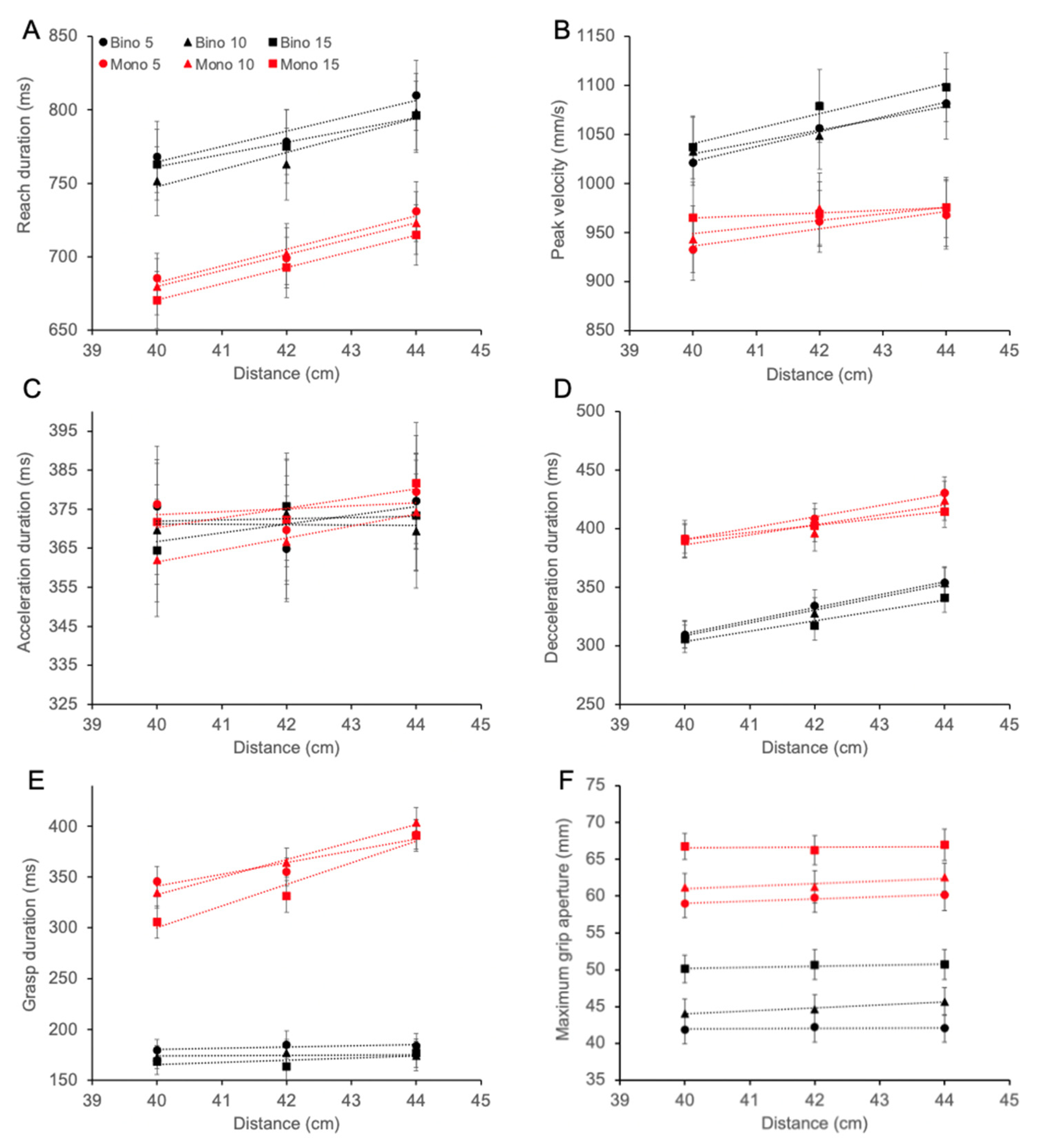
3.4. Reach Kinematics
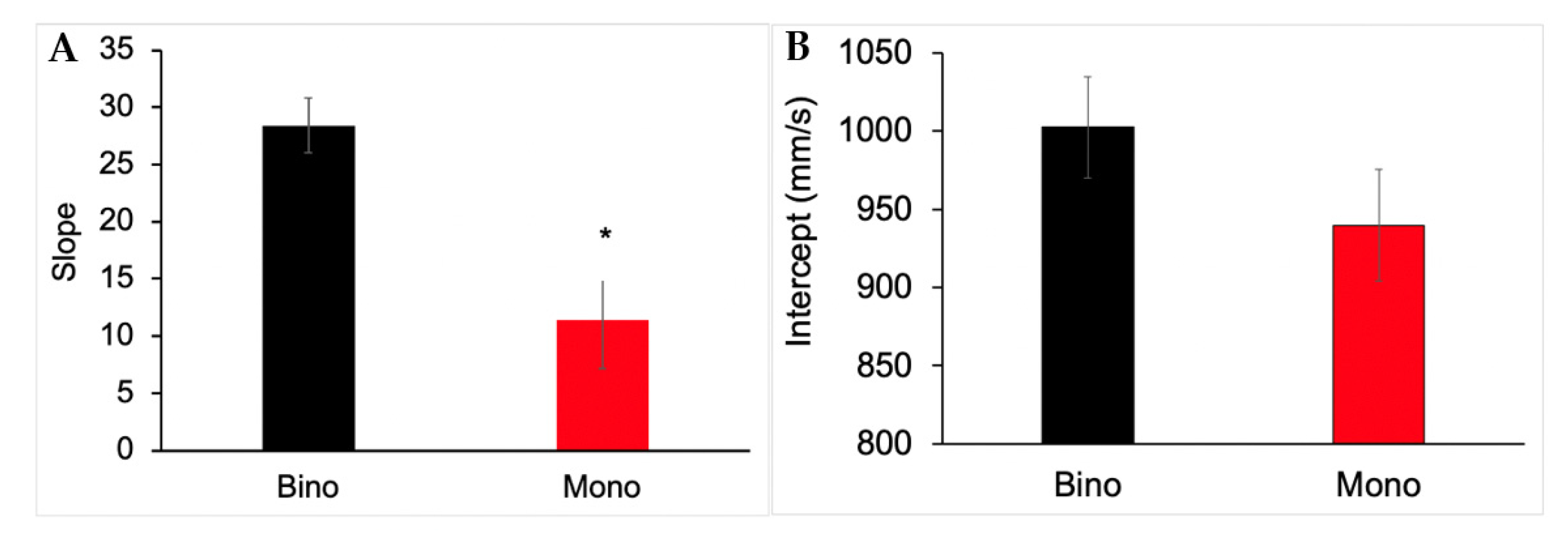
3.5. Slope and Intercept Analysis for Reach Peak Velocity
3.6. Safety Margin for Reaching and Grasping
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ono, H.; Comerford, J. Stereoscopic depth constancy. In Stability and Constancy in Visual Perception: Mechanisms and Processes; Epstein, W., Ed.; Wiley: Hoboken, NJ, USA, 1977; pp. 91–128. [Google Scholar]
- Knill, D.C. Reaching for visual cues to depth: The brain combines depth cues differently for motor control and perception. J. Vis. 2005, 5, 103–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keefe, B.D.; Watt, S.J. The role of binocular vision in grasping: A small stimulus-set distorts results. Exp. Brain Res. 2009, 194, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Goodale, M.A.; Haffenden, A.M. Interactions between the dorsal and ventral streams of visual processing. Adv. Neurol. 2003, 93, 249–267. [Google Scholar] [PubMed]
- Marotta, J.J.; Goodale, M.A. Role of familiar size in the control of grasping. J. Cogn. Neurosci. 2001, 13, 8–17. [Google Scholar] [CrossRef]
- Loftus, A.; Servos, P.; Goodale, M.A.; Mendarozqueta, N.; Mon-Williams, M. When two eyes are better than one in prehension: Monocular viewing and end-point variance. Exp. Brain Res. 2004, 158, 317–327. [Google Scholar] [CrossRef]
- Gnanaseelan, R.; Gonzalez, D.A.; Niechwiej-Szwedo, E. Binocular advantage for prehension movements performed in visually-enriched environments. Front. Hum. Neurosci. 2014, 8, 959. [Google Scholar] [CrossRef][Green Version]
- Carey, D.P.; Dijkerman, H.C.; Milner, A.D. Perception and action in depth. Conscious. Cogn. 1998, 7, 438–453. [Google Scholar] [CrossRef]
- Marotta, J.J.; DeSouza, J.F.X.; Haffenden, A.M.; Goodale, M.A. Does a monocularly presented size-contrast illusion influence grip aperture? Neuropsychologia 1998, 36, 491–497. [Google Scholar] [CrossRef]
- Goodale, M.A.; Milner, A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef]
- Mishkin, M.; Ungerleider, L.G. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav. Brain Res. 1982, 6, 57–77. [Google Scholar] [CrossRef]
- Murray, S.O.; Boyaci, H.; Kersten, D. The representation of perceived angular size in human primary visual cortex. Nat. Neurosci. 2006, 9, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, I.; Chouinard, P.A. The mechanisms of size constancy. Multisens. Res. 2015, 28, 253–283. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, R.L.; Sperandio, I.; Buckingham, G.; Chouinard, P.A.; Goodale, M.A. Grip constancy but not perceptual size constancy survives lesions of early visual cortex. Curr. Biol. 2020, 30, 3680–3686.e5. [Google Scholar] [CrossRef] [PubMed]
- Goodale, M.A. Transforming vision into action. Vis. Res. 2011, 51, 1567–1587. [Google Scholar] [CrossRef]
- Franz, V.H.; Gegenfurtner, K.R. Grasping visual illusions: Consistent data and no dissociation. Cogn. Neuropsychol. 2008, 25, 920–950. [Google Scholar] [CrossRef]
- Schenk, T.; McIntosh, R.D. Do we have independent visual streams for perception and action? Cogn. Neurosci. 2010, 1, 52–62. [Google Scholar] [CrossRef]
- Rossetti, Y.; Pisella, L.; McIntosh, R.D. Rise and fall of the two visual systems theory. Ann. Phys. Rehabil. Med. 2017, 60, 130–140. [Google Scholar] [CrossRef]
- Kravitz, D.J.; Saleem, K.S.; Baker, C.I.; Mishkin, M. A new neural framework for visuospatial processing. Nat. Rev. Neurosci. 2011, 12, 217–230. [Google Scholar] [CrossRef]
- van Polanen, V.; Davare, M. Interactions between dorsal and ventral streams for controlling skilled grasp. Neuropsychologia 2015, 79, 186–191. [Google Scholar] [CrossRef]
- Freud, E.; Plaut, D.C.; Behrmann, M. “What” is happening in the dorsal visual pathway. Trends Cogn. Sci. 2016, 20, 773–784. [Google Scholar] [CrossRef]
- Freud, E.; Culham, J.C.; Plaut, D.C.; Behrmann, M. The large-scale organization of shape processing in the ventral and dorsal pathways. Elife 2017, 6, e27576. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sperandio, I.; Goodale, M.A. Proprioceptive distance cues restore perfect size constancy in grasping, but not perception, when vision is limited. Curr. Biol. 2018, 28, 927–932.e4. [Google Scholar] [CrossRef] [PubMed]
- Servos, P.; Goodale, M.A.; Jakobson, L.S. The role of binocular vision in prehension: A kinematic analysis. Vis. Res. 1992, 32, 1513–1521. [Google Scholar] [CrossRef]
- Jackson, S.R.; Jones, C.A.; Newport, R.; Pritchard, C. A kinematic analysis of goal-directed prehension movements executed under binocular, monocular and memory-guided viewing conditions. Vis. Cogn. 1991, 4, 113–142. [Google Scholar] [CrossRef]
- Keefe, B.D.; Hibbard, P.B.; Watt, S.J. Depth-cue integration in grasp programming: No evidence for a binocular specialism. Neuropsychologia 2011, 49, 1246–1257. [Google Scholar] [CrossRef]
- Melmoth, D.R.; Grant, S. Advantages of binocular vision for the control of reaching and grasping. Exp. Brain Res. 2006, 171, 371–388. [Google Scholar] [CrossRef]
- Hibbard, P.B.; Bradshaw, M.F. Reaching for virtual objects: Binocular disparity and the control of prehension. Exp. Brain Res. 2003, 148, 196–201. [Google Scholar] [CrossRef]
- Bradshaw, M.F.; Elliott, K.M.; Watt, S.J.; Hibbard, P.B.; Davies, I.R.; Simpson, P.J. Binocular cues and the control of prehension. Spat. Vis. 2004, 17, 95–110. [Google Scholar]
- Gonzalez, D.A.; Niechwiej-Szwedo, E. The effects of monocular viewing on hand-eye coordination during sequential grasping and placing movements. Vis. Res. 2016, 128, 30–38. [Google Scholar] [CrossRef]
- Howard, I.P. Reaching and moving in 3D space. In Perceiving in Depth; Oxford University Press: Oxford, UK, 2012; Volume 3, pp. 260–275. [Google Scholar]
- Tresilian, J.R.; Mon-Williams, M.; Kelly, B.M. Increasing confidence in vergence as a cue to distance. Proc. R. Soc. Lond. B 1999, 266, 39–44. [Google Scholar] [CrossRef]
- Melmoth, D.R.; Storoni, M.; Todd, G.; Finlay, A.L.; Grant, S. Dissociation between vergence and binocular disparity cues in the control of prehension. Exp. Brain Res. 2007, 183, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Mroczkowski, C.; Niechwiej-Szwedo, E. Stereopsis contributes to the predictive control of grip forces during prehension. Exp. Brain Res. 2021, 239, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; Melmoth, D.R.; Morgan, M.J.; Finlay, A.L. Prehension deficits in amblyopia. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1139–1148. [Google Scholar] [CrossRef]
- Sakata, H.; Taira, M.; Kusunoki, M.; Murata, A.; Tsutsui, K.-i.; Tanaka, Y.; Shein, W.N.; Miyashita, Y. Nerual represenatations of three-dimensional features of manipulation objects with stereopsis. Exp. Brain Res. 1999, 128, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, S.; Peeters, R.; Kolster, H.; Todd, J.T.; Orban, G.A. The processing of three-dimensional shape from disparity in the human brain. J. Neurosci. 2009, 29, 727–742. [Google Scholar] [CrossRef]
- Durand, J.B.; Peeter, R.; Norman, J.F.; Todd, J.T.; Orban, G.A. Parietal regions processing visual 3D shape extracted from disparity. Neuroimage 2009, 46, 1114–1126. [Google Scholar] [CrossRef]
- Verhoef, B.-E.; Vogels, R.; Janssen, P. Binocular depth processing in the ventral visual pathway. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 37, 120150259. [Google Scholar] [CrossRef]
- Fischmeister, F.P.S.; Bauer, H. Neural correlates of monocular and binocular depth cues based on natural images: A LORETA analysis. Vis. Res. 2006, 46, 3373–3380. [Google Scholar] [CrossRef]
- Marotta, J.J.; Behrmann, M.; Goodale, M.A. The removal of binocular cues disrupts the calibration of grasping in patients with visual form agnosia. Exp. Brain Res. 1997, 116, 113–121. [Google Scholar] [CrossRef]
- Dijkerman, H.; Milner, A.; Carey, D. The perception and prehension of objects oriented in the depth plane: I. Effects of visual form agnosia. Exp. Brain Res. 1996, 112, 442–451. [Google Scholar] [CrossRef]
- Otto-de Haart, E.G.; Carey, D.P.; Milne, A.B. More thoughts on perceiving and grasping the Müller-Lyer illusion. Neuropsychologia 1999, 37, 1437–1444. [Google Scholar] [CrossRef]
- Kopiske, K.K.; Bruno, N.; Hesse, C.; Schenk, T.; Franz, V.H. The functional subdivision of the visual brain: Is there a real illusion effect on action? A multi-lab replication study. Cortex 2016, 79, 130–152. [Google Scholar] [CrossRef]
- Smeets, J.B.; Brenner, E. A new view on grasping. Motor. Control. 1999, 3, 237–271. [Google Scholar] [CrossRef] [PubMed]
- Haffenden, A.M.; Schiff, K.C.; Goodale, M.A. The dissociation between perception and action in the Ebbinghaus illusion: Nonillusory effects of pictorial cues on grasp. Curr. Biol. 2001, 11, 177–181. [Google Scholar] [CrossRef]
- Franz, V.H.; Fahle, M.; Bülthoff, H.H.; Gegenfurtner, K.R. Effects of visual illusions on grasping. J. Exp. Psychol. Hum. Percept. Perform. 2001, 27, 1124–1144. [Google Scholar] [CrossRef]
- Franz, V.H. Manual size estimation: A neuropsychological measure of perception? Exp. Brain Res. 2003, 151, 471–477. [Google Scholar] [CrossRef]
- Takemura, N.; Fukui, T.; Inui, T. A computational model for aperture control in reach-to-grasp movement based on predictive variability. Front. Comput. Neurosci. 2015, 9, 143. [Google Scholar] [CrossRef][Green Version]
- Rand, M.K.; Lemay, M.; Squire, L.M.; Shimansky, Y.P.; Stelmach, G.E. Role of vision in aperture closure control during reach-to-grasp movements. Exp. Brain Res. 2007, 181, 447–460. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jakobson, L.S.; Archibald, Y.M.; Carey, D.P.; Goodale, M.A. A kinematic analysis of reaching and grasping movements in a patient recovering from optic ataxia. Neuropsychologia 1991, 29, 803–809. [Google Scholar] [CrossRef]
- Schlicht, E.J.; Schrater, P.R. Effects of visual uncertainty on grasping movements. Exp. Brain Res. 2007, 182, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Wing, A.M.; Turton, A.; Fraser, C. Grasp size and accuracy of approach in reaching. J. Mot. Behav. 1986, 18, 245–260. [Google Scholar] [CrossRef]
- Washburn, M.F.; Faison, C.; Scott, R. A Comparison between the Miles A-B-C method and retinal rivalry as tests of ocular dominance. Am. J. Psychol. 1934, 46, 633–636. [Google Scholar] [CrossRef]
- Grierson, L.E.; Elliott, D. The impact of real and illusory target perturbations on manual aiming. Exp. Brain Res. 2009, 197, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.; Binsted, G.; Heath, M. The control of goal-directed limb movements: Correcting errors in the trajectory. Hum. Mov. Sci. 1999, 18, 121–136. [Google Scholar] [CrossRef]
- Glazebrook, C.; Gonzalez, D.A.; Hansen, S.; Elliot, D. The role of vision for online control of manual aiming movements in persons with autism spectrum disorders. Autism 2009, 13, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, S.; Pisu, V.; Bruno, N. Precision in grasping: Consistent with Weber’s law, but constrained by “safety margins”. Neuropsychologia 2021, 163, 108088. [Google Scholar] [CrossRef]
- Leibowitz, H.W.; Dato, R.A. Visual size-constancy as a function of distance for temporarily and permanently monocular observers. Am. J. Psychol. 1966, 79, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.O.; Banks, M.S. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 2002, 415, 429–433. [Google Scholar] [CrossRef]
- Richards, W. Configuration stereopsis: A new look at the depth-disparity relation. Spat. Vis. 2009, 22, 91–103. [Google Scholar] [CrossRef]
- Volcic, R.; Fantoni, C.; Caudek, C.; Assad, J.A.; Domini, F. Visuomotor adaptation changes stereoscopic depth perception and tactile discrimination. J. Neurosci. 2013, 33, 17081–17088. [Google Scholar] [CrossRef][Green Version]
- Mckee, S.P.; Welch, L. The precision of size constancy. Vis. Res. 1992, 32, 1447–1460. [Google Scholar] [CrossRef]
- Read, J.C.A.; Begum, S.F.; McDonald, A.; Trowbridge, J. The binocular advantage in visuomotor tasks involving tools. Iperception 2013, 4, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.; Weir, P.L.; Tremblay, L.; Weeks, D.J.; Elliott, D. Monocular and binocular vision in the control of goal-directed movement. J. Mot. Behav. 2000, 32, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, M.F.; Elliott, K.M. The role of binocular information in the “on-line” control of prehension. Spat. Vis. 2003, 16, 295–309. [Google Scholar]
- Watt, S.J.; Bradshaw, M.F. Binocular cues are important in controlling the grasp but not the reach in natural prehension movements. Neuropsychologia 2000, 38, 1473–1481. [Google Scholar] [CrossRef]
- Elliott, D.; Lyons, J.; Hayes, S.J.; Burkitt, J.J.; Hansen, S.; Grierson, L.E.M.; Foster, N.C.; Roberts, J.W.; Bennett, S.J. The multiple process model of goal-directed aiming/reaching: Insights on limb control from various special populations. Exp. Brain Res. 2020, 238, 2685–2699. [Google Scholar] [CrossRef]
- Schmidt, R.A.; Zelaznik, H.; Hawkins, B.; Frank, J.S.; Quinn, J.T., Jr. Motor-output variability: A theory for the accuracy of rapid motor acts. Psychol. Rev. 1979, 47, 415–451. [Google Scholar] [CrossRef]
- Smeets, J.B.J.; van der Kooij, K.; Brenner, E. A review of grasping as the movements of digits in space. J. Neurophysiol. 2019, 122, 1578–1597. [Google Scholar] [CrossRef]
- Tugac, N.; Gonzalez, D.; Noguchi, K.; Niechwiej-Szwedo, E. The role of somatosensory input in target localization during binocular and monocular viewing while performing a high precision reaching and placement task. Exp. Eye Res. 2019, 183, 76–83. [Google Scholar] [CrossRef]
- Niechwiej-Szwedo, E.; Goltz, H.; Chandrakumar, M.; Hirji, Z.A.; Crawford, J.D.; Wong, A.M. Effects of anisometropic amblyopia on visuomotor behaviour: II. Visually-guided reaching. Investig. Ophthalmol. Vis. Sci. 2011, 52, 795–803. [Google Scholar] [CrossRef]
- Jackson, S.R.; Newport, R.; Shaw, A. Monocular vision leads to a dissociation between grip force and grip aperture scaling during reach-to-grasp movements. Curr. Biol. 2002, 12, 237–240. [Google Scholar] [CrossRef][Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niechwiej-Szwedo, E.; Cao, M.; Barnett-Cowan, M. Binocular Viewing Facilitates Size Constancy for Grasping and Manual Estimation. Vision 2022, 6, 23. https://doi.org/10.3390/vision6020023
Niechwiej-Szwedo E, Cao M, Barnett-Cowan M. Binocular Viewing Facilitates Size Constancy for Grasping and Manual Estimation. Vision. 2022; 6(2):23. https://doi.org/10.3390/vision6020023
Chicago/Turabian StyleNiechwiej-Szwedo, Ewa, Michael Cao, and Michael Barnett-Cowan. 2022. "Binocular Viewing Facilitates Size Constancy for Grasping and Manual Estimation" Vision 6, no. 2: 23. https://doi.org/10.3390/vision6020023
APA StyleNiechwiej-Szwedo, E., Cao, M., & Barnett-Cowan, M. (2022). Binocular Viewing Facilitates Size Constancy for Grasping and Manual Estimation. Vision, 6(2), 23. https://doi.org/10.3390/vision6020023



