Immunohistochemical Analysis of a Vitreous Membrane Removed from a Patient with Incontinentia Pigmenti-Related Retinal Detachment
Abstract
:1. Introduction
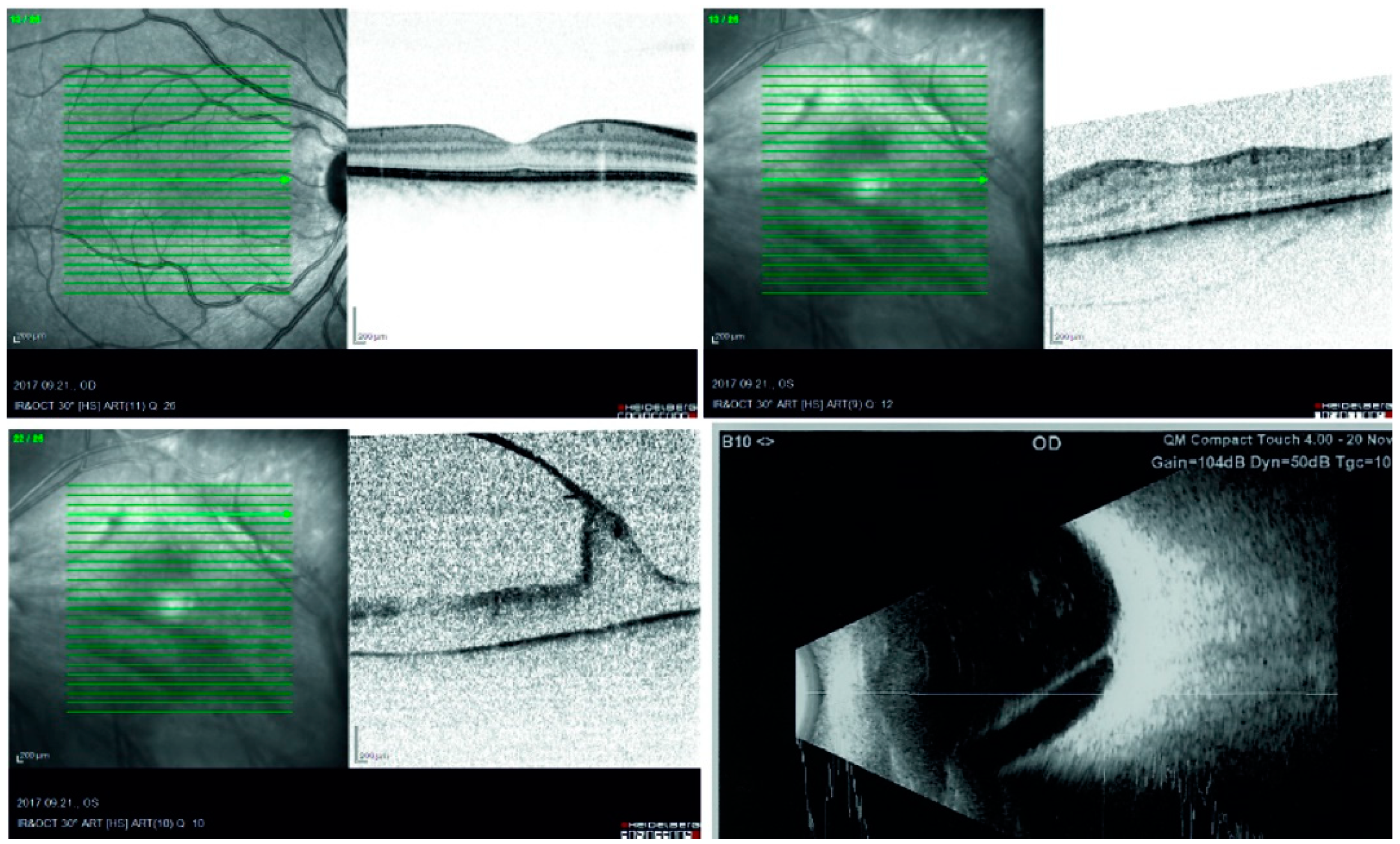
2. Case Report
3. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aradhya, S.; Woffendin, H.; Jakins, T.; Bardaro, T.; Esposito, T.; Smahi, A.; Shaw, C.; Levy, M.; Munnich, A.; D’Urso, M.; et al. A recurrent deletion in the ubiquitously expressed NEMO (IKK-gamma) gene accounts for the vast majority of incontinentia pigmenti mutations. Hum. Mol. Genet. 2001, 10, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.L.; Paller, A.S.; Chan, L.S. Incontinentia pigmenti: A review and update on the molecular basis of pathophysiology. J. Am. Acad. Dermatol. 2002, 47, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Holmström, G.; Thorén, K. Ocular manifestations of incontinentia pigmenti. Acta Ophthalmol. Scand. 2000, 78, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.G. Incontinentia pigmenti. A world statistical analysis. Arch. Dermatol. 1976, 112, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Oka, Y.; Takagi, I.; Yamana, T.; Kitano, A. The clinical features and treatment of the retinopathy in Bloch-Sulzberger syndrome (incontinentia pigmenti). Jpn. J. Ophthalmol. 1980, 24, 310–319. [Google Scholar]
- Goldberg, M.F.; Custis, P.H. Retinal and other manifestations of incontinentia pigmenti (Bloch-Sulzberger syndrome). Ophthalmology 1993, 100, 1645–1654. [Google Scholar] [CrossRef]
- Wald, K.J.; Mehta, M.C.; Katsumi, O.; Sabates, N.R.; Hirose, T. Retinal detachments in incontinentia pigmenti. Arch. Ophthalmol. 1993, 111, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.W.; Green, W.R.; Goldberg, M.F. Histopathologic and trypsin digested studies of the retina in incontinentia pigmenti. Ophthalmology 2008, 115, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Sheybani, A.; Harocopos, G.J.; Rao, P.K. Immunohistochemical study of epiretinal membranes in patients with uveitis. J. Ophthalmic Inflamm. Infect. 2012, 2, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampik, A.; Kenyon, K.R.; Michels, R.G. Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch. Ophthalmol. 1981, 99, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.M.; McLeod Scott, D.; Bhutto, I.A.; Villalonga, M.B.; Seddon, J.M.; Lutty, G.A. Idiopathic preretinal glia in aging and age-related macular degeneration. Exp. Eye Res. 2016, 150, 44–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.K.; Lee, S.Y.; Yu, H.G. Epiretinal membrane in nonexudative age-related macular degeneration: Anatomical Features, Visual Outcomes and Prognostic Factors. Retina 2016, 36, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Vinores, S.A.; Campochiaro, P.A.; McGehee, R.; Orman, W.; Hackett, S.F.; Hjelmeland, L.M. Ultrastructural and immunocytochemical changes in retinal pigment epithelium, retinal glia, and fibroblasts in vitreous culture. Investig. Ophthalmol. Vis. Sci. 1990, 12, 2529–2545. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janáky, M.; Hári Kovács, A.; Jánossy, Á.; Török, D.; Ivanyi, B.; Braunitzer, G.; Benedek, G. Immunohistochemical Analysis of a Vitreous Membrane Removed from a Patient with Incontinentia Pigmenti-Related Retinal Detachment. Vision 2020, 4, 5. https://doi.org/10.3390/vision4010005
Janáky M, Hári Kovács A, Jánossy Á, Török D, Ivanyi B, Braunitzer G, Benedek G. Immunohistochemical Analysis of a Vitreous Membrane Removed from a Patient with Incontinentia Pigmenti-Related Retinal Detachment. Vision. 2020; 4(1):5. https://doi.org/10.3390/vision4010005
Chicago/Turabian StyleJanáky, Márta, András Hári Kovács, Ágnes Jánossy, Dóra Török, Béla Ivanyi, Gábor Braunitzer, and György Benedek. 2020. "Immunohistochemical Analysis of a Vitreous Membrane Removed from a Patient with Incontinentia Pigmenti-Related Retinal Detachment" Vision 4, no. 1: 5. https://doi.org/10.3390/vision4010005
APA StyleJanáky, M., Hári Kovács, A., Jánossy, Á., Török, D., Ivanyi, B., Braunitzer, G., & Benedek, G. (2020). Immunohistochemical Analysis of a Vitreous Membrane Removed from a Patient with Incontinentia Pigmenti-Related Retinal Detachment. Vision, 4(1), 5. https://doi.org/10.3390/vision4010005



