The Horizontal Raphe of the Human Retina and its Watershed Zones
Abstract
1. Introduction
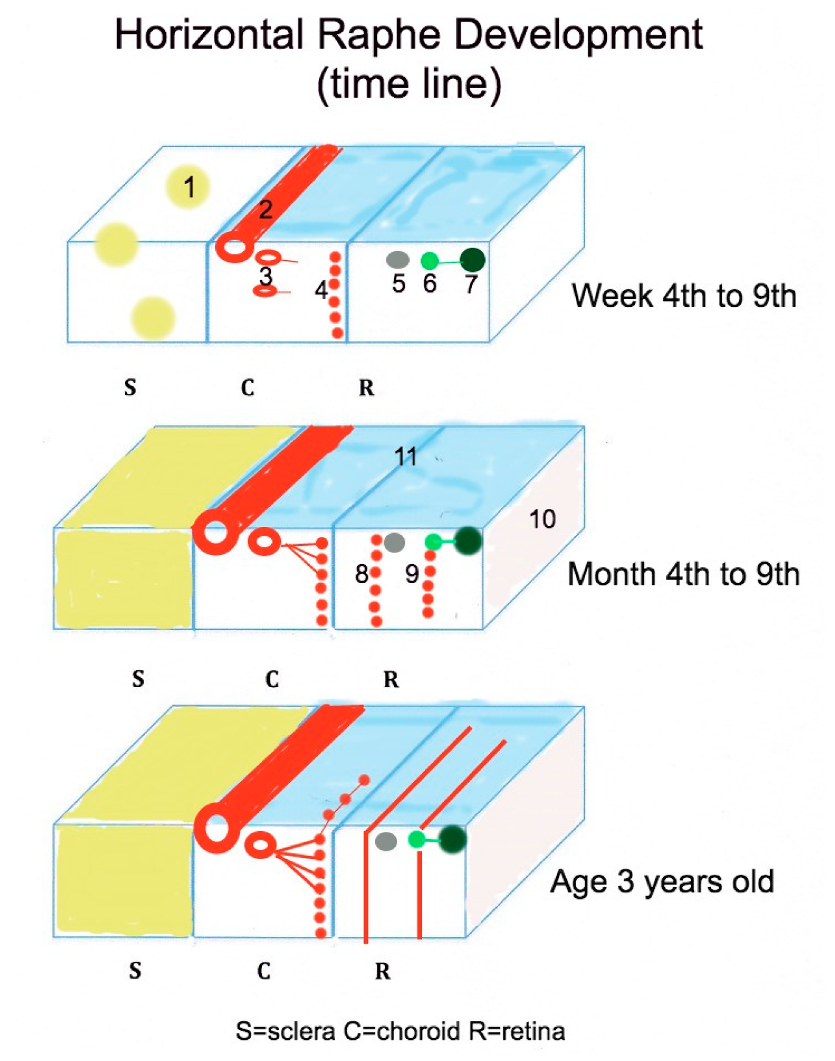
2. Developmental Aspects of the Human Horizontal Raphe
2.1. Molecular Signals Involved in Fetal Nerve Fiber Layer Axon Guidance
2.2. Morphological Organization of the Human Nerve Fiber Layer
2.3. The Impact of the Choroidal Development on Defining the Horizontal Raphe
3. Composition and Extend of the Horizontal Raphe
3.1. Postnatal Nerve Fiber Layer Development
3.2. Mature Human Retinal Nerve Fiber Layer
3.3. The Adult Nerve Fiber Layer Perfusion
3.4. The HR Watershed Zones (WSZs)
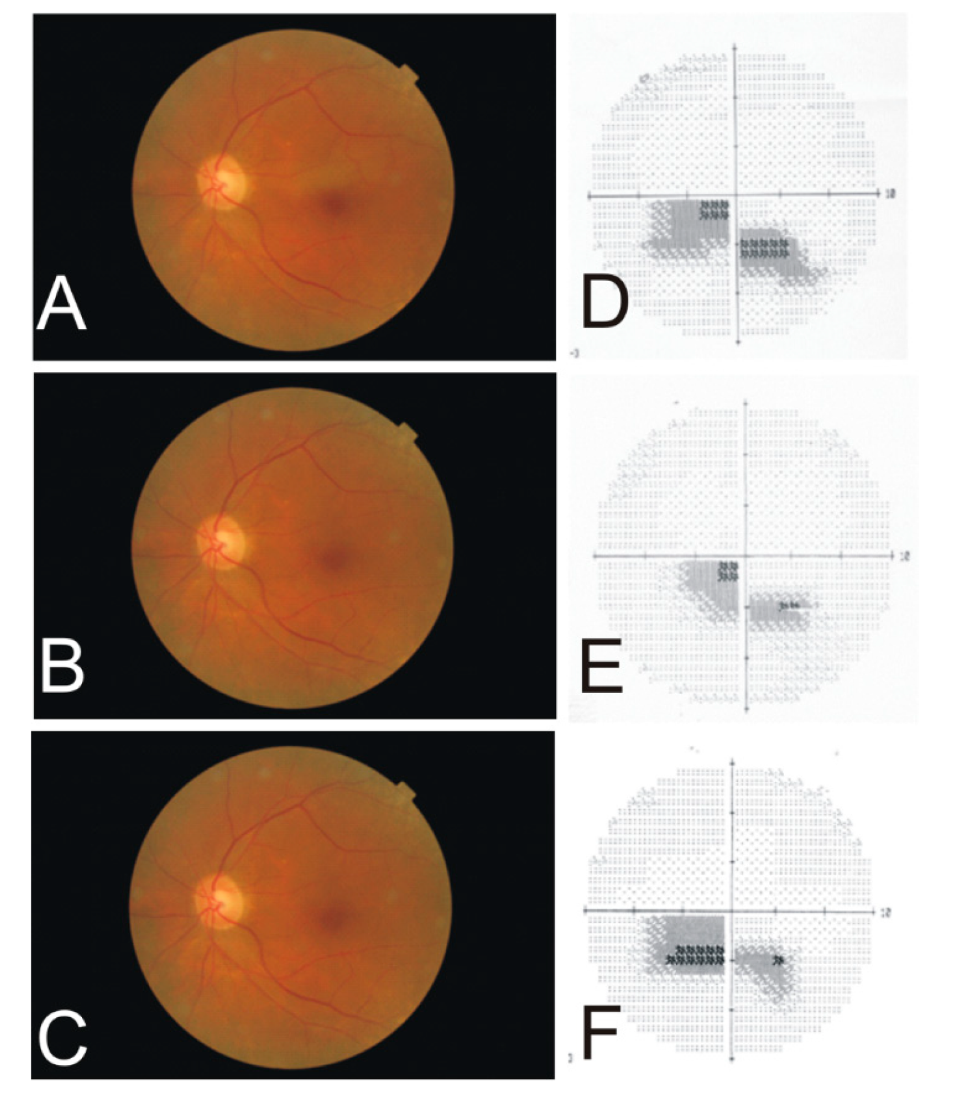
4. Clinical Cases Demonstrating the Presence of the HR
4.1. Arteritic Ischemic Optic Neuropathy
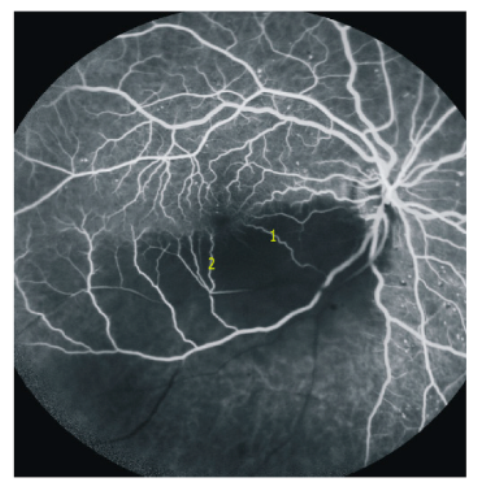
4.2. Cilio-Retinal Artery Occlusion
4.3. Branch Retinal Artery Occlusion
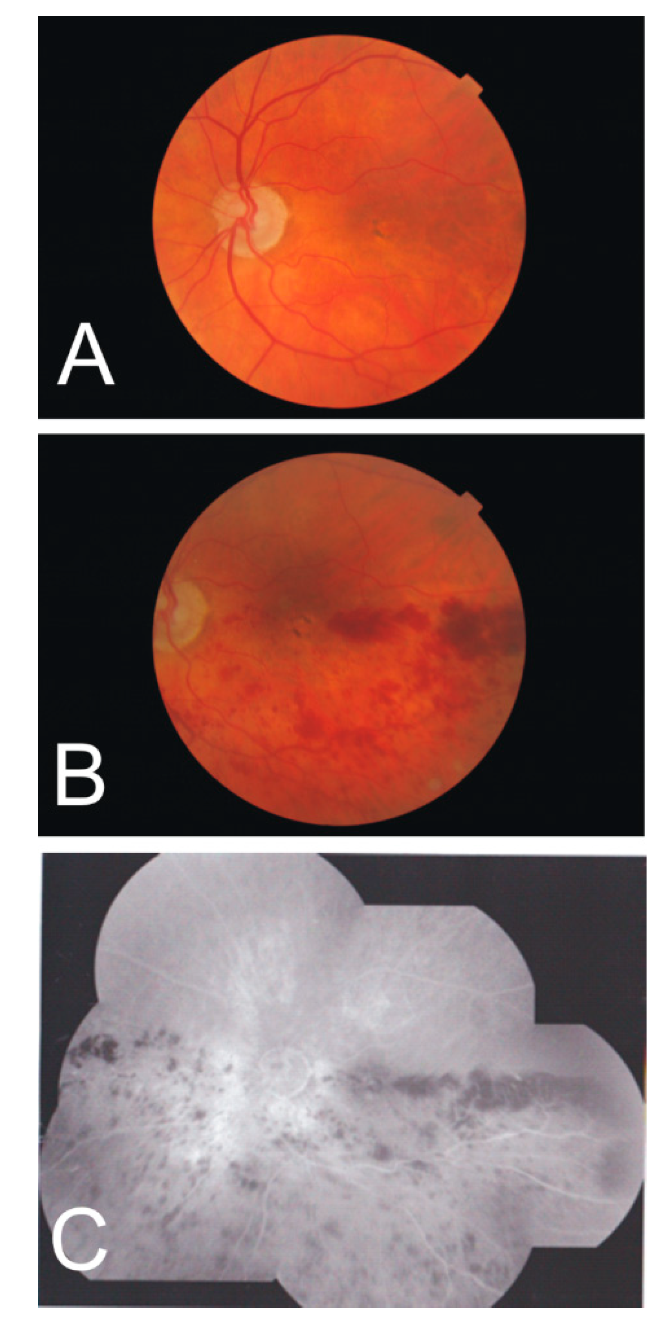
4.4. Choroidal Retinal Artery Occlusion
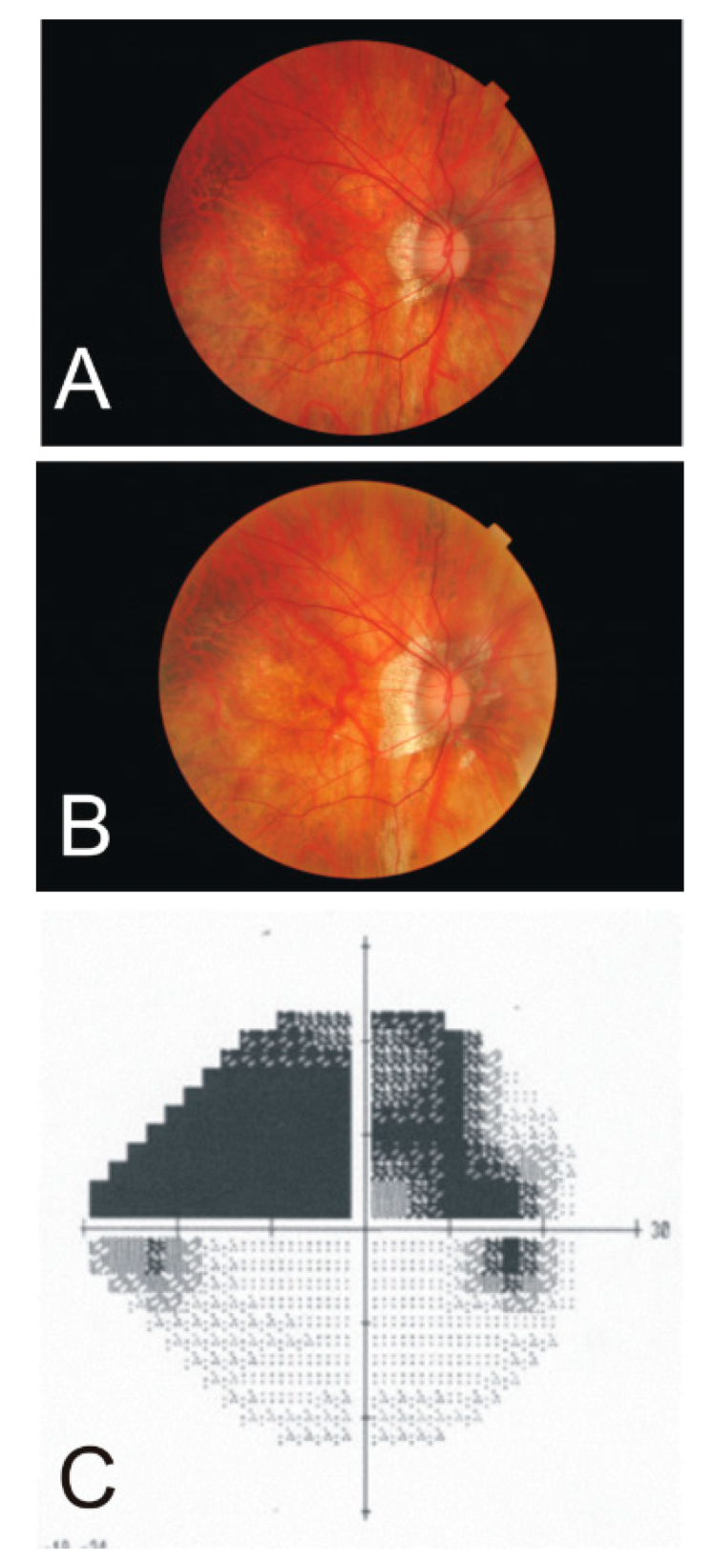
4.5. Hemiretinal Vein Occlusion
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ballantyne, A.S. The nerve fiber pattern of the human retina. Trans. Ophthalmol. Soc. UK 1947, 66, 179–191. [Google Scholar]
- Leber, T. Die Zirkulations- und Ernährungsverhältnisse des Auges. In Handbuch der gesamten Augenheilkunde; Graefe, A.C., Saemisch, E.T., Eds.; Engelmann: Leipzig, Germany, 1903. [Google Scholar]
- Polyak, S.L. The Retina; University of Chicago Press: Chicago, IL, USA, 1941; pp. 219–220. [Google Scholar]
- Posner, A.; Schlossman, A. Development of changes in visual fields associated with glaucoma. Arch. Ophthalmol. 1948, 39, 623–639. [Google Scholar] [CrossRef]
- Francois, J.; Neetens, A. Vascularization of the optic pathway: I. Lamina cribrosa and optic nerve. Br. J. Ophthalmol. 1954, 38, 472–488. [Google Scholar] [CrossRef]
- Vrabec, F. The temporal raphe of the human retina. Am. J. Ophthalmol. 1966, 62, 926–938. [Google Scholar] [CrossRef]
- Ashimatey, B.S.; King, B.J.; Burns, S.A.; Swanson, W.H. Evaluating glaucomatous abnormality in peripapillary optical coherence tomography enface visualisation of the retinal nerve fibre layer reflectance. Ophthalmic Physiol. Opt. 2018, 38, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.H.; King, B.J.; Burns, S.A. Within-subject variability in human retinal nerve fiber bundle width. PLoS ONE 2019, 14, e0223350. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yoo, B.W.; Jeoung, J.W.; Kim, H.C.; Kim, H.J.; Park, K.H. Glaucoma-diagnostic ability of ganglion cell-inner plexiform layer thickness difference across temporal raphe in highly myopic eyes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5856–5863. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yoo, B.W.; Kim, H.C.; Park, K.H. Automated detection of hemifield difference across horizontal raphe on ganglion cell—Inner plexiform layer thickness map. Ophthalmology 2015, 122, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.C.; Sharpe, G.P.; Hutchison, D.M. Imaging of the temporal raphe with optical coherence tomography. Ophthalmology 2014, 121, 2287–2288. [Google Scholar] [CrossRef]
- Guedes, V.; Schuman, J.S.; Hertzmark, E.; Wollstein, G.; Correnti, A.; Mancini, R.; Lederer, D.; Voskanian, S.; Velazquez, L.; Pakter, H.M.; et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology 2003, 110, 177–189. [Google Scholar] [CrossRef]
- Curcio, C.A.; Allen, K.A. Topography of ganglion cells in human retina. J. Comp. Neurol. 1990, 300, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Gast, T.J.; Burns, S.A. In vivo adaptive optics imaging of the temporal raphe and its relationship to the optic disc and fovea in the human retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5952–5961. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kurokawa, K.; Zhang, F.; Lee, J.J.; Miller, D.T. Imaging and quantifying ganglion cells and other transparent neurons in the living human retina. Proc. Natl. Acad. Sci. USA 2017, 114, 12803–12808. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Luo, T.; Gast, T.J.; Burns, S.A.; Malinovsky, V.E.; Swanson, W.H. Imaging Glaucomatous Damage Across the Temporal Raphe. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3496–3504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herrera, E.; Erskine, L.; Morenilla-Palao, C. Guidance of retinal axons in mammals. Semin. Cell Dev. Biol. 2017. [Google Scholar] [CrossRef]
- Oster, S.F.; Deiner, M.; Birgbauer, E.; Sretavan, D.W. Ganglion cell axon pathfinding in the retina and optic nerve. Semin. Cell Dev. Biol. 2004, 15, 125–136. [Google Scholar] [CrossRef]
- Jones, C.A.; London, N.R.; Chen, H.; Park, K.W.; Sauvaget, D.; Stockton, R.A.; Wythe, J.D.; Suh, W.; Larrieu-Lahargue, F.; Mukouyama, Y.S.; et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat. Med. 2008, 14, 448–453. [Google Scholar] [CrossRef]
- Rama, N.; Dubrac, A.; Mathivet, T.; Ní Chárthaigh, R.A.; Genet, G.; Cristofaro, B.; Pibouin-Fragner, L.; Ma, L.; Eichmann, A.; Chédotal, A. Slit2 signaling through Robo1 and Robo2 is required for retinal neovascularization. Nat. Med. 2015, 21, 483–491. [Google Scholar] [CrossRef]
- Li, S.; Huang, L.; Sun, Y.; Bai, Y.; Yang, F.; Yu, W.; Li, F.; Zhang, Q.; Wang, B.; Geng, J.G.; et al. Slit2 Promotes Angiogenic Activity Via the Robo1-VEGFR2-ERK1/2 Pathway in Both In Vivo and In Vitro Studies. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5210–5217. [Google Scholar] [CrossRef] [PubMed]
- Dufourcq, P.; Leroux, L.; Ezan, J.; Descamps, B.; Lamazière, J.M.; Costet, P.; Basoni, C.; Moreau, C.; Deutsch, U.; Couffinhal, T.; et al. Regulation of endothelial cell cytoskeletal reorganization by a secreted frizzled-related protein-1 and frizzled 4- and frizzled 7-dependent pathway: Role in neovessel formation. Am. J. Pathol. 2008, 172, 37–49. [Google Scholar] [CrossRef]
- Marcos, S.; Nieto-Lopez, F.; Sandonìs, A.; Cardozo, M.J.; Di Marco, F.; Esteve, P.; Bovolenta, P. Secreted frizzled related proteins modulate pathfinding and fasciculation of mouse retina ganglion cell axons by direct and indirect mechanisms. J. Neurosci. 2015, 35, 4729–4740. [Google Scholar] [CrossRef]
- Marcus, R.C.; Gale, N.W.; Morrison, M.E.; Mason, C.A.; Yancopoulos, G.D. Eph family receptors and their ligands distribute in opposing gradients in the developing mouse retina. Dev. Biol. 1996, 180, 786–789. [Google Scholar] [CrossRef]
- Hoshino, A.; Ratnapriya, R.; Brooks, M.J.; Chaitankar, V.; Wilken, M.S.; Zhang, C.; Starostik, M.R.; Gieser, L.; La Torre, A.; Nishio, M.; et al. Molecular anatomy of the developing human retina. Dev. Cell 2017, 43, 763–779. [Google Scholar] [CrossRef]
- Gupta, T.; Kapoor, K.; Sahni, D.; Singh, B. Mapping the time line of development in each layer of human foetal retina. J. Clin. Diagn. Res. 2016, 10, AC04–AC07. [Google Scholar] [CrossRef]
- Mann, I. The Development of the Human Eye; British Medical Association: Cambridge, UK, 1949. [Google Scholar]
- Provis, J.M.; van Driel, D.; Billson, F.A.; Russell, P. Development of the human retina: Patterns of cell distribution and redistribution in the ganglion cell layer. J. Comp. Neurol. 1985, 233, 429–451. [Google Scholar] [CrossRef]
- Provis, J.M.; van Driel, D.; Billson, F.A.; Russell, P. Human fetal optic nerve: Overproduction and elimination of retinal axons during development. J. Comp. Neurol. 1985, 238, 92–100. [Google Scholar] [CrossRef]
- Wong, R.O.; Hughes, A. Role of cell death in the topogenesis of neuronal distributions in the developing cat retinal ganglion cell layer. J. Comp. Neurol. 1987, 262, 496–511. [Google Scholar] [CrossRef]
- Henderson, Z.; Finlay, B.L.; Wikler, K.C. Development of ganglion cell topography in ferret retina. J. Neurosci. 1988, 8, 1194–1205. [Google Scholar] [CrossRef]
- Beros, J.; Rodger, J.; Harvey, A.R. Developmental retinal ganglion cell death and retinotopicity of the murine retinocollicular projection. Dev. Neurobiol. 2018, 78, 51–60. [Google Scholar] [CrossRef]
- Leventhal, A.G.; Schall, J.D.; Ault, S.J.; Provis, J.M.; Vitek, D.J. Class-specific cell death shapes the distribution and pattern of central projection of cat retinal ganglion cells. J. Neurosci. 1988, 8, 2011–2027. [Google Scholar] [CrossRef]
- So, K.F.; Campbell, G.; Lieberman, A.R. Development of the mammalian retinogeniculate pathway: Target finding, transient synapses and binocular segregation. J. Exp. Biol. 1990, 153, 85–104. [Google Scholar]
- González-Menéndez, I.; Contreras, F.; Cernuda-Cernuda, R.; García-Fernández, J.M. No loss of melanopsin-expressing ganglion cells detected during postnatal development of the mouse retina. Histol. Histopathol. 2010, 25, 73–82. [Google Scholar]
- Lia, B.; Williams, R.W.; Chalupa, L.M. Formation of retinal ganglion cell topography during prenatal development. Science 1987, 236, 848–851. [Google Scholar] [CrossRef]
- Chalupa, L.M.; Lia, B. The nasotemporal division of retinal ganglion cells with crossed and uncrossed projections in the fetal rhesus monkey. J. Neurosci. 1991, 11, 191–202. [Google Scholar] [CrossRef]
- O’Sullivan, M.L.; Puñal, V.M.; Kerstein, P.C.; Brzezinski, J.A., 4th; Glaser, T.; Wright, K.M.; Kay, J.N. Astrocytes follow ganglion cell axons to establish an angiogenic template during retinal development. Glia 2017, 65, 1697–1716. [Google Scholar] [CrossRef]
- Hasegawa, T.; McLeod, D.S.; Prow, T.; Merges, C.; Grebe, R.; Lutty, G.A. Vascular precursors in developing human retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2178–2192. [Google Scholar] [CrossRef]
- Gariano, R.F.; Iruela-Arispe, M.L.; Hendrickson, A.E. Vascular development in primate retina: Comparison of laminar plexus formation in monkey and human. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3442–3455. [Google Scholar]
- Lutty, G.A.; Hasegawa, T.; Baba, T.; Grebe, R.; Bhutto, I.; McLeod, D.S. Development of the human choriocapillaris. Eye 2010, 24, 408–415. [Google Scholar] [CrossRef]
- Sellheyer, K.; Spitznas, M. The fine structure of the developing human choriocapillaris during the first trimester. Graefes Arch. Clin. Exp. Ophthalmol. 1988, 226, 65–74. [Google Scholar] [CrossRef]
- Lutty, G.A.; McLeod, D.S. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog. Retin. Eye Res. 2018, 62, 58–76. [Google Scholar] [CrossRef]
- Zouache, M.A.; Eames, I.; Klettner, C.A.; Luthert, P.J. Form, shape and function: Segmented blood flow in the choriocapillaris. Sci. Rep. 2016, 6, 35754. [Google Scholar] [CrossRef] [PubMed]
- Eida, H.; Bhutto, I.A.; Amemiya, T. Corrosion cast demonstration of choroidal vasculature in normal Wistar Kyoto rat. Ital. J. Anat. Embryol. 2001, 106, 245–250. [Google Scholar] [PubMed]
- Rothman, A.L.; Sevilla, M.B.; Freedman, S.F.; Tong, A.Y.; Tai, V.; Tran-Viet, D.; Farsiu, S.; Toth, C.A.; El-Dairi, M.A. Assessment of retinal nerve fiber layer thickness in healthy, full-term neonates. Am. J. Ophthalmol. 2015, 159, 803–811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, A.; Purohit, R.; Lee, H.; Sheth, V.; Maconachie, G.; Papageorgiou, E.; McLean, R.J.; Gottlob, I.; Proudlock, F.A. Optic nerve head development in healthy infants and children using handheld spectral-domain optical coherence tomography. Ophthalmology 2016, 123, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.J.; Cocker, K.D.; Lau, G.; Clay, S.T.; Fielder, A.R.; Moseley, M.J. Optic disk size and optic disk-to-fovea distance in preterm and full-term infants. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4683–4686. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D.; Wilkinson, J.M. Position of the fovea centralis with respect to the optic nerve head. Optometry Vision Sci. 1992, 69, 369–377. [Google Scholar] [CrossRef]
- Yanni, S.E.; Wang, J.; Cheng, C.S.; Locke, K.I.; Wen, Y.; Birch, D.G.; Birch, E.E. Normative reference ranges for the retinal nerve fiber layer, macula, and retinal layer thicknesses in children. Am. J. Ophthalmol. 2013, 155, 354–360. [Google Scholar] [CrossRef]
- Elia, N.; Pueyo, V.; Altemir, I. Normal reference ranges of optical coherence tomography parameters in childhood. Br. J. Ophthalmol. 2012, 96, 665–670. [Google Scholar] [CrossRef]
- Hong, S.W.; Ahn, Y.J.; Kang, N.Y. Relationship between age and retinal nerve fiber layer thickness in normal children. Semin. Ophthalmol. 2017, 32, 655–660. [Google Scholar] [CrossRef]
- Lee, J.W.; Yau, G.S.; Woo, T.T.; Yick, D.W.; Tam, V.T.; Lai, J.S. Retinal nerve fiber layer thickness in myopic, emmetropic, and hyperopic children. Medicine 2015, 94, e699. [Google Scholar] [CrossRef]
- Samarawickrama, C.; Wang, J.J.; Huynh, S.C.; Pai, A.; Burlutsky, G.; Rose, K.A.; Mitchell, P. Ethnic differences in optic nerve head and retinal nerve fibre layer thickness parameters in children. Br. J. Ophthalmol. 2010, 94, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, C.; Barikian, A.; Jaroudi, M.; Massoud, V.; Tamim, H.; Noureddin, B. Spectral domain optical coherence tomography in children: Normative data and biometric correlations. BMC Ophthalmol. 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Sahoo, B.; Kumar, M.; Varshney, G.; Kumar, R. Retinal nerve fiber layer thickness in children <18 years by spectral-domain optical coherence tomography. Semin. Ophthalmol. 2013, 28, 97–102. [Google Scholar] [PubMed]
- Vajzovic, L.; Hendrickson, A.E.; O’Connell, R.V.; Clark, L.A.; Tran-Viet, D.; Possin, D.; Chiu, S.J.; Farsiu, S.; Toth, C.A. Maturation of the human fovea: Correlation of spectral-domain optical coherence tomography findings with histology. Am. J. Ophthalmol. 2012, 154, 779–789. [Google Scholar] [CrossRef]
- Alasil, T.; Wang, K.; Keane, P.A.; Lee, H.; Baniasadi, N.; de Boer, J.F.; Chen, T.C. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J. Glaucoma 2013, 22, 532–541. [Google Scholar] [CrossRef]
- Budenz, D.L.; Anderson, D.R.; Varma, R.; Schuman, J.; Cantor, L.; Savell, J.; Greenfield, D.S.; Patella, V.M.; Quigley, H.A.; Tielsch, J. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology 2007, 114, 1046–1052. [Google Scholar] [CrossRef]
- Cohen, M.J.; Kaliner, E.; Frenkel, S.; Kogan, M.; Miron, H.; Blumenthal, E.Z. Morphometric analysis of human peripapillary retinal nerve fiber layer thickness. Investig. Ophthalmol. Vis. Sci. 2008, 49, 941–944. [Google Scholar] [CrossRef]
- Bedggood, P.; Nguyen, B.; Lakkis, G.; Turpin, A.; McKendrick, A.M. Orientation of the temporal nerve fiber raphe in healthy and in glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4211–4217. [Google Scholar] [CrossRef]
- Bedggood, P.; Tanabe, F.; McKendrick, A.M.; Turpin, A. Automatic identification of the temporal retinal nerve fiber raphe from macular cube data. Biomed. Opt. Express 2016, 7, 4043–4053. [Google Scholar] [CrossRef]
- Radius, R.L.; Anderson, D.R. The course of axons through the retina and optic nerve head. Arch. Ophthalmol. 1979, 97, 1154–1158. [Google Scholar] [CrossRef]
- Minckler, D.S. The organization of nerve fiber bundles in the primate optic nerve head. Arch. Ophthalmol. 1980, 98, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Leber, T. Die Krankheiten der Netzhaut und des Sehnerven. In Handbuch der gesamten Augenheilkunde; Graefe, A.C., Saemisch, E.T., Eds.; Engelmann: Leipzig, Germany, 1877. [Google Scholar]
- Quigley, H.A.; Addicks, E.M. Quantitative studies of retinal nerve fiber layer defects. Arch. Ophthalmol. 1982, 100, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Ogden, T.E. Nerve fiber layer of the owl monkey retina: Retinotopic organization. Investig. Ophthalmol. Vis. Sci. 1983, 24, 265–269. [Google Scholar] [PubMed]
- Ogden, T.E. Nerve fiber layer of the macaque retina: Retinotopic organization. Investig. Ophthalmol. Vis. Sci. 1983, 24, 85–98. [Google Scholar] [PubMed]
- FitzGibbon, T. The human fetal retinal nerve fiber layer and optic nerve head: A DiI and DiA tracing study. Vis. Neurosci. 1997, 14, 433–447. [Google Scholar] [CrossRef]
- FitzGibbon, T.; Taylor, S.F. Retinotopy of the human retinal nerve fibre layer and optic nerve head. J. Comp. Neurol. 1996, 375, 238–251. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.; Cull, G.; Fortune, B.; Cioffi, G.A. Varicosities of intraretinal ganglion cell axons in human and nonhuman primates. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2–9. [Google Scholar] [CrossRef]
- Rutkowski, P.; May, C.A. Nutrition and Vascular Supply of Retinal Ganglion Cells during Human Development. Front. Neurol. 2016, 7, 49. [Google Scholar] [CrossRef]
- Mase, T.; Ishibazawa, A.; Nagaoka, T.; Yokota, H.; Yoshida, A. Radial peripapillary capillary network visualized using wide-field montage optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT504–OCT510. [Google Scholar] [CrossRef]
- Fryczkowski, A.W.; Sherman, M.D.; Walker, J. Observation on the lobular organization of the human Choriocapillaris. Int. Ophthalmol. 1991, 15, 109–120. [Google Scholar] [CrossRef]
- Fryczkowski, A.W.; Sherman, M.D. Scanning electron microscopy of human ocular vascular casts: The submacular Choriocapillaris. Acta Anat. 1988, 132, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Posterior ciliary artery circulation in health and disease: The Weisenfeld lecture. Investig. Ophthalmol. Vis. Sci. 2004, 45, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Olver, J.M. Functional Anatomy of the choroidal circulation: Methyl methacrylate casting of human choroid. Eye 1990, 4, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Yang, H.; Chan-Ling, T. Vascularization of the human fetal retina: Roles of vasculogenesis and angiogenesis. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1217–1228. [Google Scholar]
- Provis, J.M.; Hendrickson, A.E. The foveal avascular region of developing human retina. Arch. Ophthalmol. 2008, 126, 507–511. [Google Scholar] [CrossRef]
- Cogan, D.G. Development and senescence of the human retinal vasculature. Trans. Ophthalmol. Soc. UK 1963, 83, 465–489. [Google Scholar]
- Michaelson, I.C.; Friedenwald, J.S. Retinal Circulation in Man and Animals; Charles, C., Ed.; Thomas: Springfield, IL, USA, 1954. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, C.A.; Rutkowski, P. The Horizontal Raphe of the Human Retina and its Watershed Zones. Vision 2019, 3, 60. https://doi.org/10.3390/vision3040060
May CA, Rutkowski P. The Horizontal Raphe of the Human Retina and its Watershed Zones. Vision. 2019; 3(4):60. https://doi.org/10.3390/vision3040060
Chicago/Turabian StyleMay, Christian Albrecht, and Paul Rutkowski. 2019. "The Horizontal Raphe of the Human Retina and its Watershed Zones" Vision 3, no. 4: 60. https://doi.org/10.3390/vision3040060
APA StyleMay, C. A., & Rutkowski, P. (2019). The Horizontal Raphe of the Human Retina and its Watershed Zones. Vision, 3(4), 60. https://doi.org/10.3390/vision3040060




