What Neuroscientific Studies Tell Us about Inhibition of Return
Abstract
1. Introduction
2. Behavioral Manifestations
2.1. Causes and Consequences
2.2. Spatio-Temporal Properties
2.3. IOR as Foraging Facilitator
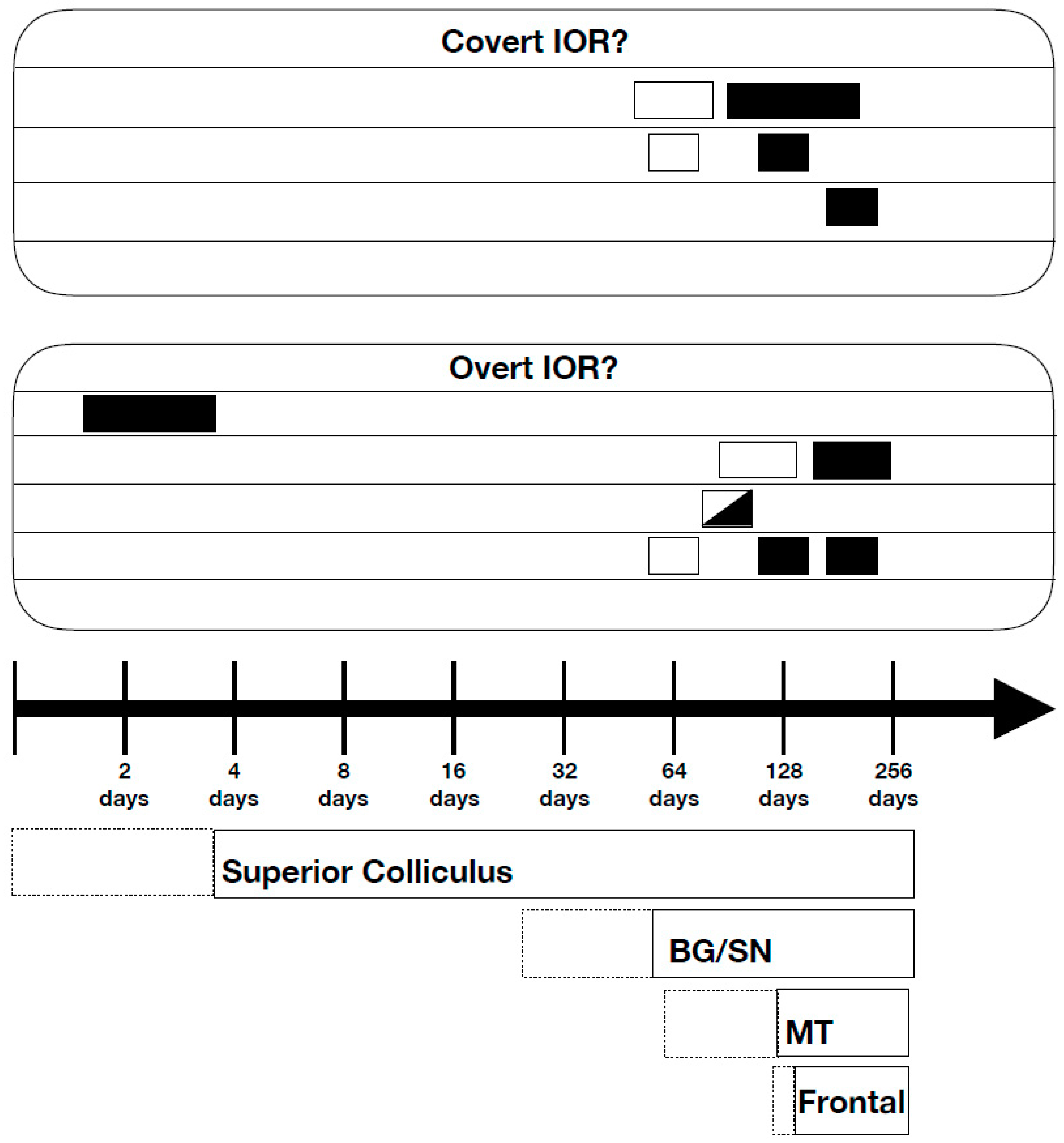
3. Neuroscience
3.1. Patient Studies
3.2. Developmental Studies
3.3. Human Brain Imaging
3.4. Manipulations Aimed at Exploring the Roles of Neural Structures and Pathways
3.5. Monkey Neurophysiology
3.6. Computational Modeling
4. Conclusions
Funding
Conflicts of Interest
References
- Posner, M.I.; Cohen, Y. Components of Visual Orienting. Attent. Perform. X Control Lang. Processes 1984, 32, 531–556. [Google Scholar]
- Posner, M.I.; Rafal, R.D.; Choate, L.; Vaughan, J. Inhibition of return: Neural basis and function. Cogn. Neuropsychol. 1985, 2, 211–228. [Google Scholar] [CrossRef]
- Klein, R.M. Inhibition of return. Trends Cogn. Sci. 2000, 4, 138–147. [Google Scholar] [CrossRef]
- Dukewich, K.; Klein, R.M. Inhibition of return: A phenomenon in search of a definition and a theoretical framework. Attent. Percept. Psychophys. 2015, 77, 1647–1658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hilchey, M.D.; Klein, R.M.; Satel, J. Returning to “Inhibition of Return” by dissociating long-term oculomotor IOR from short-term sensory adaptation and other nonoculomotor “inhibitory” cueing effects. J. Exp. Psychol. Hum. Percept. Perform. 2014, 40, 1606–1616. [Google Scholar] [CrossRef]
- Taylor, T.; Klein, R.M. Visual and motor effects in inhibition of return. J. Exp. Psychol. Hum. Percept. Perform. 2000, 6, 1639–1655. [Google Scholar] [CrossRef]
- Berlucchi, G. Inhibition of return: A phenomenon in search of a mechanism and a better name. Cogn Neuropsychol. 2006, 23, 1065–1074. [Google Scholar] [CrossRef]
- Rafal, R.D.; Calabresi, P.A.; Brennan, C.W.; Sciolto, T.K. Saccade preparation inhibits reorienting to recently attended locations. J. Exp. Psychol. Hum. Percept. Perform. 1989, 15, 673–685. [Google Scholar] [CrossRef]
- Klein, R.M.; Christie, J.; Morris, E. Vector averaging of inhibition of return. Psychon. Bull.Rev. 2005, 12, 295–300. [Google Scholar] [CrossRef]
- Spence, C.; Lloyd, D.; McGlone, F.; Nicholls, M.E.R.; Driver, J. Inhibition of return is supramodal: A demonstration between all possible pairings of vision, touch, and audition. Exp. Brain Res. 2000, 134, 42–48. [Google Scholar] [CrossRef]
- Chica, A.B.; Klein, R.M.; Rafal, R.D.; Hopfinger, J.B. Endogenous saccade preparation does not produce Inhibition of Return: Failure to replicate Rafal, Calabresi, Brennan, & Sciolto (1989). J. Exp. Psychol. Hum. Percept. Perform. 2010, 36, 1193–1206. [Google Scholar] [PubMed]
- Klein, R.M.; Redden, R.S. Two “Inhibitions of Return” Bias Orienting Differently. In Spatial Biases in Perception and Cognition; Hubbard, T., Ed.; Cambridge University Press: Cambridge, UK, 2018; pp. 295–306. [Google Scholar]
- Samuel, A.G.; Kat, D. Inhibition of return: A graphical meta-analysis of its time course and an empirical test of its temporal and spatial properties. Psychon. Bull. Rev. 2003, 10, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Lupiáñez, J. Inhibition of return. In Attention and Time; Nobre, A.C., Coull, J.T., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 17–34. [Google Scholar]
- Bennett, P.J.; Pratt, J. The spatial distribution of inhibition of return. Psychol. Sci. 2001, 12, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yan, C.; Klein, R.M.; Wang, Z. Inhibition of return revisited: Localized inhibition on top of a pervasive bias. Psychon. Bull. Rev. 2018, 25, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.J.; Hilchey, M.D.; Klein, R.M. Inhibition of return is at the midpoint of simultaneous cues. Attent. Percept. Psychophys. 2013, 75, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.J.; Hilchey, M.D.; Mishra, R.; Klein, R.M. Eye movements are primed toward the centre of multiple stimuli even when the interstimulus distances are too large to generate saccade averaging. Exp. Brain Res. 2015, 233, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Maylor, E.A.; Hockey, R. Inhibitory component of externally controlled covert orienting in visual space. J. Exp. Psychol. Hum. Percept. Perform. 1985, 11, 777–787. [Google Scholar] [CrossRef]
- Pertzov, Y.; Zohary, E.; Avidan, G. Rapid formation of spatiotopic representations as revealed by inhibition of return. J. Neurosci. 2010, 30, 8882–8887. [Google Scholar] [CrossRef]
- Hilchey, M.D.; Klein, R.M.; Satel, J.; Wang, Z. Oculomotor inhibition of return: How soon is it “recoded” into spatiotopic coordinates? Attent. Percept. Psychophy. 2012, 74, 1145–1153. [Google Scholar] [CrossRef]
- Yan, C.; He, T.; Klein, R.M.; Wang, Z. Predictive remapping gives rise to environmental inhibition of return. Psychon. Bull. Rev. 2016, 23, 1860–1866. [Google Scholar] [CrossRef]
- Tipper, S.P.; Driver, J.; Weaver, B. Object-centred inhibition of return of visual attention. Q. J. Exp. Psychol. A 1991, 43, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klein, R.M. Searching for inhibition of return in visual search: A review. Vis. Res. 2010, 50, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Redden, R.S.; Klages, J.; Klein, R.M. The effect of scene removal on inhibition of return in a cue target task. Attent. Percept. Psychophys. 2017, 79, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.M. Inhibitory tagging system facilitates visual search. Nature 1988, 334, 430. [Google Scholar] [CrossRef]
- Klein, R.M.; MacInnes, W.J. Inhibition of return is a foraging facilitator in visual search. Psychol. Sci. 1999, 10, 346–352. [Google Scholar] [CrossRef]
- Briand, K.A.; Szapiel, S.V.; Sereno, A.B. Disruption of reflexive visual orienting in an individual with a collicular lesion. J. Clin. Exp. Neuropsychol. 2003, 28, 145–166. [Google Scholar]
- Sapir, A.; Soroker, N.; Berger, A.; Henik, A. Inhibition of return in spatial attention: Direct evidence for collicular generation. Nat. Neurosci. 1999, 2, 1053–1054. [Google Scholar] [CrossRef]
- Tipper, S.P.; Rafal, R.; Reuter-Lorenz, P.A.; Starrveldt, Y.; Ro, T.; Egly, R. Object-based facilitation and inhibition from visual orienting in the human split brain. J. Exp. Psychol. Hum. Percept. Perform. 1997, 23, 1522–1532. [Google Scholar] [CrossRef]
- Smith, D.T.; Ball, K.; Swalwell, R.; Schenk, T. Object-based attentional facilitation and inhibition are neuropsychologically dissociated. Neuropsychologia 2016, 80, 9–16. [Google Scholar] [CrossRef]
- Sapir, A.; Hayes, A.; Henik, A.; Danziger, S.; Rafal, R. Parietal lobe lesions disrupt saccadic remapping of inhibitory location tagging. J. Cogn. Neurosci. 2004, 16, 503–509. [Google Scholar] [CrossRef]
- Bourgeois, A.; Chica, A.B.; Migliaccio, R.; de Schotten, M.T.; Bartolomeo, P. Cortical control of inhibition of return: Evidence from patients with inferior parietal damage and visual neglect. Neuropsychologia 2012, 50, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.T.; Rorden, C.; Jackson, S.R. Exogenous orienting of attention depends upon 805 the ability to execute eye movements. Curr. Biol. 2004, 14, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.T.; Schenk, T.; Rorden, C. Saccade preparation is required for exogenous attention but not endogenous attention or IOR. J. Exp. Psychol. Hum. Percept. Perform. 2012, 38, 1438. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, Ł.; Paszulewicz, J.; Bielas, J.; Wolski, P. Is saccade preparation required for inhibition of return (IOR)? Neurosci. Lett. 2018, 665, 13–17. [Google Scholar] [CrossRef]
- Casteau, S.; Smith, D.T. Associations and Dissociations between Oculomotor Readiness and Covert attention. Vision 2019, 3, 17. [Google Scholar] [CrossRef]
- Klein, R.M. On the role of endogenous orienting in the inhibitory aftermath of exogenous orienting. In Developing Individuality in the Human Brain: A Tribute to Michael I. Posner; Mayr, U., Awh, E., Keele, S., Eds.; APA Books: Washington, DC, USA, 2005; pp. 45–64. [Google Scholar]
- Johnson, M.H. Cortical maturation and the development of visual attention in early infancy. J. Cogn. Neurosci. 1990, 2, 81–95. [Google Scholar] [CrossRef]
- Valenza, E.L.; Simion, F.L.; Umilta, C.L. Inhibition of return in newborn infants. Infant Behav. Dev. 1994, 17, 293–302. [Google Scholar] [CrossRef]
- Simion, F.; Valenza, E.; Umilta, C.; Dalla, B. Inhibition of return in newborns is temporo-nasal asymmetrical. Infant Behav. Dev. 1995, 8, 189–194. [Google Scholar] [CrossRef]
- Johnson, M.H.; Posner, M.I.; Rothbart, M.K. Facilitation of saccades toward a covertly attended location in early infancy. Psychol. Sci. 1994, 5, 90–93. [Google Scholar] [CrossRef]
- Martín-Arévalo, E.; Chica, A.B.; Lupiáñez, J. No single electrophysiological marker for facilitation and inhibition of return: A review. Behav. Brain Res. 2016, 300, 1–10. [Google Scholar] [CrossRef]
- Satel, J.; Hilchey, M.D.; Wang, Z.; Story, R.; Klein, R.M. The effects of ignored versus foveated cues upon inhibition of return: An event-related potential study. Attent. Percept. Psychophys. 2013, 75, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Satel, J.; Hilchey, M.D.; Wang, Z.; Reiss, C.S.; Klein, R.M. In search of a reliable electrophysiological marker of oculomotor inhibition of return. Psychophysiology 2014, 51, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Satel, J.; Wang, Z.; Hilchey, M.D.; Klein, R.M. Examining the dissociation of retinotopic and spatiotopic inhibition of return with event-related potentials. Neurosci. Lett. 2012, 524, 40–44. [Google Scholar] [CrossRef]
- McDonald, J.J.; Hickey, C.; Green, J.J.; Whitman, J.C. Inhibition of return in the covert deployment of attention: Evidence from human electrophysiology. J. Cogn. Neurosci 2009, 21, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.M.; Crouse, M.D.; Green, J.J. Evidence for an attentional component of inhibition of return in visual search. Psychophysiology 2017, 54, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Klein, R.M.; Satel, J.; Xu, P.; Yao, D. Electrophysiological explorations of the cause and effect of inhibition of return in a cue-target paradigm: A spatio-temporal theory. Brain Topogr. 2011, 24, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Regan, D. Chromatic adaptation and steady-state evoked potentials. Vis. Res. 1968, 8, 149–158. [Google Scholar] [CrossRef]
- Morgan, S.T.; Hansen, J.C.; Hillyard, S.A.; Posner, M. Selective attention to stimulus location modulates the steady-state visual evoked potential. Neurobiology 1996, 93, 4770–4774. [Google Scholar] [CrossRef]
- Müller, M.M.; Picton, T.W.; Valdes-Sosa, P.; Riera, J.; Teder-Sälejärvi, W.A.; Hillyard, S.A. Effects of spatial selective attention on the steady-state visual evoked potential in the 20–28 Hz range. Cogn. Brain Res. 1998, 6, 249–261. [Google Scholar] [CrossRef]
- Li, A.-S.; Zhang, G.-L.; Miao, C.-G.; Wang, S.; Zhang, M.; Zhang, Y. The time course of inhibition of return: Evidence from steady-state visual evoked potentials. Front. Psychol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Klein, R.M. Orienting and inhibition of return. In The Cognitive Neurosciences, 3rd ed.; Gazzaniga, M.S., Ed.; MIT Press: Cambridge, MA, USA, 2004; pp. 545–560. [Google Scholar]
- Müller, N.G.; Kleinschmidt, A. Temporal dynamics of the attentional spotlight: Neuronal correlates of attentional capture and inhibition of return in early visual cortex. J. Cogn. Neurosci. 2007, 19, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Mizzi, R.; Michael, G.A. Exploring visual attention functions of the human extrageniculate pathways through behavioral cues. Psychol. Rev. 2016, 123, 740–757. [Google Scholar] [CrossRef] [PubMed]
- Jóhannesson, Ó.I.; Tagu, J.; Kristjánsson, Á. Asymmetries of the visual system and their influence on visual performance and oculomotor dynamics. Eur. J. Neurosci. 2018, 48, 3426–3445. [Google Scholar] [CrossRef]
- Sumner, P.; Nachev, P.; Vora, N.; Husain, M.; Kennard, C. Distinct cortical and collicular mechanisms of inhibition of return revealed with S cone stimuli. Curr. Biol. 2004, 14, 2259–2263. [Google Scholar] [CrossRef]
- Ro, T.; Farnè, A.; Chang, E. Inhibition of return and the human frontal eye fields. Exp. Brain Res. 2003, 150, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Chica, A.B.; Bartolomeo, P.; Valero-Cabré, A. Dorsal and ventral parietal contributions to spatial orienting in the human brain. J. Neurosci. 2011, 31, 8143–8149. [Google Scholar] [CrossRef] [PubMed]
- Van Koningsbruggen, M.G.; Gabay, S.; Sapir, A.; Henik, A.; Rafal, R.D. Hemispheric asymmetry in the remapping and maintenance of visual saliency maps: A TMS study. J. Cogn. Neurosci. 2010, 22, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.; Chica, A.B.; Valero-Cabré, A.; Bartolomeo, P. Cortical control of inhibition of return: Causal evidence for task-dependent modulations by dorsal and ventral parietal regions. Cortex 2013, 49, 2229–2238. [Google Scholar] [CrossRef]
- Bourgeois, A.; Chica, A.B.; Valero-Cabre, A.; Bartolomeo, P. Cortical Control of Inhibition of Return: Exploring the Causal Contributions of the Left Parietal Cortex. Cortex 2013, 49, 2927–2934. [Google Scholar] [CrossRef]
- Gabay, S.; Leibovich, T.; Ben-Simon, A.; Henik, A.; Segev, R. Inhibition of return in the archer fish. Nat. Commun. 2013, 4, 1657. [Google Scholar] [CrossRef]
- Saban, W.; Sekely, L.; Klein, R.M.; Gabay, S. Endogenous orienting in the archer fish. Proc. Nat. Acad. Sci. 2017, 114, 7577–7581. [Google Scholar] [CrossRef] [PubMed]
- Dorris, M.C.; Klein, R.M.; Everling, S.; Munoz, D.P. Contribution of the primate superior colliculus to inhibition of return. J. Cogn. Neurosci. 2002, 14, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Fecteau, J.H.; Munoz, D.P. Correlates of capture of attention and inhibition of return across stages of visual processing. J. Cogn. Neurosci. 2005, 17, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Mirpour, K.; Bisley, J.W. Anticipatory remapping of attentional priority across the entire visual field. J. Neurosci. 2012, 32, 16449–16457. [Google Scholar] [CrossRef] [PubMed]
- Mirpour, K.; Bolandnazar, Z.; Bisley, J.W. Neurons in FEF keep track of items that have been previously fixated in free viewing visual search. J. Neurosci. 2019, 39, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Itti, L.; Koch, C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vis. Res. 2000, 40, 1489–1506. [Google Scholar] [CrossRef]
- Krasovskaya, S.; MacInnes, J. Salience models: A computational cognitive neuroscience review. Vision 2019. In press. [Google Scholar]
- Trappenberg, T.P.; Dorris, M.C.; Munoz, D.P.; Klein, R.M. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. J. Cogn. Neurosci. 2001, 13, 256–271. [Google Scholar] [CrossRef]
- Satel, J.; Wang, Z.; Klein, R.M.; Trappenberg, T.P. Modeling inhibition of return (IOR) as short-term depression of early sensory input to the superior colliculus. Vis. Res. 2011, 51, 987–996. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Satel, J.; Trappenberg, T.P.; Klein, R.M. Behavioral affereffects of a saccade explored in a dynamic neural field model of the superior colliculus. J. Eye Mov. Res. 2011, 4, 1–16. [Google Scholar]
- Lim, A.; Eng, V.; Janssen, S.M.J.; Satel, J. Sensory adaptation and inhibition of return: Dissociating multiple inhibitory cueing effects. Exp. Brain Res. 2018, 236, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.J.; Farrell, S.; Ellis, L.A.; Gilchrist, I.D. The mechanism underlying inhibition of saccadic return. Cogn. Psychol. 2009, 59, 180–202. [Google Scholar] [CrossRef] [PubMed]
- MacInnes, W.J. Multiple diffusion models to compare saccadic and manual responses for inhibition of return. Neural Comput. 2017, 29, 804–824. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.L.; Ratcliff, R. Psychology and neurobiology of simple decisions. Trends Neurosci. 2004, 27, 161–168. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satel, J.; Wilson, N.R.; Klein, R.M. What Neuroscientific Studies Tell Us about Inhibition of Return. Vision 2019, 3, 58. https://doi.org/10.3390/vision3040058
Satel J, Wilson NR, Klein RM. What Neuroscientific Studies Tell Us about Inhibition of Return. Vision. 2019; 3(4):58. https://doi.org/10.3390/vision3040058
Chicago/Turabian StyleSatel, Jason, Nicholas R. Wilson, and Raymond M. Klein. 2019. "What Neuroscientific Studies Tell Us about Inhibition of Return" Vision 3, no. 4: 58. https://doi.org/10.3390/vision3040058
APA StyleSatel, J., Wilson, N. R., & Klein, R. M. (2019). What Neuroscientific Studies Tell Us about Inhibition of Return. Vision, 3(4), 58. https://doi.org/10.3390/vision3040058




