1. Introduction
Attention is a broad term that refers to the allocation of processing resources in space, time and to activities [
1]. The allocation of attention in space, referred to as orienting or spatial attention, can be controlled exogenously—or bottom-up—by external stimuli in the environment and endogenously—or top-down—by our internally generated expectations or intentions [
2]. Regardless of the mode of control, the distribution of our cognitive resources among the many competing stimuli around and within us controls which stimuli we consciously perceive. When attention is allocated effectively, these should be the stimuli most relevant to us at the moment.
Studies using visual stimuli that are near the threshold of perceptibility (i.e., very brief and/or faint) have found that spatial attention increases the likelihood that a stimulus will be consciously perceived (e.g., Chica et al. [
3]), and that this effect is largest when attention is elicited exogenously [
4]. This suggests that reflexive spatial attention, and to a lesser extent voluntary spatial attention, improve the likelihood that visual stimuli will be perceived. The effect of attention on the accuracy of perception is less clear: in the same studies as above, spatial attention was found to have little to no effect on the accuracy of responses for stimuli while controlling for self-reported conscious perception. Conversely, studies using stimuli above the threshold of perception have found that spatial attention influences how many properties of stimuli are perceived, including contrast [
5,
6], colour saturation [
7], spatial frequency [
8,
9], and the size of gaps in line objects [
9]. These findings suggest that attention affects how stimuli are perceived, beyond the likelihood that they are perceived at all.
A powerful method for studying the effects of attention generally, and spatial attention specifically, on conscious perception is to use a continuous response variable. Given that many stimulus properties are continuous (e.g., contrast, rotation, tone) and not binary (e.g., present/not present), it is possible to measure responses in a similarly continuous manner. Paradigms using continuous response variables are sometimes referred to as infinite-alternative forced choice (∞-AFC), as participants are able to make responses at any point across a continuous spectrum. This approach has some important advantages over traditional two-alternative forced choice (2-AFC) paradigms, wherein participants are limited to making one of two responses (e.g., “Which stimulus had higher contrast? (Left/Right)”): first, it allows researchers to measure accuracy as a spectrum instead of a hard binary (i.e., correct or incorrect), providing richer data. Second, it allows us to explore how different factors improve or impair the overall accuracy of perception as opposed to how it shifts our thresholds for comparison judgements.
One of the first perception studies to employ an ∞-AFC paradigm was Prinzmetal et al. [
10], where the authors used several such measures to investigate the effects of dual-task interference on the accuracy of perception. In one experiment, the authors devised a paradigm where coloured targets (small dots) were presented briefly on either the left or right side of the screen while a second task (a 3 × 3 letter array search) divided their attention, and participants were required to indicate the colour of the target on a colour wheel using the computer cursor. The authors used this paradigm to measure the impact of various manipulations of attention on the accuracy of colour responses, finding that presenting the 3 × 3 search array for the interfering task on the opposite side of the screen as the target, increasing the difficulty of the interfering task, and presenting the interfering task and colour target simultaneously (as opposed to sequentially) all had detrimental effects on the accuracy of responses via the colour wheel. These findings, along with the findings of the other experiments in their study, led Prinzmetal et al. [
10] to conclude that accuracy of visual perception was improved by various manipulations of attention.
In the years since Prinzmetal et al. [
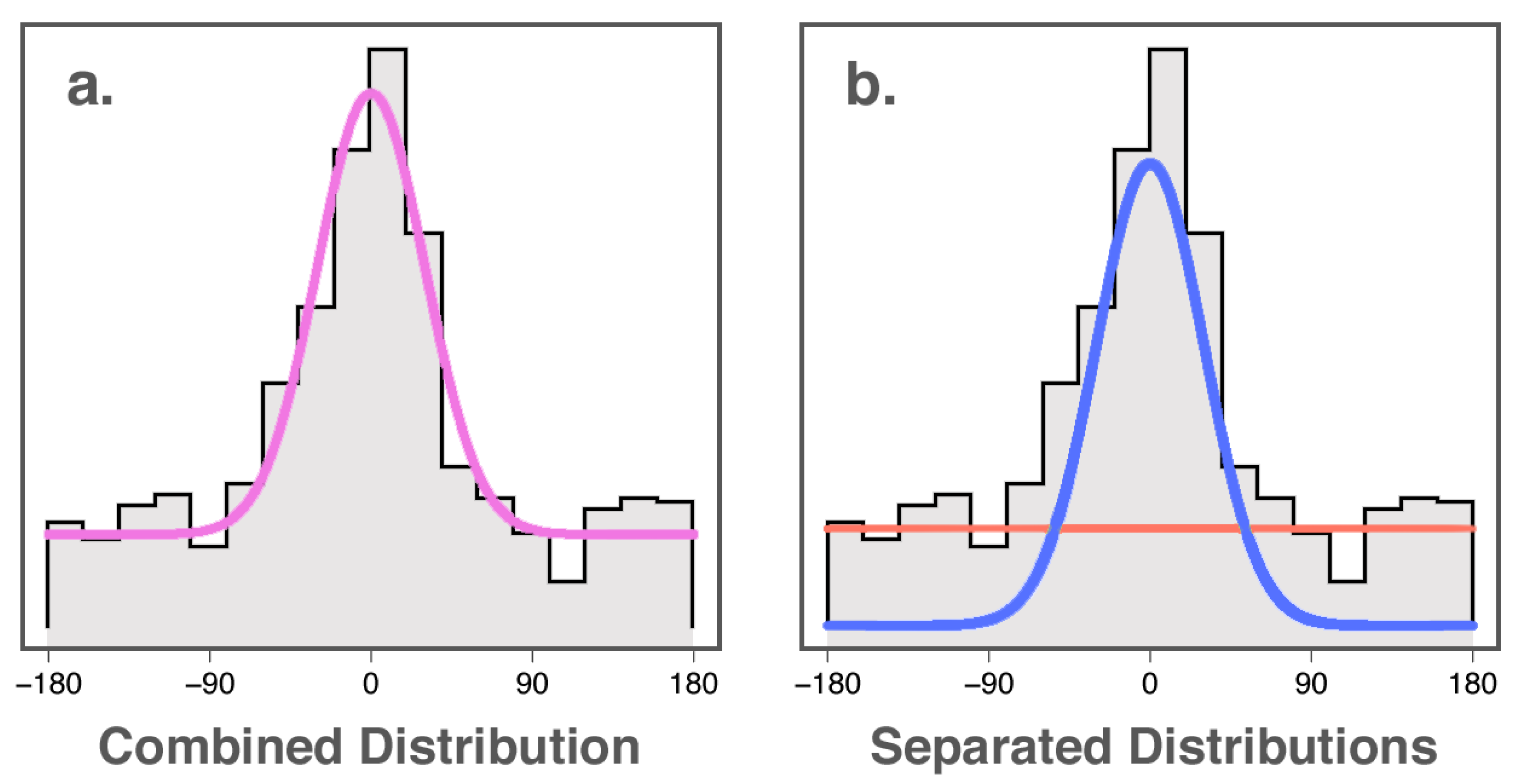
10], new statistical methods have been developed that allow the separation of “accuracy” of colour wheel responses into two sub-components: probability (the likelihood that a stimulus will be perceived), and fidelity (the accuracy with which perceived stimuli are encoded). Interested in the maintenance of colour in working memory, Zhang and Luck [
11] developed a mixture-modelling approach to analysing colour wheel data based on the response distributions that would be expected for remembering and blind guessing. If participants retained no memory of the target colour, the authors reasoned that their responses would be expected to be distributed uniformly along the colour spectrum (red line in
Figure 1b). If they did remember the target colour, however, responses would be expected to follow a von Mises distribution (normal distribution adjusted for a circle) centred around the location on the colour wheel where the colour of the target was drawn from (blue line in
Figure 1b). Thus, the combined distribution of angular error on the colour wheel (
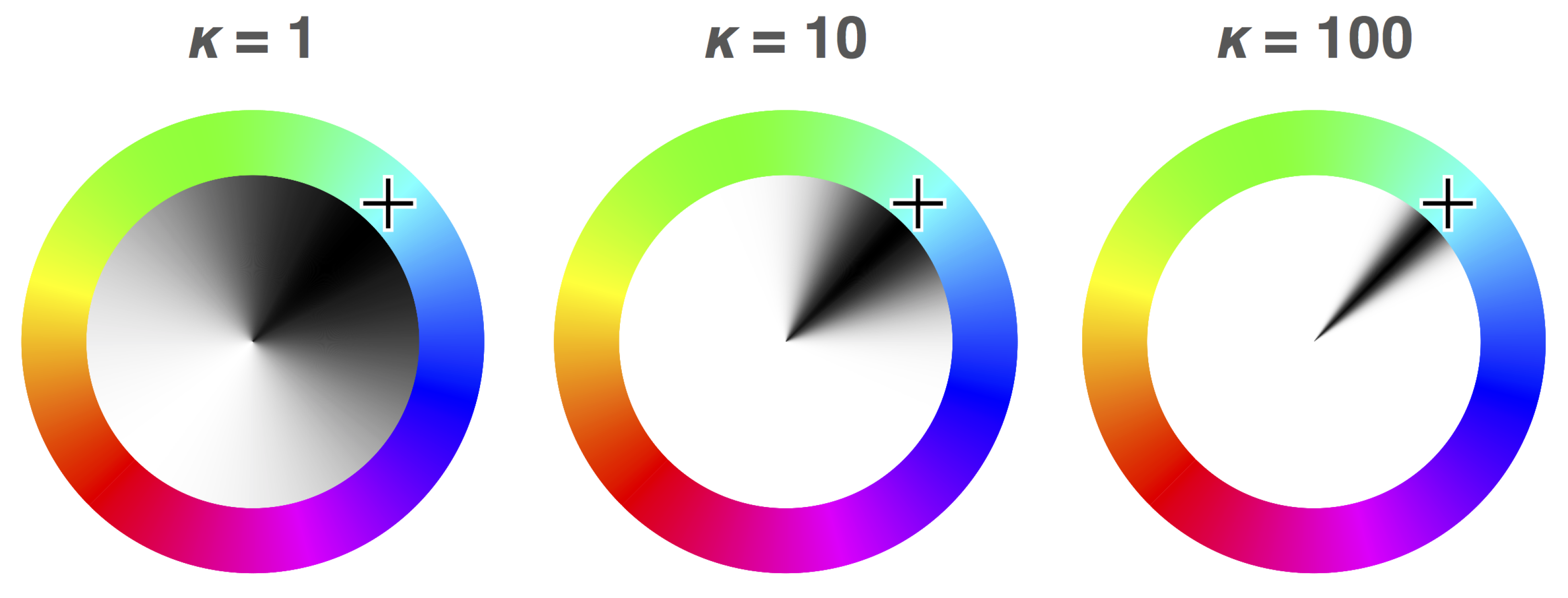
Figure 1a) is able to be modelled as being a mixture of these two distributions in some proportion, giving us two parameters: the proportion of the error best explained by blind guessing, and the concentration parameter of the error distribution for non-guessing responses. The probability of memory is defined in this model as the proportion of non-guessing responses. The fidelity of memory is defined in this model as the concentration parameter
(kappa) of the von Mises distribution of the angular error for non-guessing responses: if responses centred around the true colour have a wide spread, fidelity of memory is inferred to be poor (
Figure 2). Likewise, if the distribution of responses is concentrated around the true colour, fidelity of memory is inferred to be high. This method of analysis is elaborated on in Lawrence [
12].
Despite being developed to isolate the probability and fidelity of encoding in working memory, the method of analysis developed by Zhang and Luck [
11] can likewise be applied to the study of conscious perception. Consider that a colour must first be perceived in order to enter working memory: if factors that affect working memory storage and retrieval (e.g., memory load) are held constant while factors that affect perception (e.g., attention) are manipulated, then any effects of experimental manipulations on the probability or fidelity of responses must therefore be at the perceptual level. Thus, the colour wheel and mixed modelling paradigm can be used to investigate the effects of various types of attention on (a) the likelihood that stimuli will be consciously perceived, and (b) the accuracy with which they are perceived. An important advantage of this method over some used in prior studies is that it does not rely on the accuracy of participants’ meta-awareness of whether they perceived something to determine the probability of perception, instead using statistical inference on the distributions of responses along the colour wheel to determine the proportion of blind guessing.
In recent years, researchers have called the utility of the Zhang and Luck (ZL) model into question in the context of working memory, finding that models accounting for the number of colour stimuli held simultaneously in memory vastly outperform the simple ZL mixture model in terms of model fit [
13,
14]. Despite this, we believe that the ZL model is well-suited for studying the effects of attention on the perception of colour. In terms of model selection, the working memory models that have been found to outperform the ZL mixture model do so by accounting for stimulus set size in various ways [
13], and are thus ill-suited for tasks where only one colour stimulus is presented per trial. Additionally, the ZL model reflects our theoretical expectation that angular error for brief single-colour stimuli will arise from two processes: blind guessing when the colour target is not perceived at all, and imprecision when the colour target is perceived.
To date, little research has been done using the Zhang and Luck [
11] colour wheel method to investigate the effects of spatial attention on conscious perception: to our knowledge, the only two attentional studies to use this analysis have been Redden et al. [
15] and Redden et al. [
16]. These two studies integrated the colour wheel into temporal order judgement (TOJ) tasks as a diagnostic to verify that attention was being biased towards the intended locations in space, and manipulated spatial attention endogenously via between-block instructions that informed participants where colour probes were more likely to appear. Redden et al. [
15] found that endogenous attention improved both the probability and fidelity of colour encoding in participants that used the probe bias instructions to direct their attention. Redden et al. [
16] likewise found that endogenous attention improved the perception of colour targets, but found that the specific effects on fidelity and probability of encoding varied depending on the colour probe duration. To date, no studies using the mixed-modelling colour wheel method have been done exploring the influence of endogenous or exogenous spatial cues on the perception of colour, nor have any studies explored the effects of exogenous attention on the probability and fidelity of conscious perception. Here, in two experiments, we aim to address these gaps in the literature.
In our first experiment, we used alphanumeric characters as endogenous cues in a Posner-like cueing paradigm [
2] to direct attention to the left side, right side, or neither side of the screen, followed by a target at either the left or right location. As such, targets could be presented at the location indicated by the cue (validly cued targets), at the location opposite from that indicated (invalidly cued targets) or following an uninformative cue (neutrally cues targets). Spatial cues were valid on 80% of the trials on which they occurred. The stimulus onset asynchrony between cues and targets (SOA) was 800 ms for all trials in this experiment.
In our second experiment, we used spatially-uninformative bright flashes as exogenous cues in a Posner cueing paradigm otherwise similar to Experiment 1. Past research on exogenous spatial attention has found that spatial cues facilitate responding to targets when cue-target intervals are shorter than 200–300 ms and inhibit responding to targets at longer cue-target intervals. This latter phenomenon is known as “Inhibition of Return” (IOR, see Klein [
17] for a review). To explore how IOR affects the perception of colour, we varied the SOA between 100 ms and 800 ms within blocks, giving us a short SOA for which we would expect valid cues to facilitate processing at the cued location, and a long SOA, for which we would expect them to inhibit processing at the cued location. In both experiments, participants were required to make detection responses to targets as quickly as possible, and subsequently indicate the colours of the targets they had detected as accurately as possible on an RGB colour wheel.
Based on the findings of past research, we hypothesized that, for endogenous cues, participants would have faster reaction times to validly cued targets than invalidly cued ones, and that there would be a facilitatory effect at the cued location on at least one of the two properties of colour encoding (probability and fidelity). For exogenous cues, we predicted faster reaction times to targets at the cued location when the SOA was short (facilitation) and slower reaction times when the SOA was long (inhibition). Given the lack of existing literature on the topic, we did not have any concrete hypotheses about how exogenous attention would affect the probability and fidelity of encoding of colour stimuli.
2. Experiment 1
2.1. Methods
2.1.1. Participants
Participants were recruited from a local undergraduate participant pool and included a total of 81 individuals. All participants gave their informed consent prior to participating, and were compensated via their choice of course credit (one percentage point per hour) or 15$ per hour for their participation. The experiment was conducted in accordance with the Declaration of Helsinki, and ethical approval for the experiment was provided by the Dalhousie University Social Sciences and Humanities Research Ethics Board (file number: R04/05.2011-2496).
Due to variability in the time participants took to complete each trial, 10 participants failed to complete the entire experiment within the allotted time and were consequently removed from analysis. Following exclusions, the data of 71 participants were included in the final analysis (23 male, 7 left-handed, Mage = 20.1, age range: 18–30 years).
2.1.2. Materials
The experiment was coded in the Python programming language using the pyglet library, and run on a Mac Mini computer with a 2 GHz processor running Mac OS X 10.5.7 (Apple Inc., Cupertino, CA). The experiment code, along with animations illustrating the paradigms in action and instructions on how to replicate, can be found on our lab’s GitHub page (
https://github.com/TheKleinLab/colour_wheel_cueing). Stimuli were displayed using a 19-inch CRT monitor at a resolution of 1024 × 768 pixels and a refresh rate of 120 Hz. Responses were collected via USB keyboard and mouse.
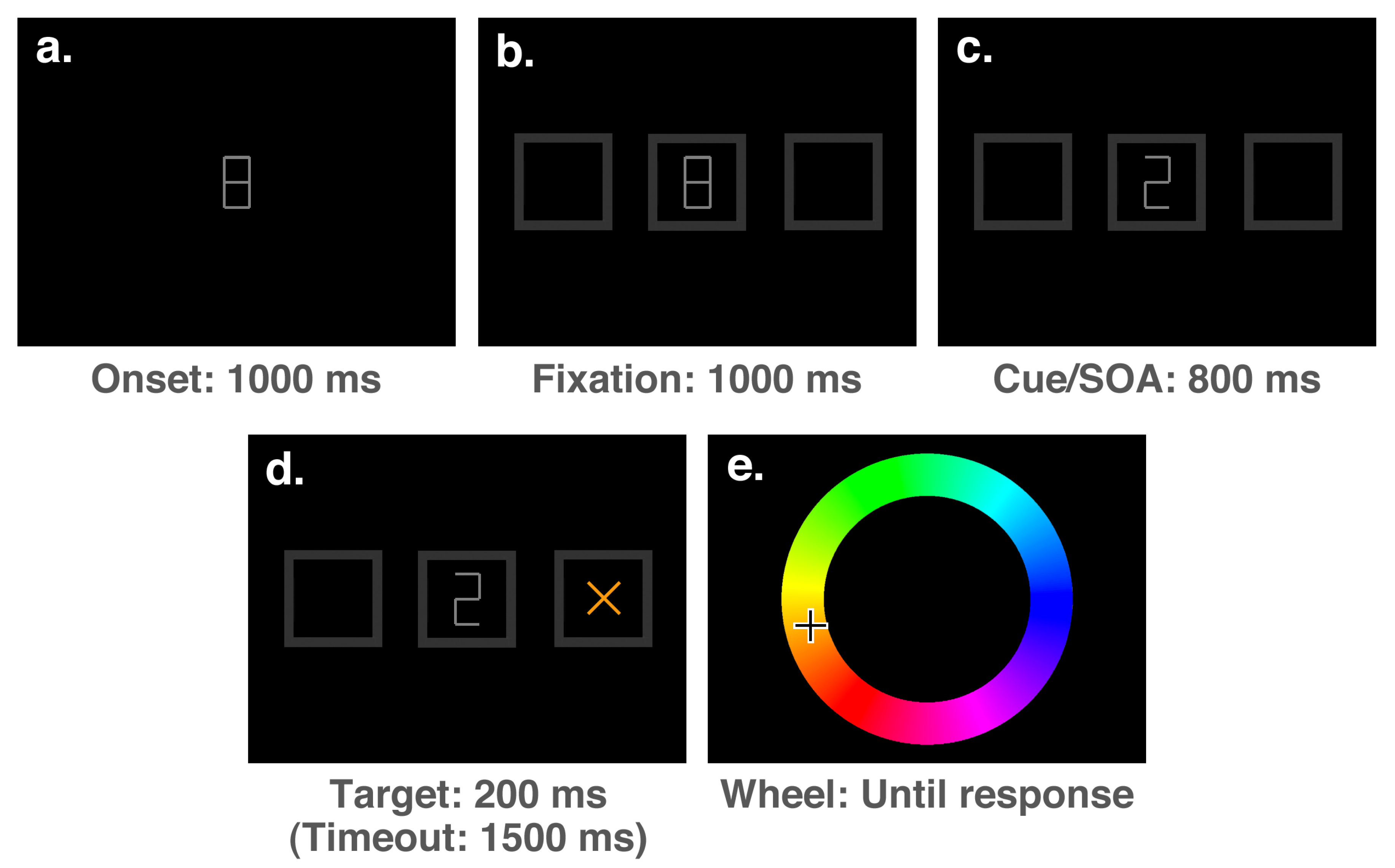
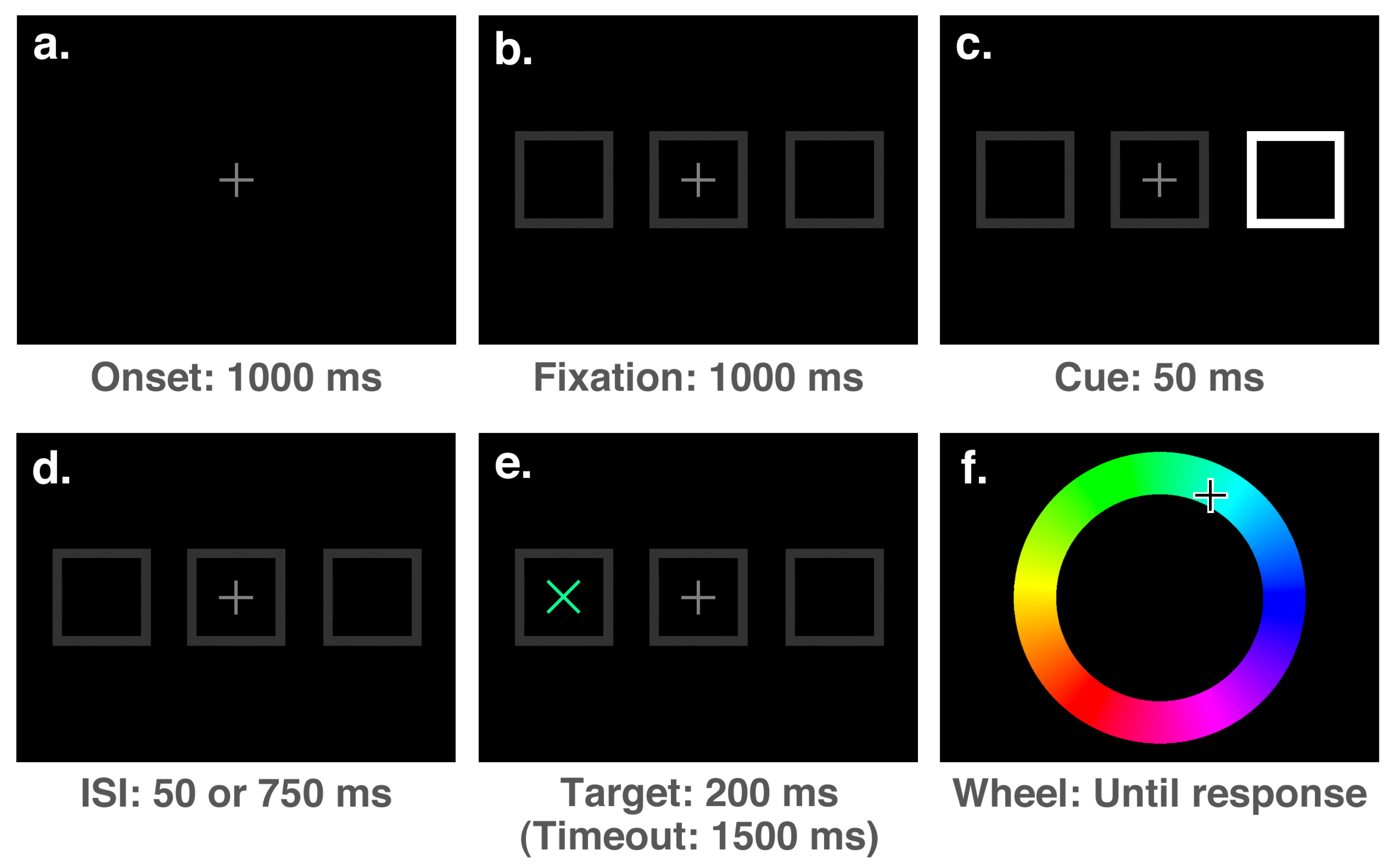
The stimuli presented at each stage of a trial can be seen in
Figure 3. Each trial of the experiment started with the presentation of a medium grey (50% white) fixation stimulus (a number “8” drawn with straight lines, measuring
of visual angle in width and
in height with a line thickness of
) at the centre of the screen for a duration of 1000 ms (see
Figure 3a). The fixation period was followed by the onset of three dark grey (20% white) boxes aligned horizontally (each measuring
and with a line thickness of
): one at the centre surrounding the fixation stimulus, and two peripheral boxes with centres offset from fixation by 7° (
Figure 3b). After an interval of 1000 ms, this display was followed by the onset of a cue, which consisted of the removal of either the upper-left and lower-right lines of the “8”, rendering a “2”, the lower-left and upper-right lines, rendering a “5”, or the top and bottom lines, rendering an “H”. The “2” and “5” stimuli were presented as spatial cues on 5 out of every 6 trials, accurately indicating the target location on 4 out of 5 spatial cue trials (i.e., 80% of the time). On the remaining 1/6th of trials, the “H” stimulus was presented as a neutral cue, providing no spatial prediction. Following the cue onset by 800 ms, a target (an “x”, measuring
with a line thickness of
) with a colour selected randomly from an RGB colour wheel appeared in either the left or right box and remained on-screen for a duration of 200 ms (
Figure 3d). The full colour spectrum from which target colours were selected is illustrated accurately in both
Figure 2 and
Figure 3e. After a speeded detection response to the target was made by pressing the space bar, or a timeout interval of 1500 ms had elapsed since the target onset, the screen was cleared and an RGB colour wheel (an annulus with an outer diameter of
and a thickness of
, with angle of rotation varying randomly between trials) and mouse cursor were presented on the screen (
Figure 3e). The screen was cleared once a location on the colour wheel had been clicked, ending the trial.
On 20% of trials no target was presented, serving as catch trials to discourage anticipatory responding. Thus, 6 (4 valid cues + 1 invalid cue + 1 neutral cue) × 2 (target location) × 5 (20% catch trials) = 60 trials were necessary to complete the design.
2.1.3. Procedure
After participants had read and signed the consent forms for the study, participants were given verbal instructions on how to perform the task. The mapping of numeric cues to locations in space was counterbalanced between participants: half the participants were instructed that the central cue “2” meant that the target would likely appear on the right and “5” indicated it would likely appear on the left, and the other half were told the inverse. All participants were instructed that, if an “H” appeared, the target was equally likely to appear on the left or right.
Participants completed seven test blocks of 60 trials each, for a total of 420 trials. Prior to the test blocks, participants completed a practice block consisting of 30 trials randomly selected from the full 60 of a test block. Participants were allowed to take a break every 30 trials.
2.2. Results
All analysis was performed using version 3.5.1 of the R statistical software [
18], in conjunction with the Stan platform for Bayesian statistical modelling [
19] via RStan 2.18.2. In the interest of reproducible science, the raw data and analysis scripts necessary to reproduce the results and visualisations reported in this manuscript have been made openly available via the Open Science Framework, as described in the
Supplementary Materials.
A hierarchical Bayesian mixed-effects model was used for data analysis, the details of which can be found in
Appendix A. Bayesian statistics is an approach that aims to approximate the probability distributions of one’s parameters of interest. This is in contrast to frequentist statistics, where statistical inference is typically made based on the probability of obtaining one’s current data with repeated observations, assuming that the null hypothesis is true (see Chapter 1 of McElreath [
20] for a more comprehensive explanation). We selected a Bayesian model for our data because we believe it to be a more robust and suitable method of implementing the Zhang and Luck [
11] mixture model analysis because it avoids many of the problems inherent in frequentist statistics, and because we believe the results of a Bayesian model to be more intuitive to interpret than those of traditional statistical tests. We used a hierarchical model because it accounts for between-subject variation and makes the results robust against differing trial counts between participants.
Because the Bayesian approach gives us distributions of probability for our estimates of parameters and effects (known as Bayesian posterior distributions), the way in which we interpret them differs from how we interpret frequentist results. For example, we are not beholden to the black-and-white decision-making of the Neyman–Pearson framework, which requires that we choose the rate at which we want to obtain false positives across our careers (typically 0.05, or 95% confidence), then reject the null hypothesis whenever our p-value is less then our cutoff and accept the null when the p-value is greater than it. Instead, because posterior distributions represent the probability of the parameter of interest being within a given range of values, we can instead talk about the credibility of effects.
In frequentist statistics, confidence intervals counter-intuitively represent the interval that, if calculated for repeated hypothetical samples, would contain the true parameter value a certain percentage of the time (e.g., 95% of the time for a 95% CI). In this study, we will instead discuss our findings in terms of Highest Density Intervals (HDIs), which represent the range of values that we believe contain the true population mean with a certain level of likelihood, given our data. Because a difference between distributions of zero represents the absence of a cueing effect, we will refer to effects as “highly credible” when their 95% HDI does not contain zero, “weakly credible” when their 95% HDI contains zero but their 50% HDI does not, and non-credible when their 50% HDI contains zero.
The probability distributions for the effects of cueing (valid versus invalid) and values of the parameters across cueing conditions are visualised using “violin plots”, which represent the shape of the probability distribution for a given parameter or effect along the x-axis. That is, the width of the shape at any given value on the y-axis represents how likely that value is to be the true population mean. In addition, each “violin” contains a black dot representing the median value, a thin black line representing the range covered by the 95% HDI, and a thicker black line representing the range covered by the 50% HDI.
After excluding participants who had not completed all blocks of the experiment, data was pre-processed prior to modelling with Stan. All practice blocks were dropped from the data, as were all trials on which participants failed to make a detection response to targets (misses, 0.32% of target trials), made detection responses on catch trials where no targets were presented (false alarms, 3.02% of catch trials), or made detection responses on target trials before targets appeared (pre-target responses, 1.22% of target trials). Reaction times, which were right-skewed, were log-transformed to make their distribution approximately normal. For each participant, the median absolute deviation around the median (MADM) for log reaction times for each cue condition was computed, and any trials in that condition with s that deviated from the median by more than five times the MADM were considered outliers and excluded from subsequent analysis. Using this procedure, 2.37% of the remaining trials were excluded.
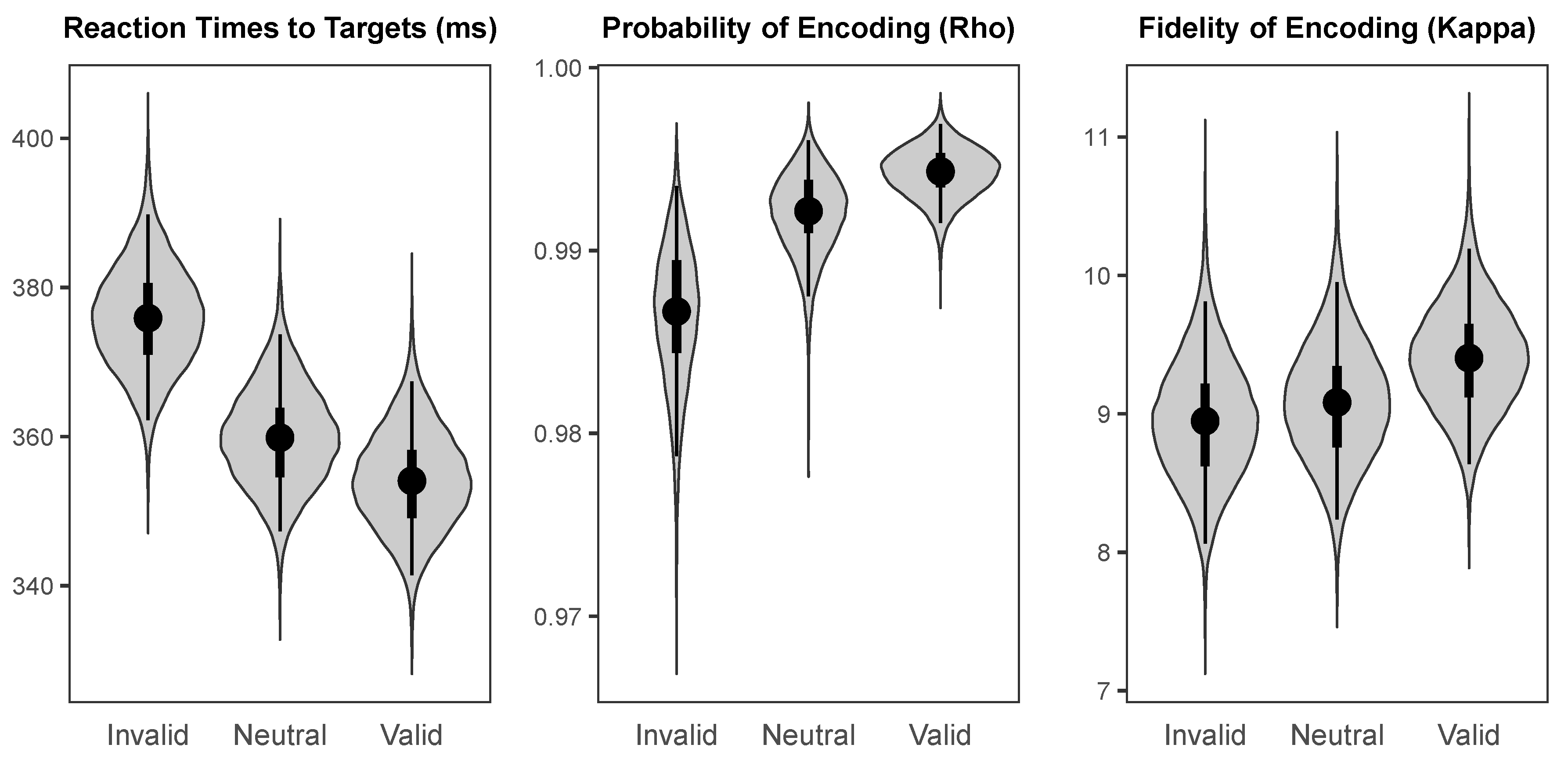
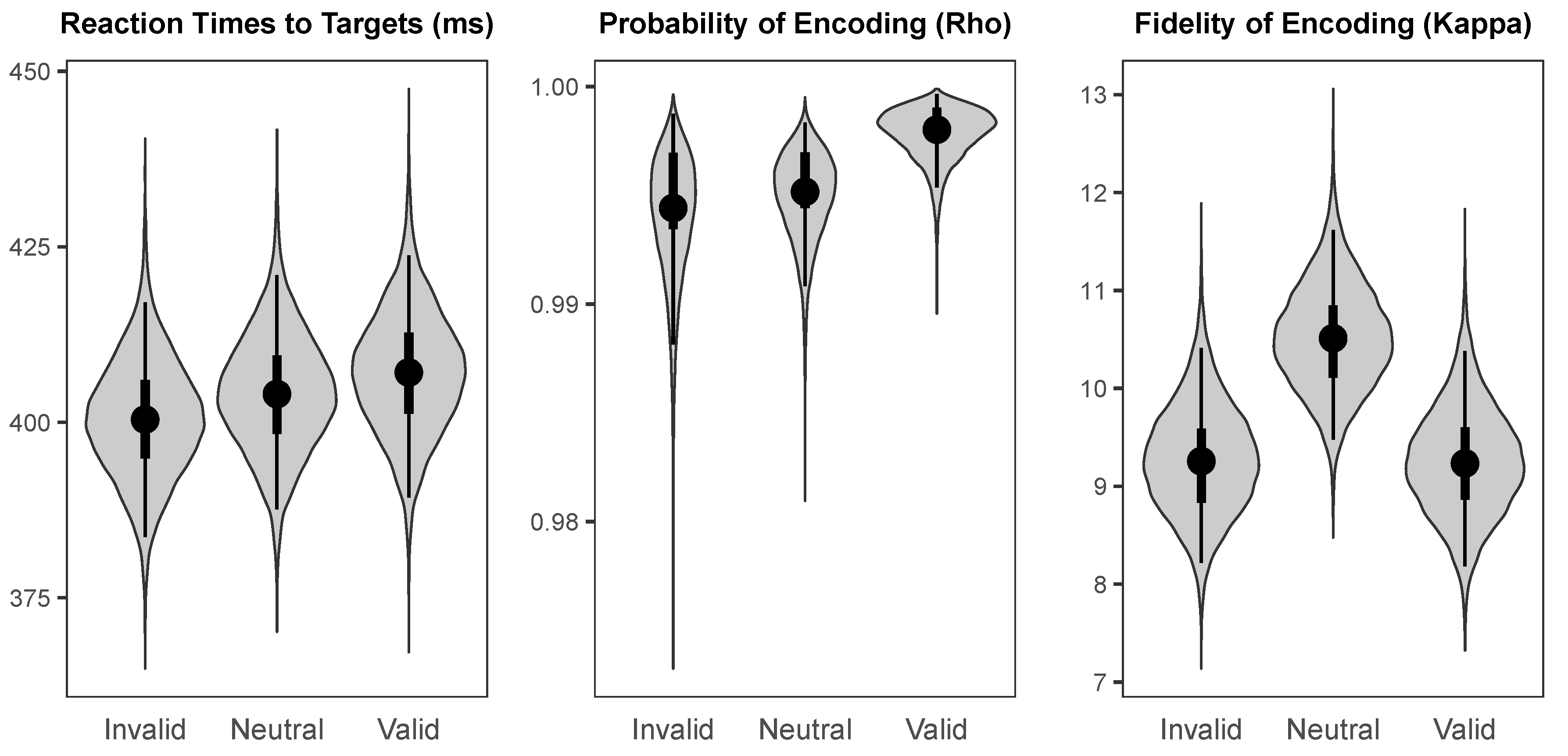
The probability distributions for the parameters of interest in each cueing condition are shown in
Figure 4. The median intercept for reaction times to targets was 359.86 ms (
HDI95% = [347.30 ms, 373.70 ms]). The intercept for probability of colour encoding was near-ceiling (Median = 0.992,
HDI95% = [0.987, 0.996]), and the intercept for fidelity of colour encoding was likewise high (Median = 9.08,
HDI95% = [8.24, 9.95]).
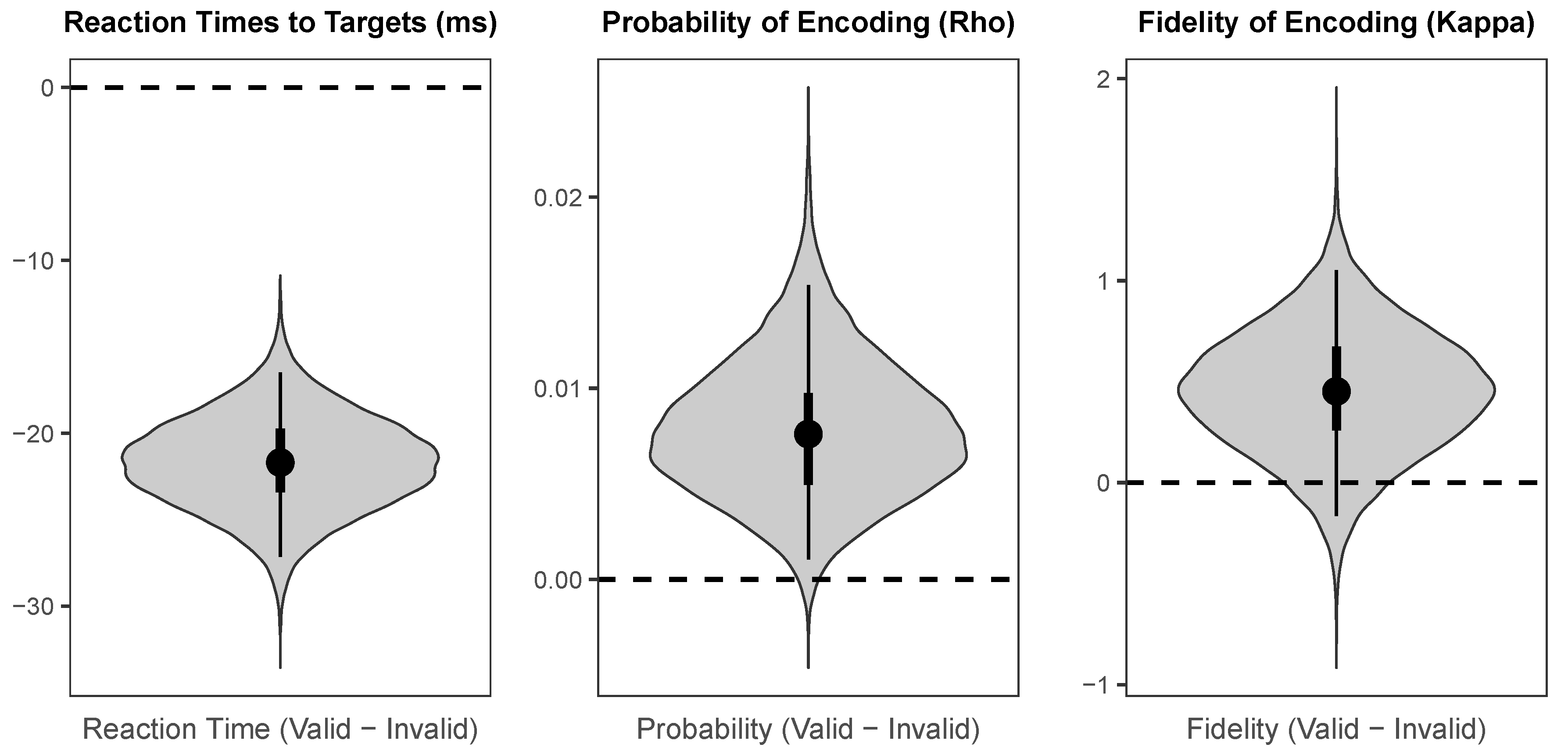
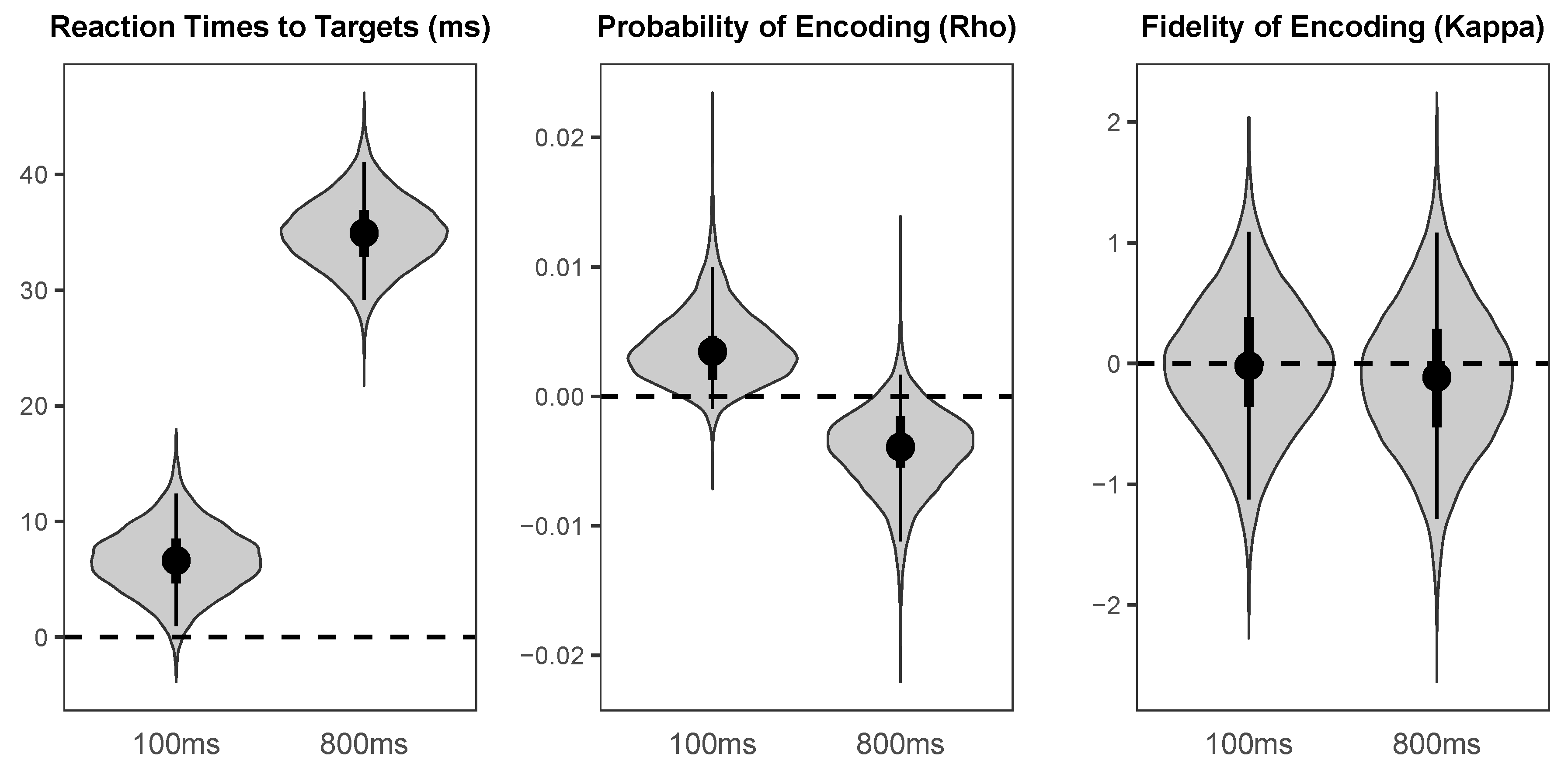
As illustrated in
Figure 5, there was a large credible benefit of cueing on reaction times to targets (Median = −21.70 ms,
HDI95% = [−27.15 ms, −16.47 ms]), a small credible benefit of cueing on probability of colour encoding (Median = 0.008,
HDI95% = [0.001, 0.015]), and a small weakly credible benefit of cue validity of fidelity of colour encoding (Median = 0.45,
HDI95% = [−0.17, 1.05],
HDI50% = [0.26, 0.67]).
2.3. Discussion
In agreement with a substantial literature, the results from the speeded detection responses demonstrate that endogenous spatial attention speeds target detection. Moreover, the mixed-model analysis of the colour wheel response data found that valid endogenous cues credibly improved the probability that target colours were perceived at all, despite the overall probability of colour perception being near–ceiling. Additionally, our model provides some limited evidence that valid endogenous cues improved the fidelity with which colours were encoded. This pattern of results is consistent with the findings of Redden et al. [
15] and Redden et al. [
16], both of which found that biasing attention to one of two target locations in TOJ tasks using between-block instructions improved the probability and/or fidelity of the encoding of colour information. Building on these papers, our study provides the first evidence for the effects of endogenous spatial cueing on the probability and fidelity of colour encoding. These results are consistent with the finding that spatial attention alters the sensitivity of neurons in the V4 region of the visual system [
21,
22], which is the region largely responsible for the perception of colour [
23].
4. General Discussion
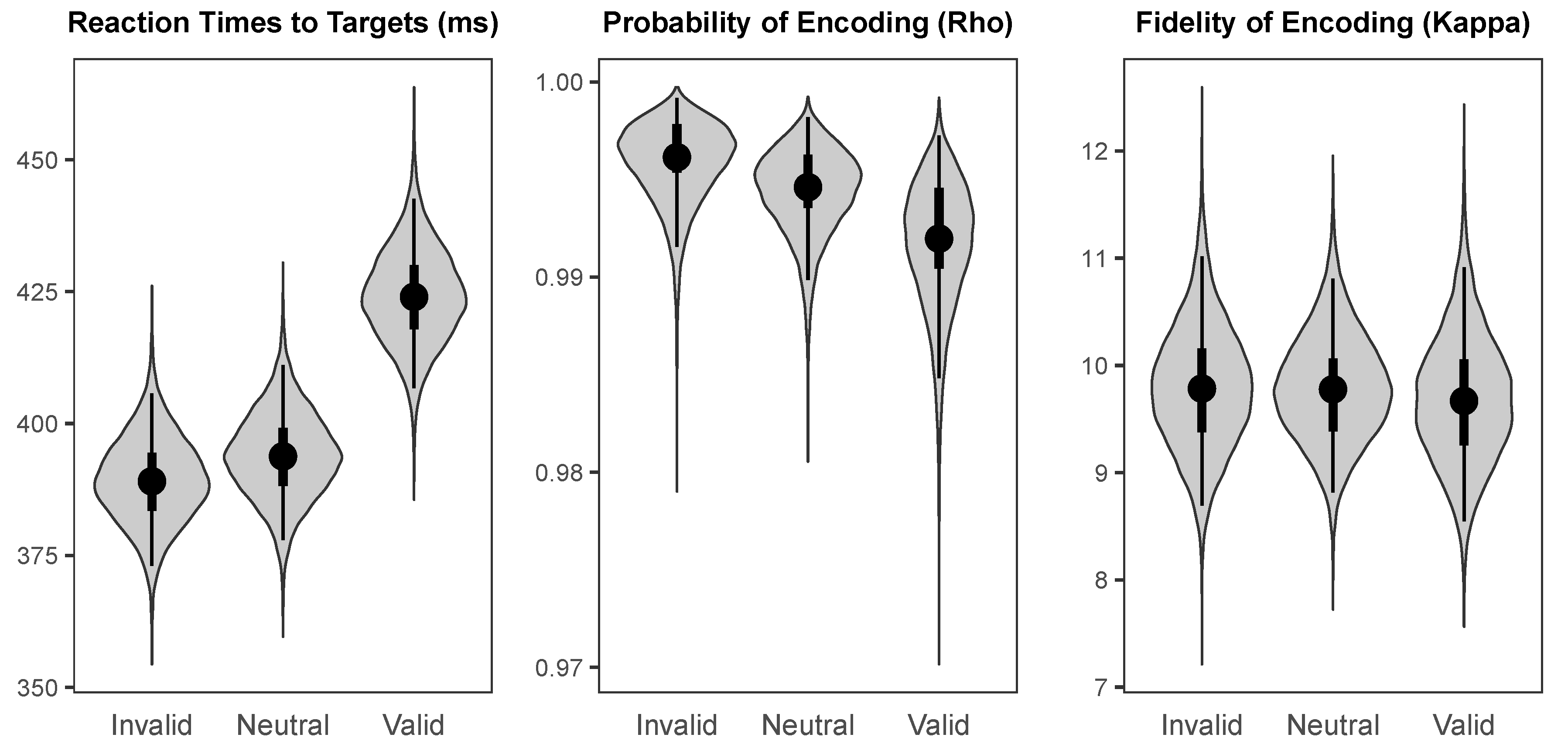
Together, our findings from Experiments 1 and 2 provide preliminary evidence that both endogenous and exogenous cueing influence the accuracy with which colour information is encoded into conscious awareness. Specifically, we found that endogenous spatial cueing improved both the probability and fidelity of colour encoding, whereas exogenous spatial cueing appears to affect probability but not fidelity. Although these effects were small, and additional research is needed to increase our confidence in these findings, our Bayesian models determined our observed effects to be weakly-to-highly credible and thus deserving of consideration. Additionally, our experiments showcase the utility of using continuous response variables in attention research, which can provide rich information for answering questions about how various factors influence the encoding of stimuli.
In the introduction, we noted that if factors affecting working memory storage and retrieval are held constant, then we can attribute the effects of our attentional manipulation to perception. In both of our experiments, however, the interval between target presentation and colour wheel onset varied depending on how quickly participants made a detection response to each target. As pointed out by an anonymous reviewer, this raises the possibility that our observed effects on probability and/or fidelity could be due to memory rather than perception, because slower detection responses in some cue conditions would result in greater opportunity for decay of colour information already encoded into working memory. For a variety of reasons, we believe that this is highly unlikely. Firstly, with a single colour stimulus per trial to hold in memory and brief intervals between targets and responses, decay seems unlikely. Secondly, the appearance of the colour wheel ~400 ms (the speed of the typical detection response) after the target provided the opportunity to make the colour response, but this response was usually made a second or more later. Hence, relative to the overall time between target removal and colour response, the RT differences (10 ms to 35 ms) provide little opportunity for differences in decay. Thirdly, the pattern of results at the 100 ms SOA in E2 demonstrated a dissociation between reaction times, probability, and fidelity, which would not be expected if our probability and fidelity effects were due to differences in colour decay associated with mean reaction times. Finally, the previously mentioned TOJ studies [
15,
16] found effects of attention on the probability and fidelity of colour perception in paradigms that held the target-response interval constant across trials, providing precedent for effects of attention on colour perception unrelated to working memory.
In discussing the study of the effects of attention on conscious experience, Shulman [
28] recommended that researchers use paradigms that allow the isolation of the effects of attention on different perceptual processes in order to better understand the interactions between attention and perception. By allowing researchers to separately measure the effects of attention on the probability and fidelity of colour encoding, the mixed-modeling colour wheel paradigm does exactly this, and provides attention researchers with a powerful, information-rich tool for studying visual perception.
Across both experiments, we found the overall probability of perception to be near-ceiling and the fidelity of perception to be fairly high. Although it is interesting to study the effects of attention on perception in all contexts, a paradigm in which overall perceptual performance was lower might show larger or clearer effects of attentional manipulations on the probability and fidelity of perception. There are a number of possible ways this might be done, including making targets smaller, more brief, or presented at a greater eccentricity from fixation. Additionally, if the effects of an attentional manipulation happen at the locus of decay in working memory (i.e., attention reduces the decay of perceived stimuli), increasing the target-wheel interval to at least 1000 ms might help ensure sufficient decay to detect such effects.