Image Stabilization in Central Vision Loss: The Horizontal Vestibulo-Ocular Reflex
Abstract
1. Introduction
2. Methods
2.1. Participants
2.1.1. AMD Group
2.1.2. Control Group
2.2. Equipment
2.3. Procedure
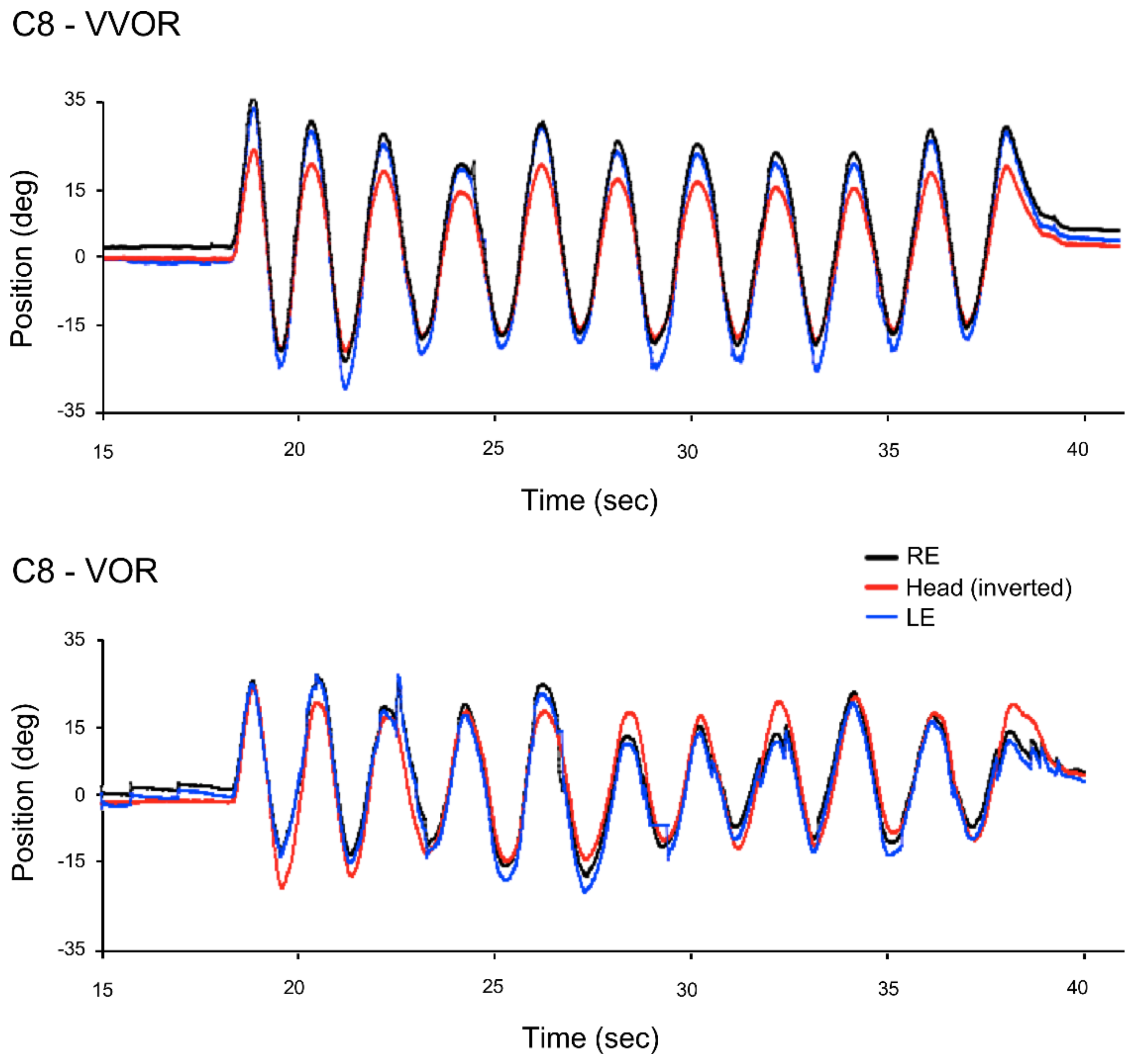
2.4. Data Analysis
2.5. Gain
2.6. Saccades
3. Results
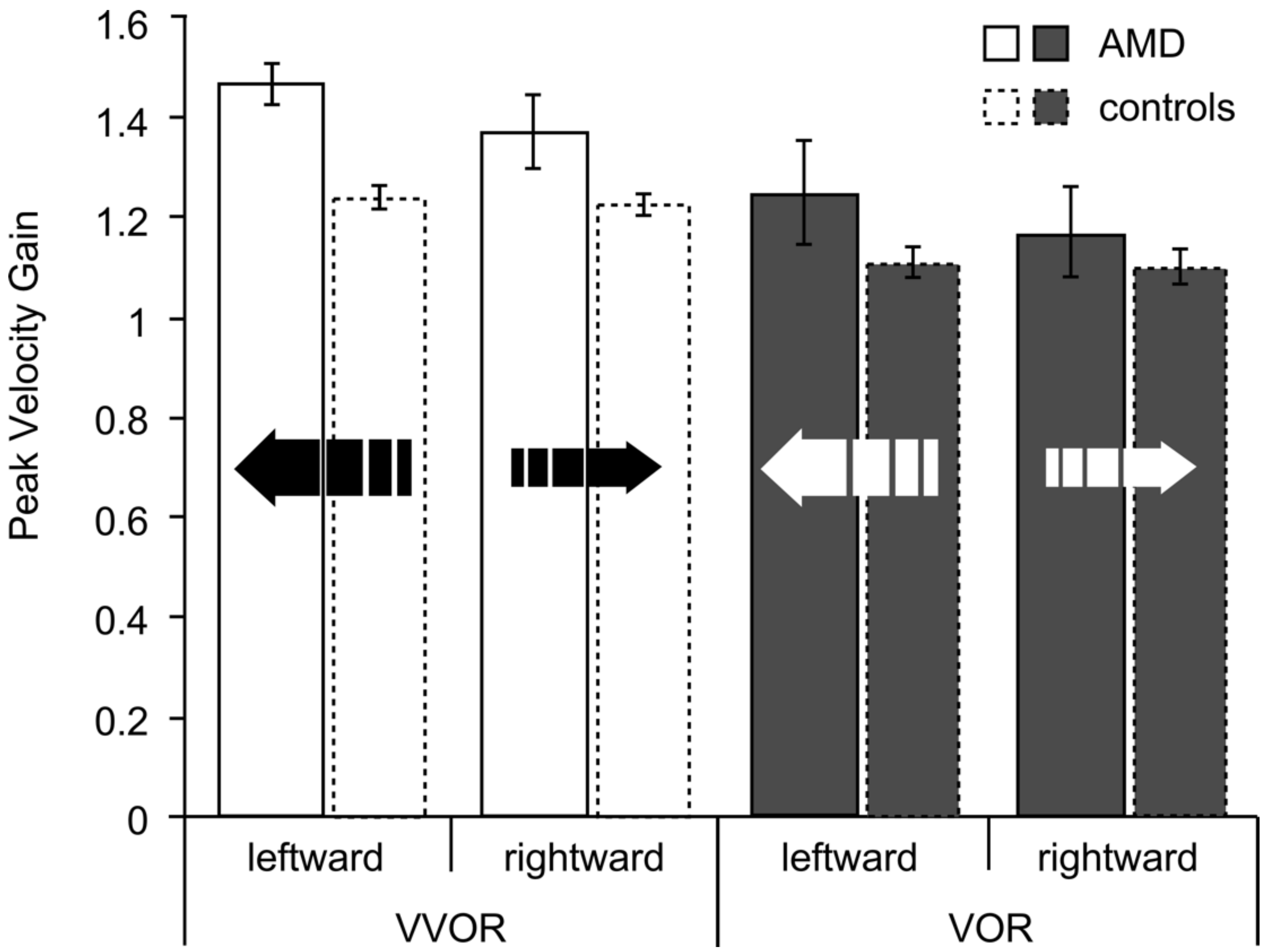
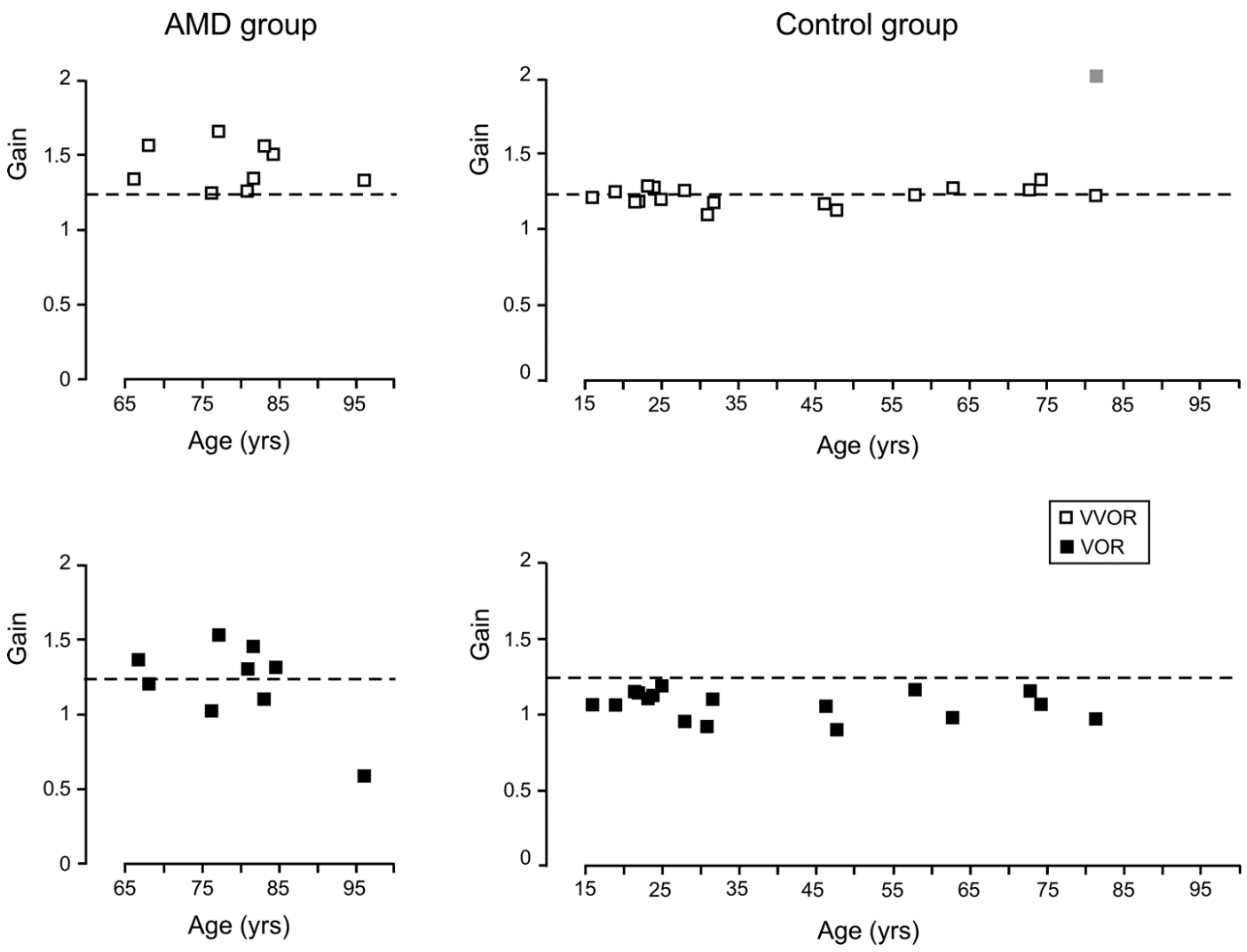
3.1. Gain
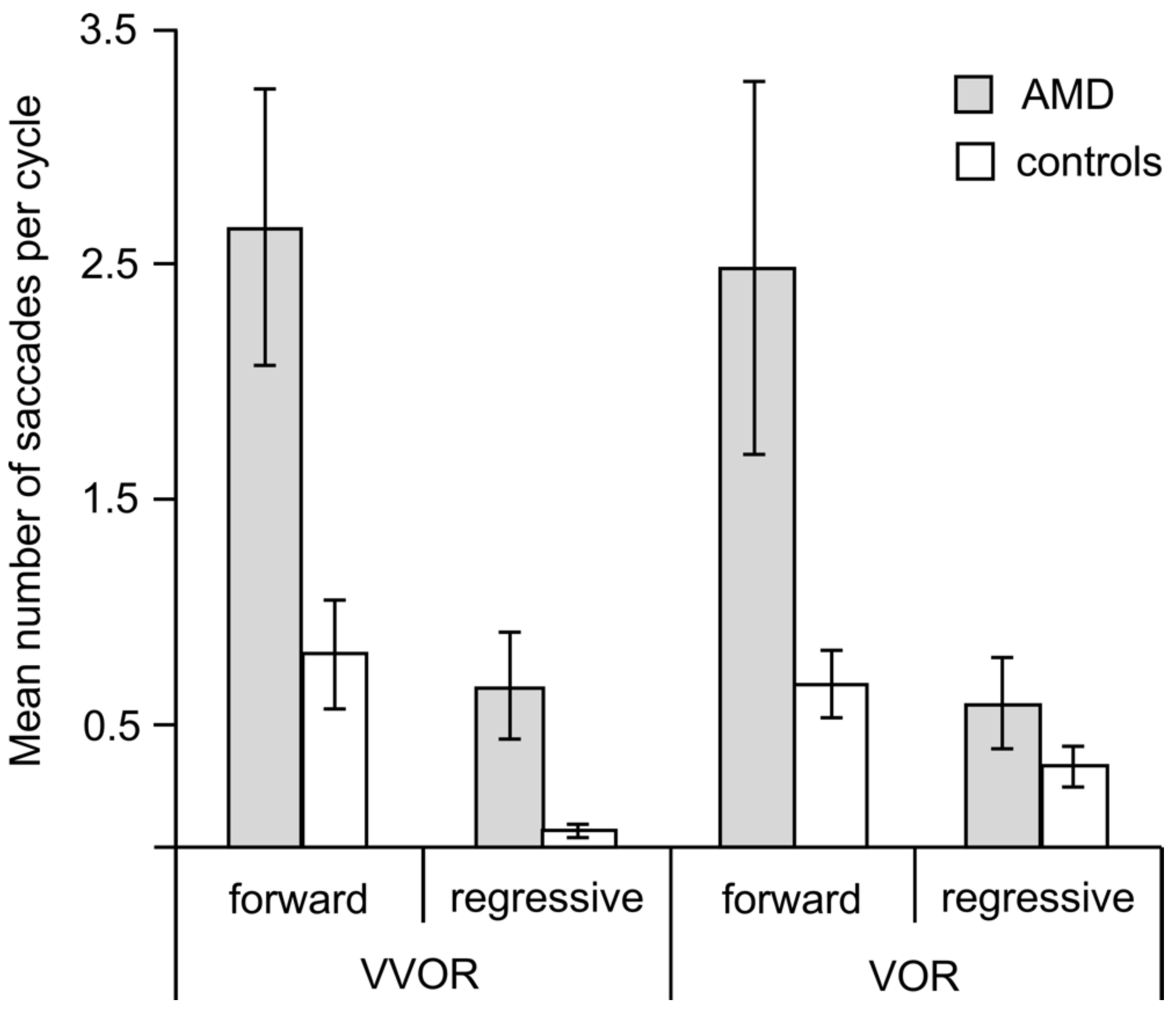
3.2. Saccades
3.3. Acuity and Age
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, 106–116. [Google Scholar] [CrossRef]
- Curcio, C.A.; Medeiros, N.E.; Millican, L.C. Photoreceptor Loss in Age-related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1236–1249. [Google Scholar]
- Fine, S.L.; Berger, J.W.; Maguire, M.G.; Ho, A.C. Age-related macular degeneration. N. Engl. J. Med. 2000, 342, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Lovie-Kitchin, J.; Feigl, B. Assessment of age-related maculopathy using subjective vision tests. Clin. Exp. Optom. 2005, 88, 292–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mones, J.; Rubin, G.S. Contrast sensitivity as an outcome measure in patients with subfoveal choroidal neovascularisation due to age-related macular degeneration. Eye 2004, 19, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Cheng, A.S.; Vingrys, A.J. Color vision deficits in intermediate age-related macular degeneration. Optom. Vis. Sci. 2014, 91, 932–938. [Google Scholar] [CrossRef] [PubMed]
- O’Neill-Biba, M.; Sivaprasad, S.; Rodriguez-Carmona, M.; Wolf, J.E.; Barbur, J.L. Loss of chromatic sensitivity in AMD and diabetes: A comparative study. Ophthalmic Physiol. Opt. 2010, 30, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, P.N.; Robman, L.D.; Varsamidis, M.; Aung, K.Z.; Makeyeva, G.A.; Guymer, R.H.; Vingrys, A.J. Visual function tests as potential biomarkers in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9457–9469. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, P.N.; Robman, L.D.; Varsamidis, M.; Aung, K.Z.; Makeyeva, G.; Busija, L.; Vingrys, A.J.; Guymer, R.H. Relationship between clinical macular changes and retinal function in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5213–5220. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.R.; Clark, M.E.; Scott, I.U.; Walter, L.E.; Quillen, D.A.; Brigell, M.G. Twelve-month natural history of dark adaptation in patients with AMD. Optom. Vis. Sci. 2014, 91, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Wilson, E.; Locke, K.G.; Edwards, A.O. Shape discrimination in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2055–2062. [Google Scholar]
- Seiple, W.; Rosen, R.B.; Garcia, R.M. Abnormal fixation in individuals with age-related macular degeneration when viewing an image of a face. Optom. Vis. Sci. 2013, 90, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Tejeria, L.; Harper, R.A.; Artes, P.H.; Dickinson, C.M. Face recognition in age related macular degeneration: Perceived disability, measured disability, and performance with a bioptic device. Br. J. Ophthalmol. 2002, 86, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Tarita-Nistor, L.; González, E.G.; Markowitz, S.M.; Steinbach, M.J. Binocular function in patients with age-related macular degeneration: A review. Can. J. Ophthalmol. 2006, 41, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Timberlake, G.T.; Mainster, M.A.; Peli, E.; Augliere, R.A.; Essock, E.A.; Arend, L.E. Reading with a macular scotoma. I. Retinal location of scotoma and fixation area. Investig. Ophthalmol. Vis. Sci. 1986, 7, 1137–1147. [Google Scholar]
- Petre, K.L.; Hazel, C.A.; Fine, E.M.; Rubin, G.S. Reading with eccentric fixation is faster in inferior visual field than in left visual field. Optom. Vis. Sci. 2000, 77, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Crossland, M.D.; Engel, S.A.; Legge, G.E. The Preferred Retinal Locus in Macular Disease: Toward a Consensus Definition. Retina 2011, 31, 2109–2114. [Google Scholar] [CrossRef] [PubMed]
- Von Noorden, G.K.; Mackensen, G. Phenomenology of Eccentric Fixation. Am. J. Ophthalmol. 1962, 53, 642–661. [Google Scholar] [CrossRef]
- White, J.M.; Bedell, H.E. The Oculomotor Reference in Humans with Bilateral Macular Disease. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1149–1161. [Google Scholar]
- Crossland, M.D.; Rubin, G.S. The use of an infrared eyetracker to measure fixation stability. Optom. Vis. Sci. 2002, 79, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Tarita-Nistor, L.; González, E.G.; Markowitz, S.M.; Steinbach, M.J. Fixation characteristics of patients with macular degeneration recorded with the MP-1 microperimeter. Retina 2008, 28, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Chung, S.T.L. Characteristics of fixational eye movements in people with macular disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5125–5133. [Google Scholar] [CrossRef] [PubMed]
- Shanidze, N.; Ghahghaei, S.; Verghese, P. Accuracy of Eye Position for Saccades and Smooth Pursuit. J. Vis. 2016, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.G.; Cummings, R.W.; Swieson, L.R. Saccade control without a fovea. Vis. Res. 1991, 12, 2209–2218. [Google Scholar] [CrossRef]
- González, E.; Tarita-Nistor, L.; Mandelcorn, E.; Mandelcorn, M.; Steinbach, M.J. Mechanisms of Image Stabilization in Central Vision Loss: Smooth Pursuit. Optom. Vis. Sci. 2018, 95, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Pidcoe, P.E.; Wetzel, P.A. Oculomotor Tracking Strategy in Normal Subjects with and without Simulated Scotoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 169–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shanidze, N.; Fusco, G.; Potapchuk, E.; Heinen, S.; Verghese, P. Smooth Pursuit Eye Movements in Patients with Macular Degeneration. J. Vis. 2016, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Shanidze, N.; Heinen, S.; Verghese, P. Monocular and binocular smooth pursuit in central field loss. Vis. Res. 2017, 141, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Valmaggia, C.; Charlier, J.; Gottlob, I. Optokinetic Nystagmus in Patients with Central Scotomas in Age Related Macular Degeneration. Br. J. Ophthalmol. 2001, 85, 169–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demer, J.L.; Goldberg, J.; Porter, F.I.; Jenkins, H.A. Sensorimotor adaptation to telescopic spectacles. In Low Vision: Principles and Applications; Woo, G.C., Ed.; Springer: New York, NY, USA, 1987; pp. 216–231. [Google Scholar]
- Demer, J.L.; Goldberg, J.; Porter, F.I.; Schmidr, K. Validation of physiologic predictors of successful telescopic spectacle use in low vision. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2826–2834. [Google Scholar]
- Leigh, R.J.; Zee, D.S. The Neurology of Eye Movements, 5th ed.; Oxford University Press: New York, NY, USA, 2015; pp. 55–168. [Google Scholar]
- Sharpe, J.A. Neural control of ocular motor system. In Walsh and Hoyt’s Clinical Neuroophthalmology; Miller, N.R., Newman, N.J., Eds.; Lippincott, Williams and Wilkins: Baltimore, MD, USA, 1998; Volume 1, pp. 1101–1167. [Google Scholar]
- Kelders, W.P.A.; Kleinrensink, G.J.; van der Geest, J.N.; Feenstra, L.; de Zeeuw, C.I.; Frens, M.A. Compensatory increase of the cervico-ocular reflex with age in healthy humans. J. Physiol. 2003, 553, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Viirre, E.; Tweed, D.; Milner, K.; Vilis, T. A Reexamination of the Gain of the Vestibuloocular Reflex. J. Neurophysiol. 1986, 56, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.A.; Goldberg, H.J.; Lo, A.W.; Herishanu, Y.O. Visual vestibular interaction in multiple sclerosis. Neurology 1981, 31, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.C.; Schultheis, L.W.; Robinson, D.A. Voluntary, non-visual control of the human vestibulo-ocular reflex. Acta Otolaryngol. 1976, 81, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.L.; Sharpe, J.A. The initial vestibulo-ocular reflex and its visual enhancement and cancellation in humans. Exp. Brain Res. 1994, 99, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.A.; Lo, A.W. Voluntary and visual control of the vestibuloocular reflex after cerebral hemidecortication. Ann. Neurol. 1981, 10, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, G.E.; Leigh, R.J.; Bruce, E.N.; Huebner, W.P.; Lanska, D.J. Performance of the human vestibulo-ocular reflex during locomotion. J. Neurophysiol. 1989, 62, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, T.P.; Aalto, H.; Pyykko, I.M.; Juhola, I.M.; Jantt, P. Changes in Vestibulo-ocular Reflex of Elderly People. Acta Otolaryngol. Suppl. 1997, 85, 108–110. [Google Scholar] [CrossRef]
- Ferris, F.L.; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical Classification of Age-related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Chylack, L.T.; Leske, M.C.; McCarthy, D.; Khu, P.; Kashiwagi, T.; Sperduto, R. Lens Opacities Classification System II (LOCS II). Arch. Ophthalmol. 1989, 107, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.; Eizenman, M.; Cheung, B.S.K. Combined head and eye tracking system for dynamic testing of the vestibular system. IEEE Trans. Biomed. Eng. 1996, 43, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- DiScenna, A.O.; Das, V.; Zivotofsky, A.Z.; Seidman, S.H.; Leigh, R.J. Evaluation of a Video Tracking Device for Measurement of Horizontal and Vertical Eye Rotations During Locomotion. J. Neurosci. Methods 1995, 58, 89–94. [Google Scholar] [CrossRef]
- González, E.G.; Teichman, J.; Lillakas, L.; Markowitz, S.N.; Steinbach, M.J. Fixation Stability Using Radial Gratings in Patients with Age-related Macular Degeneration. Can. J. Ophthalmol. 2006, 41, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.C.; Leigh, R.J.; Zee, D.S.; Abel, L.A. The effect of the rotational magnification of corrective spectacles on the quantitative evaluation of the VOR. Acta Otolaryngol. 1985, 100, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Collewijn, H.; Martins, A.J.; Steinman, R.M. Compensatory eye movements during active and passive head movements: Fast adaptation to changes in visual magnification. J. Physiol. 1983, 340, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Hine, T.; Thorn, F. Compensatory eye movements during active head rotation for near targets: Effects of imagination, rapid head oscillation and vergence. Vis. Res. 1987, 27, 1639–1657. [Google Scholar] [CrossRef]
- Preston, K.L.; Finocchio, D.V. Development of the vestibuloocular and optokinetic reflexes. In Early Visual Development, Normal and Abnormal; Simons, K., Ed.; Oxford University Press: New York, NY, USA, 1993; pp. 80–88. [Google Scholar]
- Sherman, K.R.; Keller, E.L. Vestibulo-ocular reflexes of adventitiously and congenitally blind adults. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1159. [Google Scholar]
- Johnston, A.; Wright, M.J. Lower thresholds of motion for gratings as a function of eccentricity and contrast. Vis. Res. 1985, 25, 179–185. [Google Scholar] [CrossRef]
- Tyler, C.W.; Torres, J. Frequency response characteristics for sinusoidal movement in the fovea and periphery. Percept. Psychophys. 1972, 12, 232–236. [Google Scholar] [CrossRef]
- Cheung, S.H.; Legge, G.E. Functional and Cortical Adaptations to Central Vision Loss. Vis. Neurosci. 2005, 22, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Guez, J.E.; Le Gargasson, J.F.; Rigaudiere, F.; O’Regan, J. Is There a Systematic Location for the Pseudo-fovea in Patients with Central Scotoma? Vis. Res. 1993, 33, 1271–1279. [Google Scholar] [CrossRef]
- Tarita-Nistor, L.; Eizenman, E.; Landon-Brace, N.; Markowitz, S.N.; Steinbach, M.J.; González, E.G. Identifying the absolute locations of the PRLs during binocular viewing. Optom. Vis. Sci. 2015, 92, 863–872. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.P.; Davis, L.L.; Kevorkian, K.F. Dynamic analysis of age-related responses of the vestibulo-ocular reflex. Adv. Otorhinolaryngol. 1990, 45, 194–202. [Google Scholar] [PubMed]
- Paige, G.D. Senescence of human visual-vestibular interactions: Smooth pursuit, optokinetic, and vestibular control of eye movements with aging. Exp. Brain Res. 1994, 98, 355–372. [Google Scholar] [CrossRef] [PubMed]


| Acuity (log MAR) | Stage of AMD (Beckman Initiative Scale) | ||||||
|---|---|---|---|---|---|---|---|
| ID | Age (Years) | RE | LE | Stereo (arc s−1) | Time Since Diagnosis (Years) | RE | LE |
| P1 | 67 | 0.34 | 1 | 400 | 2 | late | late |
| P2 | 67 | 0.44 | 0.86 | no stereo | 3 | early | late |
| P3 | 76 | 0.57 | 1.2 | 3600 | 3 | late | intermediate |
| P4 | 77 | 0.24 | 0.7 | 3600 | 1 | late | late |
| P5 | 81 | 0.3 | 0.44 | 400 | 1 | late | late |
| P6 | 81 | 0.7 | 2 | 3600 | 7 | late | late |
| P7 | 82 | 0.4 | 1 | no stereo | 3 | late | late |
| P8 | 83 | 0.98 | 1 | 800 | 4 | late | late |
| P9 | 96 | 0.22 | 0.64 | 400 | 5 | early | late |
| Acuity (log MAR) | |||
|---|---|---|---|
| ID | Age (Years) | RE | LE |
| C1 | 16 | 0.06 | 0.04 |
| C2 | 19 | −0.1 | −0.1 |
| C3 | 23 | −0.26 | −0.28 |
| C4 | 23 | 0 | −0.1 |
| C5 | 23 | 0.06 | 0.1 |
| C6 | 24 | −0.22 | −0.24 |
| C7 | 25 | −0.1 | −0.3 |
| C8 | 28 | −0.1 | −0.1 |
| C9 | 31 | −0.22 | −0.22 |
| C10 | 31 | −0.26 | −0.22 |
| C11 | 46 | −0.24 | −0.24 |
| C12 | 48 | −0.1 | −0.06 |
| C13 | 58 | −0.1 | 0 |
| C14 | 63 | −0.1 | −0.1 |
| C15 | 73 | −0.04 | −0.06 |
| C16 | 74 | 0.08 | 0.04 |
| C17 | 81 | −0.1 | −0.1 |
| PRL Location Relative to Scotoma in Visual Field | PRL Distance to Fovea (deg) | Directional Preponderance of Gain | BCEA (deg2) | ||||
|---|---|---|---|---|---|---|---|
| ID | RE | LE | RE | VVOR | VOR | RE | LE |
| P1 | inferior | left | 2 | left | left | 0.44 | 0.44 |
| P2 | central | inferior | 0 | left | left | 0.16 | 2.80 |
| P3 | inferior | central | 3 | left | left | 1.67 | 0.46 |
| P4 | central | central | 0 | right | left | 1.23 | 2.76 |
| P5 | central | central | 3 | left | left | 0.24 | 0.12 |
| P6 | left | left | 7 | left | left | 2.75 | 2.34 |
| P7 | inferior | left | 3 | right | left | 1.89 | 1.92 |
| P8 | inferior | inferior | 4 | left | left | 1.98 | 3.16 |
| P9 | central | inf/left | 2 | left | right | 0.15 | 0.94 |
| VVOR | VOR | |||
|---|---|---|---|---|
| Group | Rightward | Leftward | Rightward | Leftward |
| AMD | 48.86 (11.25) | 47.95 (9.39) | 50.81 (12.76) | 48.12 (16.75) |
| Control | 58.12 (13.51) | 59.65 (14.69) | 54.54 (15.98) | 55.60 (18.35) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, E.G.; Shi, R.; Tarita-Nistor, L.; Mandelcorn, E.D.; Mandelcorn, M.S.; Steinbach, M.J. Image Stabilization in Central Vision Loss: The Horizontal Vestibulo-Ocular Reflex. Vision 2018, 2, 19. https://doi.org/10.3390/vision2020019
González EG, Shi R, Tarita-Nistor L, Mandelcorn ED, Mandelcorn MS, Steinbach MJ. Image Stabilization in Central Vision Loss: The Horizontal Vestibulo-Ocular Reflex. Vision. 2018; 2(2):19. https://doi.org/10.3390/vision2020019
Chicago/Turabian StyleGonzález, Esther G., Runjie Shi, Luminita Tarita-Nistor, Efrem D. Mandelcorn, Mark S. Mandelcorn, and Martin J. Steinbach. 2018. "Image Stabilization in Central Vision Loss: The Horizontal Vestibulo-Ocular Reflex" Vision 2, no. 2: 19. https://doi.org/10.3390/vision2020019
APA StyleGonzález, E. G., Shi, R., Tarita-Nistor, L., Mandelcorn, E. D., Mandelcorn, M. S., & Steinbach, M. J. (2018). Image Stabilization in Central Vision Loss: The Horizontal Vestibulo-Ocular Reflex. Vision, 2(2), 19. https://doi.org/10.3390/vision2020019





