The Effect of Stimulus Size and Eccentricity on Attention Shift Latencies
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1 Participants
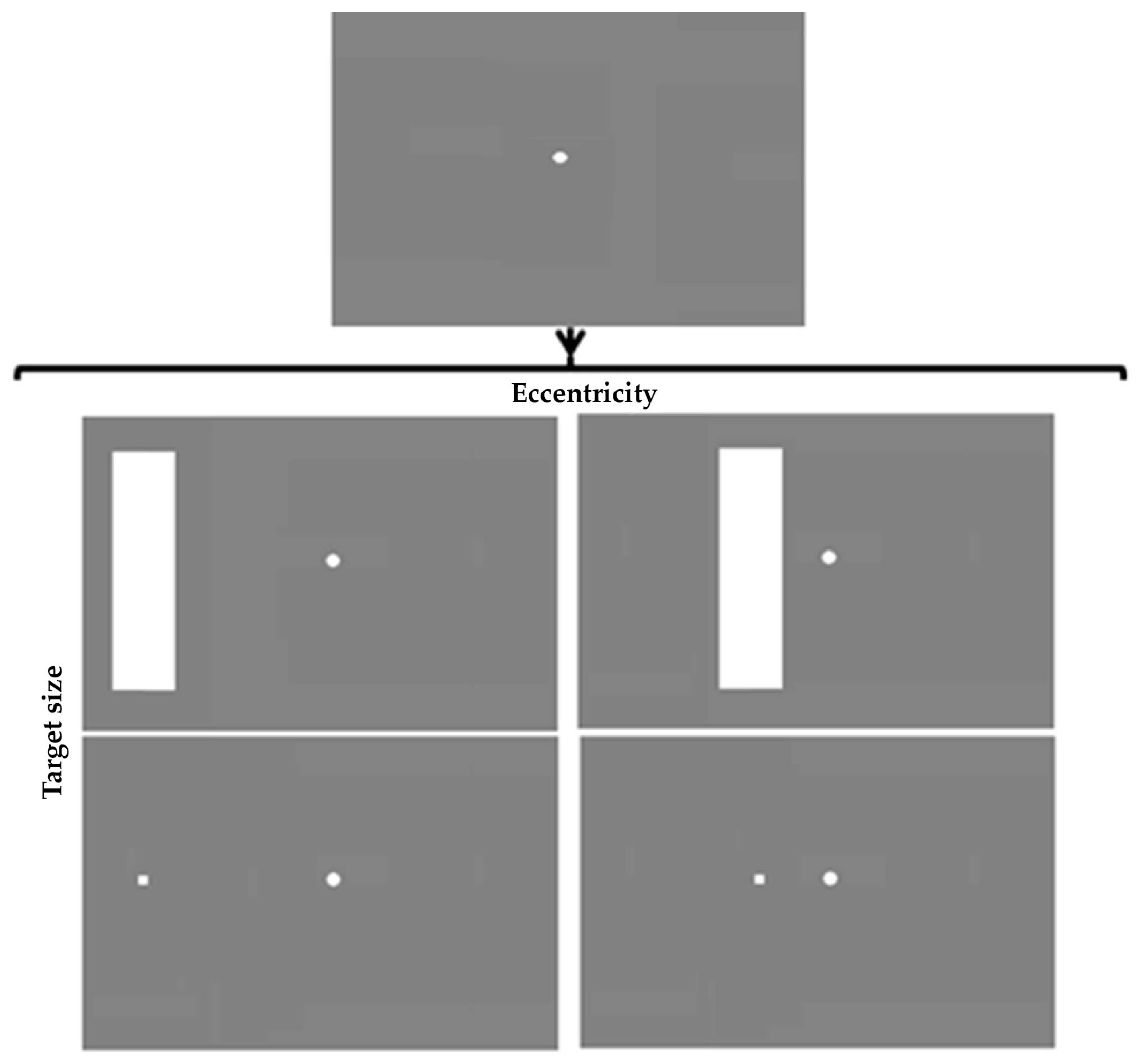
4.2 Design and Stimuli
4.3 Procedure
4.4 Gaze-Contingent Eye-Tracking
4.5 Eye-Tracking Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wass, S.; Smith, T.J.; Johnson, M.H. Parsing eye-tracking data of variable quality to provide accurate fixation duration estimates in infants and adults. Behav. Res. Methods 2012, 45, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gredebäck, G.; Johnson, S.P.; von Hofsten, C. Eye tracking in infancy research. Dev. Neuropsychol. 2009, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Braddick, O.; Anker, S.; Curran, W.; Andrew, R.; Wattam-Bell, J.; Braddick, F. Neurobiological models of visuospatial cognition in children with williams syndrome: Measures of dorsal-stream and frontal function. Dev. Neuropsychol. 2003, 23, 139–172. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Braddick, O.; Anker, S.; Nardini, M.; Birtles, D.; Rutherford, M.A.; Mercuri, E.; Dyet, L.; Edwards, A.D.; Cowan, F.M. Cortical vision, mri and developmental outcome in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F292–F297. [Google Scholar] [CrossRef] [PubMed]
- Elsabbagh, M.; Volein, A.; Holmboe, K.; Tucker, L.; Csibra, G.; Baron-Cohen, S.; Bolton, P.; Charman, T.; Baird, G.; Johnson, M.H. Visual orienting in the early broader autism phenotype: Disengagement and facilitation. J. Child Psychol. Psychiatry 2009, 50, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Gliga, T.; Jones, E.J.; Bedford, R.; Charman, T.; Johnson, M.H. From early markers to neuro-developmental mechanisms of autism. Dev. Rev. 2014, 34, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Atkinson, J.; Braddick, O.; Anker, S.; Cowan, F.; Rutherford, M.; Pennock, J.; Dubowitz, L. Visual function in full-term infants with hypoxic-ischaemic encephalopathy. Neuropediatrics 1997, 28, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Haataja, L.; Guzzetta, A.; Anker, S.; Cowan, F.; Rutherford, M.; Andrew, R.; Braddick, O.; Cioni, G.; Dubowitz, L. Visual function in term infants with hypoxic-ischaemic insults: Correlation with neurodevelopment at 2 years of age. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 80, F99–F104. [Google Scholar] [CrossRef] [PubMed]
- Braddick, O.; Atkinson, J.; Hood, B.; Harkness, W.; Jackson, G.; Vargha-Khademt, F. Possible blindsight in infants lacking one cerebral hemisphere. Nature 1992, 360, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Hood, B.; Braddick, O.; Wattam-Bell, J. Infants control of fixation shifts with single and competing targets-mechanisms of shifting attention. Perception 1988, 17, 367–368. [Google Scholar]
- Atkinson, J.; Hood, B.; Wattam-Bell, J.; Braddick, O. Changes in infants’ ability to switch visual attention in the first three months of life. Perception 1992, 21, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Hood, B.; Atkinson, J. Disengaging visual attention in the infant and adult. Infant Behav. Dev. 1993, 16, 405–422. [Google Scholar] [CrossRef]
- Atkinson, J.; Braddick, O. Visual attention in the first years: Typical development and developmental disorders. Dev. Med. Child Neurol. 2012, 54, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Butcher, P.R.; Kalverboer, A.F.; Geuze, R.H. Infants’ shifts of gaze from a central to a peripheral stimulus: A longitudinal study of development between 6 and 26 weeks. Infant Behav. Dev. 2000, 23, 3–21. [Google Scholar] [CrossRef]
- Kulke, L.; Atkinson, J.; Braddick, O. Automatic detection of attention shifts in infancy: Eye tracking in the fixation shift paradigm. PLoS ONE 2015, 10, e0142505. [Google Scholar] [CrossRef] [PubMed]
- Kulke, L.; Atkinson, J.; Braddick, O. Neural mechanisms of attention become more specialised during infancy: Insights from combined eye tracking and EEG. Dev. Psychobiol. 2017, 59, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Kulke, L.; Atkinson, J.; Braddick, O. Neural differences between covert and overt attention studied using eeg with simultaneous remote eye tracking. Front. Hum. Neurosci. 2016, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Farroni, T.; Simion, F.; Umiltà, C.; Barba, B.D. The gap effect in newborns. Dev. Sci. 1999, 2, 174–186. [Google Scholar] [CrossRef]
- Matsuzawa, M.; Shimojo, S. Infants’ fast saccades in the gap paradigm and development of visual attention. Infant Behav. Dev. 1997, 20, 449–455. [Google Scholar] [CrossRef]
- Colombo, J. The development of visual attention in infancy. Annu. Rev. Psychol. 2001, 52, 337–367. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; Posner, M.I.; Rothbart, M.K. Components of visual orienting in early infancy: Contingency learning, anticipatory looking, and disengaging. J. Cogn. Neurosci. 1991, 3, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Elsabbagh, M.; Fernandes, J.; Jane Webb, S.; Dawson, G.; Charman, T.; Johnson, M.H. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol. Psychiatry 2013, 74, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Braddick, O. Early development of the control of visual attention. Perception 1985, 14, A25. [Google Scholar]
- Hunnius, S.; Geuze, R.H. Gaze shifting in infancy: A longitudinal study using dynamic faces and abstract stimuli. Infant Behav. Dev. 2004, 27, 397–416. [Google Scholar] [CrossRef]
- Aslin, R.N.; Salapatek, P. Saccadic localization of visual targets by the very young human infant. Percept. Psychophys. 1975, 17, 293–302. [Google Scholar] [CrossRef]
- Kulke, L. Cortical Mechanisms of Visual Attention in Typically Developing Infants and Adults; University College London: London, UK, 2015. [Google Scholar]
- Csibra, G.; Johnson, M.H.; Tucker, L.A. Attention and oculomotor control: A high-density erp study of the gap effect. Neuropsychologia 1997, 35, 855–865. [Google Scholar] [CrossRef]
- Kulke, L.; Wattam-Bell, J. Combining event-related potentials and eye-tracking to assess the effect of attention on cortical response. Perception 2013, 42, 219. [Google Scholar]
- Tripathy, S.P.; Cavanagh, P. The extent of crowding in peripheral vision does not scale with target size. Vis. Res. 2002, 42, 2357–2369. [Google Scholar] [CrossRef]
- Johnson, C.A.; Keltner, J.L.; Balestrery, F. Effects of target size and eccentricity on visual detection and resolution. Vis. Res. 1978, 18, 1217–1222. [Google Scholar] [CrossRef]
- Bles, M.; Schwarzbach, J.; De Weerd, P.; Goebel, R.; Jansma, B.M. Receptive field size-dependent attention effects in simultaneously presented stimulus displays. Neuroimage 2006, 30, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.E.; Ross, L.E. Saccade latency in children and adults: Effects of warning interval and target eccentricity. J. Exp. Child Psychol. 1977, 23, 539–549. [Google Scholar] [CrossRef]
- Hodgson, T.L. The location marker effect. Exp. Brain Res. 2002, 145, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.; Ostendorf, F.; Kraft, A.; Ploner, C.J. Saccades to spatially extended targets: The role of eccentricity. Neuroreport 2004, 15, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.C.; Hoyt, W.F. The representation of the visual field in human striate cortex: A revision of the classic holmes map. Arch. Ophthalmol. 1991, 109, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H.; Wiesel, T.N. Uniformity of monkey striate cortex: A parallel relationship between field size, scatter, and magnification factor. J. Comp. Neurol. 1974, 158, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.H.; Tehovnik, E.J. Neural mechanisms underlying target selection with saccadic eye movements. Prog. Brain Res. 2005, 149, 157–171. [Google Scholar] [PubMed]
- Collins, C.E.; Lyon, D.C.; Kaas, J.H. Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 285, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Neggers, S.; Raemaekers, M.; Lampmann, E.; Postma, A.; Ramsey, N. Cortical and subcortical contributions to saccade latency in the human brain. Eur. J. Neurosci. 2005, 21, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Heinze, H.J.; Mangun, G.R.; Burchert, W.; Hinrichs, H.; Scholz, M.; Munte, T.F.; Gos, A.; Scherg, M.; Johannes, S.; Hundeshagen, H.; et al. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature 1994, 372, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, S.A.; Anllo-Vento, L. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. USA 1998, 95, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.A.; Wurtz, R.H. What the brain stem tells the frontal cortex. Ii. Role of the sc-md-fef pathway in corollary discharge. J. Neurophysiol. 2004, 91, 1403–1423. [Google Scholar] [CrossRef] [PubMed]
- Guitton, D.; Buchtel, H.A.; Douglas, R. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp. Brain Res. 1985, 58, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Pierrot-Deseilligny, C.; Rivaud, S.; Gaymard, B.; Agid, Y. Cortical control of reflexive visually-guided saccades. Brain 1991, 114, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Rafal, R.D.; Machado, L.; Ro, T.; Ingle, H. Looking forward to looking: Saccade preparation and control of the visual grasp reflex. In Attention & Performance XVIII; Monsell, S., Driver, J., Eds.; MIT Press: Cambridge, MA, USA, 2000; Volume XVIII, pp. 155–174. [Google Scholar]
- Miller, E.K. The neural basis of top-down control of visual attention in the prefrontal cortex. In Control of Cognitive Processes: Attention and Performance; Monsell, S., Driver, J., Eds.; MIT Press: Cambridge, MA, USA, 2000; Volume XVIII, p. 511. [Google Scholar]
- Henik, A.; Rafal, R.D.; Rhodes, D. Endogenously generated and visually guided saccades after lesions of the human frontal eye fields. J. Cogn. Neurosci. 1994, 6, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Peelen, M.V.; Heslenfeld, D.J.; Theeuwes, J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage 2004, 22, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Shipp, S. The brain circuitry of attention. Trends Cogn. Sci. 2004, 8, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kimchi, R. Perceptual organization and visual attention. Progress Brain Res. 2009, 176, 15–33. [Google Scholar]
- Morey, R.D.; Rouder, J.N. Bayesfactor: Computation of bayes factors for common designs, version 0.9.12-2; Available online: https://cran.r-project.org/web/packages/BayesFactor/index.html (accessed on 7 December 2017).
- R Development Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Liang, F.; Paulo, R.; Molina, G.; Clyde, M.A.; Berger, J.O. Mixtures of g priors for bayesian variable selection. J. Am. Stat. Assoc. 2012, 103, 410–423. [Google Scholar] [CrossRef]
- Hanes, D.P.; Wurtz, R.H. Interaction of the frontal eye field and superior colliculus for saccade generation. J. Neurophysiol. 2001, 85, 804–815. [Google Scholar] [PubMed]
- Crowne, D.P. The frontal eye field and attention. Psychol. Bull. 1983, 93, 232. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M. Eye movement control during visual object processing: Effects of initial fixation position and semantic constraint. Can. J. Exp. Psychol. 1993, 47, 79. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.W.; Kowler, E.; Sharma, A.; Chubb, C. Saccadic localization of random dot targets. Vis. Res. 1998, 38, 895–909. [Google Scholar] [CrossRef]
- Ploner, C.J.; Ostendorf, F.; Dick, S. Target size modulates saccadic eye movements in humans. Behav. Neurosci. 2004, 118, 237. [Google Scholar] [CrossRef] [PubMed]
- Wässle, H.; Grünert, U.; Röhrenbeck, J.; Boycott, B.B. Cortical magnification factor and the ganglion cell density of the primate retina. Nature 1989, 341, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Virsu, V.; Näsänen, R.; Osmoviita, K. Cortical magnification and peripheral vision. J. Opt. Soc. Am. A 1987, 4, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Cowey, A. Cortical maps and visual perception the grindley memorial lecture. Q. J. Exp. Psychol. 1979, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yeshurun, Y.; Carrasco, M. Attention improves or impairs visual performance by enhancing spatial resolution. Nature 1998, 396, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.; Atienza, M.; Gomez, G.; Vazquez, M. Response latencies and event-related potentials during the gap paradigm using saccadic responses in human subjects. Int. J. Psychophysiol. 1996, 23, 91–99. [Google Scholar] [CrossRef]
| 12.9° Eccentricity | 5° Eccentricity | ||
|---|---|---|---|
| 0.33° target | Mean | 275 | 272 |
| SD | 37.9 | 52.1 | |
| 3.1° × 13.2° target | Mean | 261 | 290 |
| SD | 45.1 | 51.1 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulke, L. The Effect of Stimulus Size and Eccentricity on Attention Shift Latencies. Vision 2017, 1, 25. https://doi.org/10.3390/vision1040025
Kulke L. The Effect of Stimulus Size and Eccentricity on Attention Shift Latencies. Vision. 2017; 1(4):25. https://doi.org/10.3390/vision1040025
Chicago/Turabian StyleKulke, Louisa. 2017. "The Effect of Stimulus Size and Eccentricity on Attention Shift Latencies" Vision 1, no. 4: 25. https://doi.org/10.3390/vision1040025
APA StyleKulke, L. (2017). The Effect of Stimulus Size and Eccentricity on Attention Shift Latencies. Vision, 1(4), 25. https://doi.org/10.3390/vision1040025





