Locomotion and Postural Control in Young Adults with Autism Spectrum Disorders: A Novel Kinesiological Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Experimental Procedure
2.2. sEMG Activity
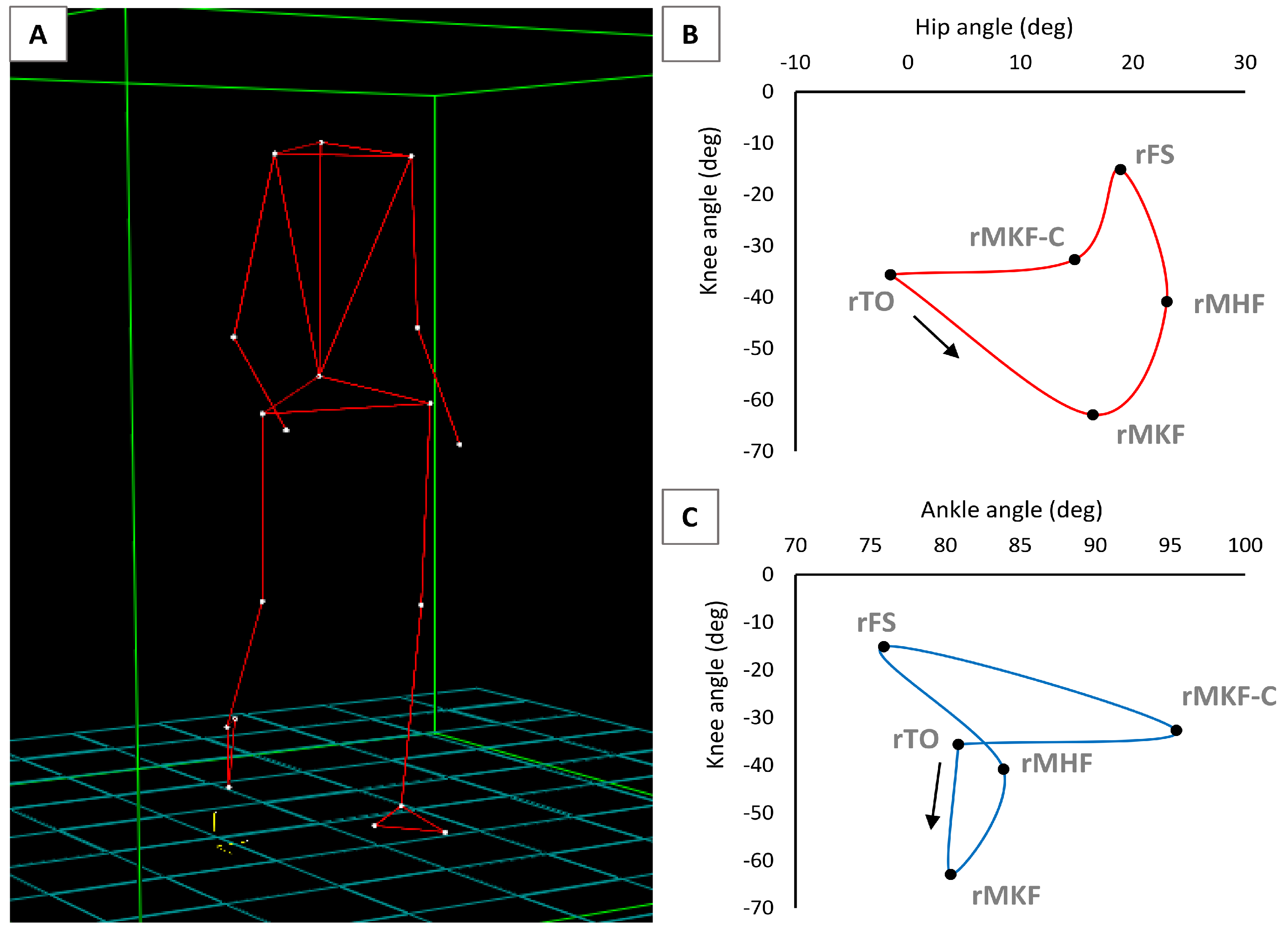
2.3. Gait Analysis and Synchronised sEMG
2.4. Postural Control and Synchronised sEMG
2.5. Statistical Analysis
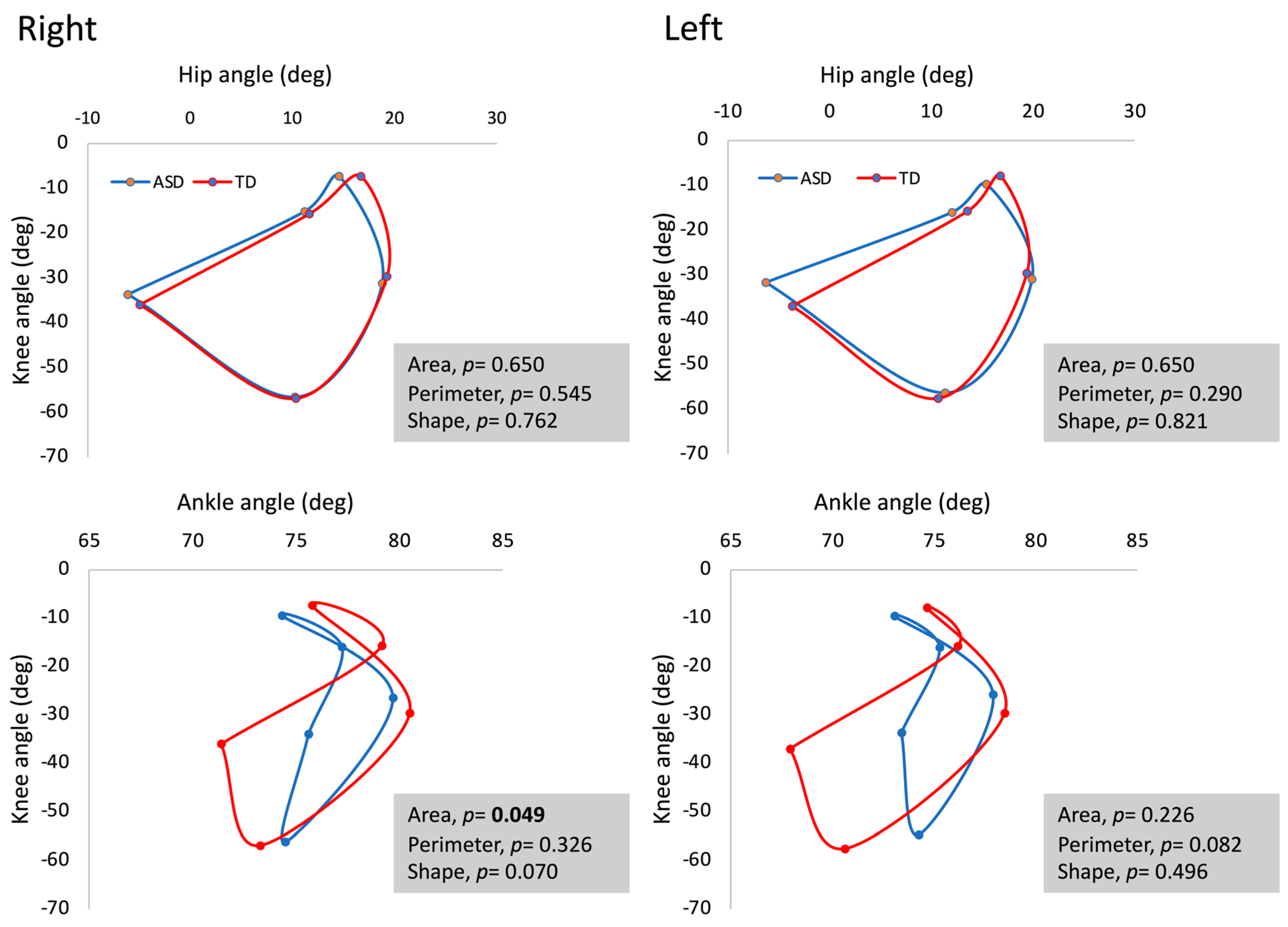
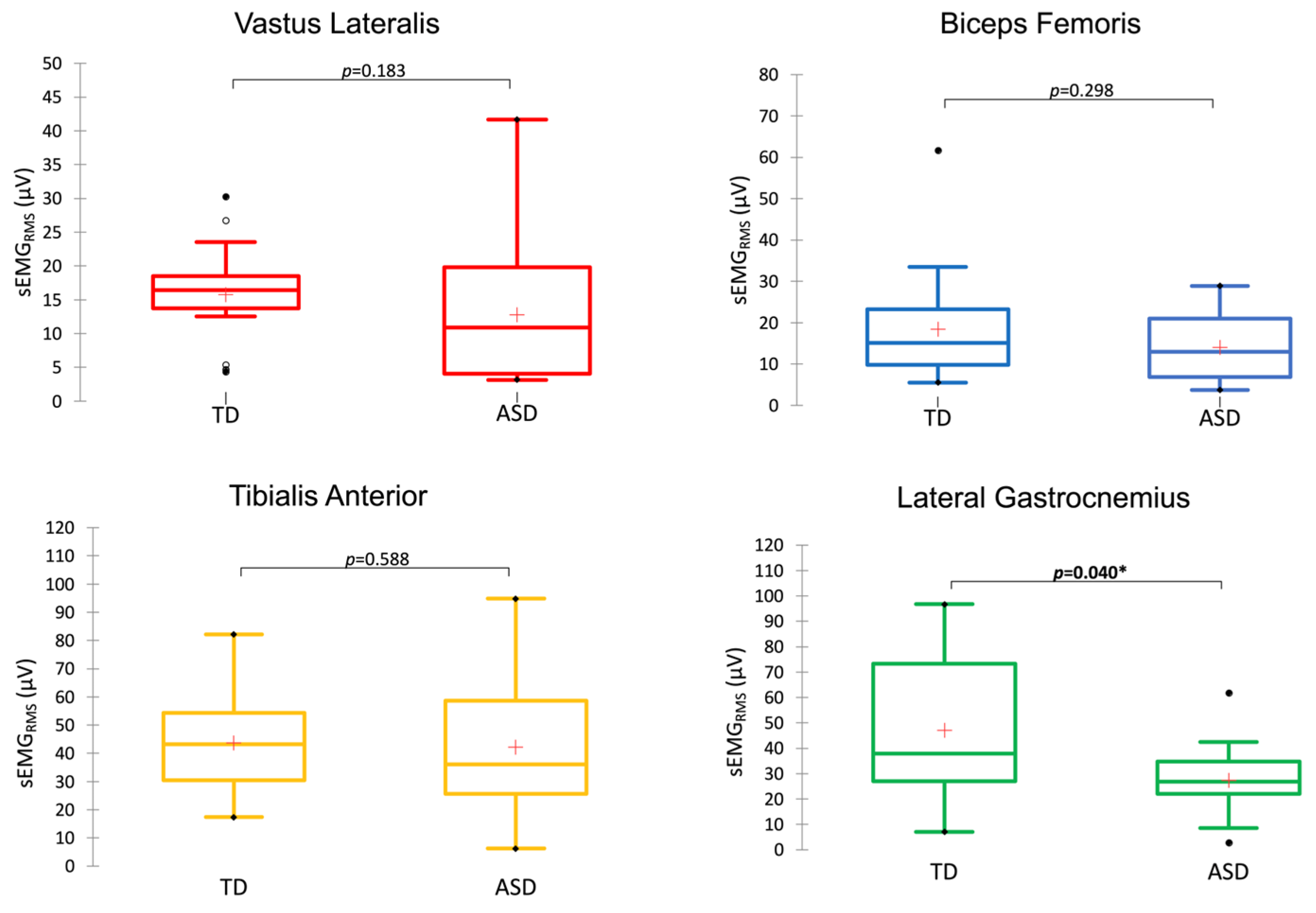
3. Results
4. Discussion
4.1. Walking at Standardised Speed
4.2. Postural Control
4.3. Mechanical and Neuromuscular Interactions
4.4. Predictive Ability of the Measured Variables
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global Prevalence of Autism: A Systematic Review Update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, M.W.; Wang, Z.; Schmitt, L.M.; Tsai, P.; Sweeney, J.A. The Role of Cerebellar Circuitry Alterations in the Pathophysiology of Autism Spectrum Disorders. Front. Neurosci. 2015, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Thye, M.D.; Bednarz, H.M.; Herringshaw, A.J.; Sartin, E.B.; Kana, R.K. The Impact of Atypical Sensory Processing on Social Impairments in Autism Spectrum Disorder. Dev. Cogn. Neurosci. 2018, 29, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Minshew, N.J.; Williams, D.L. The New Neurobiology of Autism: Cortex, Connectivity, and Neuronal Organization. Arch. Neurol. 2007, 64, 945. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.J.; Gerig, G.; Lewis, J.D.; Soda, T.; Styner, M.A.; Vachet, C.; Botteron, K.N.; Elison, J.T.; Dager, S.R.; Estes, A.M.; et al. Altered Corpus Callosum Morphology Associated with Autism over the First 2 Years of Life. Brain 2015, 138, 2046–2058. [Google Scholar] [CrossRef]
- Glickstein, M.; Doron, K. Cerebellum: Connections and Functions. Cerebellum 2008, 7, 589–594. [Google Scholar] [CrossRef]
- Barbeau, E.B.; Meilleur, A.S.; Zeffiro, T.A.; Mottron, L. Comparing Motor Skills in Autism Spectrum Individuals with and without Speech Delay. Autism Res. 2015, 8, 682–693. [Google Scholar] [CrossRef]
- Schmahmann, J. The Cerebellar Cognitive Affective Syndrome. Brain 1998, 121, 561–579. [Google Scholar] [CrossRef]
- Weiss, M.J.; Moran, M.F.; Parker, M.E.; Foley, J.T. Gait Analysis of Teenagers and Young Adults Diagnosed with Autism and Severe Verbal Communication Disorders. Front. Integr. Neurosci. 2013, 7, 33. [Google Scholar] [CrossRef]
- O’Hearn, K.; Lynn, A. Age Differences and Brain Maturation Provide Insight into Heterogeneous Results in Autism Spectrum Disorder. Front. Hum. Neurosci. 2023, 16, 957375. [Google Scholar] [CrossRef]
- Fournier, K.A.; Hass, C.J.; Naik, S.K.; Lodha, N.; Cauraugh, J.H. Motor Coordination in Autism Spectrum Disorders: A Synthesis and Meta-Analysis. J. Autism Dev. Disord. 2010, 40, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Pauk, J.; Zawadzka, N.; Wasilewska, A.; Godlewski, P. Gait Deviations in Children with Classic High-Functioning Autism and Low-Functioning Autism. J. Mech. Med. Biol. 2017, 17, 1750042. [Google Scholar] [CrossRef]
- Wilson, R.B.; Elashoff, D.; Gouelle, A.; Smith, B.A.; Wilson, A.M.; Dickinson, A.; Safari, T.; Hyde, C.; Jeste, S.S. Quantitative Gait Analysis in Duplication 15q Syndrome and Nonsyndromic ASD. Autism Res. 2020, 13, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.J.; Ringleb, S.I.; Bobzien, J.; Haegele, J.A. Walking Lower Extremity Biomechanics of Adolescents with Autism Spectrum Disorder. J. Biomech. 2021, 119, 110332. [Google Scholar] [CrossRef] [PubMed]
- Vernazza-Martin, S.; Martin, N.; Vernazza, A.; Lepellec-Muller, A.; Rufo, M.; Massion, J.; Assaiante, C. Goal Directed Locomotion and Balance Control in Autistic Children. J. Autism Dev. Disord. 2005, 35, 91–102. [Google Scholar] [CrossRef]
- Sigman, M. What are the core deficits in autism? In Atypical Cognitive Deficits in Developmental Disorders: Implications for Brain Function; Lawrence Erlbaum Associates, Inc.: Mahwah, NJ, USA, 1994; pp. 139–157. ISBN 978-1-138-96412-9. [Google Scholar]
- Bojanek, E.K.; Wang, Z.; White, S.P.; Mosconi, M.W. Postural Control Processes during Standing and Step Initiation in Autism Spectrum Disorder. J. Neurodev. Disord. 2020, 12, 1. [Google Scholar] [CrossRef]
- Travers, B.G.; Powell, P.S.; Klinger, L.G.; Klinger, M.R. Motor Difficulties in Autism Spectrum Disorder: Linking Symptom Severity and Postural Stability. J. Autism Dev. Disord. 2013, 43, 1568–1583. [Google Scholar] [CrossRef]
- Kern, J.K.; Geier, D.A.; Adams, J.B.; Troutman, M.R.; Davis, G.; King, P.G.; Young, J.L.; Geier, M.R. Autism Severity and Muscle Strength: A Correlation Analysis. Res. Autism Spectr. Disord. 2011, 5, 1011–1015. [Google Scholar] [CrossRef]
- Doumas, M.; McKenna, R.; Murphy, B. Postural Control Deficits in Autism Spectrum Disorder: The Role of Sensory Integration. J. Autism Dev. Disord. 2016, 46, 853–861. [Google Scholar] [CrossRef]
- Greffou, S.; Bertone, A.; Hahler, E.-M.; Hanssens, J.-M.; Mottron, L.; Faubert, J. Postural Hypo-Reactivity in Autism is Contingent on Development and Visual Environment: A Fully Immersive Virtual Reality Study. J. Autism Dev. Disord. 2012, 42, 961–970. [Google Scholar] [CrossRef]
- Calhoun, M.; Longworth, M.; Chester, V.L. Gait Patterns in Children with Autism. Clin. Biomech. 2011, 26, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Lebiedowska, M.K.; Thomas, S.L.; Stanhope, S.J.; Denckla, M.B.; Rumsey, J. Locomotion of Autistic Adults. Arch. Neurol. 1993, 50, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Chester, V.L.; Calhoun, M. Gait Symmetry in Children with Autism. Autism Res. Treat. 2012, 2012, 576478. [Google Scholar] [CrossRef] [PubMed]
- Buac, M.; Ibarra, G.; Torres, R.; Onal, S.; Gladfelter, A.; Wang, Z. The Urgent Need for Neuroscience Research to Consider Culture When Assessing the Development of Gait in Autistic Children: A Scoping Review. J. Integr. Neurosci. 2023, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Stins, J.F.; Emck, C. Balance Performance in Autism: A Brief Overview. Front. Psychol. 2018, 9, 901. [Google Scholar] [CrossRef]
- Valagussa, G.; Trentin, L.; Signori, A.; Grossi, E. Toe Walking Assessment in Autism Spectrum Disorder Subjects: A Systematic Review. Autism Res. 2018, 11, 1404–1415. [Google Scholar] [CrossRef]
- Armitano, C.N.; Bennett, H.J.; Haegele, J.A.; Morrison, S. Assessment of the Gait-Related Acceleration Patterns in Adults with Autism Spectrum Disorder. Gait Posture 2020, 75, 155–162. [Google Scholar] [CrossRef]
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Wojciechowski, E.; Mudge, A.; Burns, J. Spatiotemporal and Plantar Pressure Patterns of 1000 Healthy Individuals Aged 3–101 Years. Gait Posture 2017, 58, 78–87. [Google Scholar] [CrossRef]
- Cavanagh, P.R. The Biomechanics of Lower Extremity Action in Distance Running. Foot Ankle 1987, 7, 197–217. [Google Scholar] [CrossRef]
- Di Giminiani, R.; Di Lorenzo, D.; La Greca, S.; Russo, L.; Masedu, F.; Totaro, R.; Padua, E. Angle-Angle Diagrams in the Assessment of Locomotion in Persons with Multiple Sclerosis: A Preliminary Study. Appl. Sci. 2022, 12, 7223. [Google Scholar] [CrossRef]
- Hershler, C.; Milner, M. Angle-Angle Diagrams in the Assessment of Locomotion. Am. J. Phys. Med. 1980, 59, 109–125. [Google Scholar] [PubMed]
- Accardo, P.J.; Monasterio, E.; Oswald, D. Toe Walking in Autism. In Comprehensive Guide to Autism; Patel, V.B., Preedy, V.R., Martin, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 519–532. ISBN 978-1-4614-4787-0. [Google Scholar]
- Horak, F.B.; Nashner, L.M. Central Programming of Postural Movements: Adaptation to Altered Support-Surface Configurations. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.D.; Zajac, F.E. A Biomechanical Analysis of Muscle Strength as a Limiting Factor in Standing Posture. J. Biomech. 1993, 26, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Morasso, P. Integrating Ankle and Hip Strategies for the Stabilization of Upright Standing: An Intermittent Control model. Front. Comput. Neurosci. 2022, 16, 956932. [Google Scholar] [CrossRef]
- Nashner, L.M.; McCollum, G. The Organization of Human Postural Movements: A Formal Basis and Experimental Synthesis. Behav. Brain Sci. 1985, 8, 135–150. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Lord, C.; Luyster, R.J.; Gotham, K.; Guthrie, W. Autism Diagnostic Observation Schedule, (ADOS-2), Part II: Toddler Modulei, 2nd ed.; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Paquet, A.; Olliac, B.; Golse, B.; Vaivre-Douret, L. Nature of Motor Impairments in Autism Spectrum Disorder: A Comparison with Developmental Coordination Disorder. J. Clin. Exp. Neuropsychol. 2019, 41, 1–14. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Zeni, J.A.; Richards, J.G.; Higginson, J.S. Two Simple Methods for Determining Gait Events during Treadmill and Overground Walking Using Kinematic Data. Gait Posture 2008, 27, 710–714. [Google Scholar] [CrossRef]
- Masedu, F.; Angelozzi, M.; Di Giminiani, R.; Valenti, M. The Use of Fractal Dimension Methods in Clinical Epidemiology: An Application for Postural Assessment. Epidemiol. Biostat. Public Health 2013, 10, 9. [Google Scholar] [CrossRef]
- Marsden, C.D.; Merton, P.A.; Morton, H.B. Human Postural Responses. Brain 1981, 104, 513–534. [Google Scholar] [CrossRef]
- Krause, A.; Lee, K.; Freyler, K.; Bührer, T.; Gollhofer, A.; Ritzmann, R. Whole-Body Vibration Impedes the Deterioration of Postural Control in Patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 31, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Ben Maaoui, K.; Jahrami, H.; AlMarzooqi, M.A.; Boukhris, O.; Messai, B.; Clark, C.C.T.; Glenn, J.M.; Ghazzaoui, H.A.; Bragazzi, N.L.; et al. Attenuating Muscle Damage Biomarkers and Muscle Soreness after an Exercise-Induced Muscle Damage with Branched-Chain Amino Acid (BCAA) Supplementation: A Systematic Review and Meta-Analysis with Meta-Regression. Sports Med. Open 2024, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.J.; Jones, T.; Valenzuela, K.A.; Haegele, J.A. Inter and Intra-Limb Coordination Variability during Walking in Adolescents with Autism Spectrum Disorder. Clin. Biomech. 2021, 89, 105474. [Google Scholar] [CrossRef] [PubMed]
- Nobile, M.; Perego, P.; Piccinini, L.; Mani, E.; Rossi, A.; Bellina, M.; Molteni, M. Further Evidence of Complex Motor Dysfunction in Drug Naïve Children with Autism Using Automatic Motion Analysis of Gait. Autism 2011, 15, 263–283. [Google Scholar] [CrossRef]
- Li, Y.; Mache, M.A.; Todd, T.A. Complexity of Center of Pressure in Postural Control for Children With Autism Spectrum Disorders Was Partially Compromised. J. Appl. Biomech. 2019, 35, 190–195. [Google Scholar] [CrossRef]
- Li, Y.; Mache, M.A.; Todd, T.A. Automated Identification of Postural Control for Children with Autism Spectrum Disorder Using a Machine Learning Approach. J. Biomech. 2020, 113, 110073. [Google Scholar] [CrossRef]
- Stania, M.; Emich-Widera, E.; Kazek, B.; Kamieniarz, A.; Swatowska-Wenglarczyk, M.; Juras, G. Modulation of center-of-pressure signal in children on the autism spectrum: A case-control study. Gait Posture 2023, 103, 67–72. [Google Scholar] [CrossRef]
- Travers, B.G.; Mason, A.H.; Gruben, K.G.; Dean, D.C.; McLaughlin, K. Standing Balance on Unsteady Surfaces in Children on the Autism Spectrum: The Effects of IQ. Res. Autism Spectr. Disord. 2018, 51, 9–17. [Google Scholar] [CrossRef]
- Lim, Y.H.; Lee, H.C.; Falkmer, T.; Allison, G.T.; Tan, T.; Lee, W.L.; Morris, S.L. Effect of Visual Information on Postural Control in Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 4731–4739. [Google Scholar] [CrossRef]
- Cham, R.; Iverson, J.M.; Bailes, A.H.; Jennings, J.R.; Eack, S.M.; Redfern, M.S. Attention and Sensory Integration for Postural Control in Young Adults with Autism Spectrum Disorders. Exp. Brain Res. 2021, 239, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Day, B.L.; Steiger, M.J.; Thompson, P.D.; Marsden, C.D. Effect of Vision and Stance Width on Human Body Motion When Standing: Implications for Afferent Control of Lateral Sway. J. Physiol. 1993, 469, 479–499. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Taylor, W.R.; Madigan, M.L.; Nussbaum, M.A. The Spectral Content of Postural Sway during Quiet Stance: Influences of Age, Vision and Somatosensory Inputs. J. Electromyogr. Kinesiol. 2012, 22, 131–136. [Google Scholar] [CrossRef]
- Slobounov, S.; Hallett, M.; Cao, C.; Newell, K. Modulation of Cortical Activity as a Result of Voluntary Postural Sway Direction: An EEG Study. Neurosci. Lett. 2008, 442, 309–313. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giminiani, R.; La Greca, S.; Marinelli, S.; Attanasio, M.; Masedu, F.; Mazza, M.; Valenti, M. Locomotion and Postural Control in Young Adults with Autism Spectrum Disorders: A Novel Kinesiological Assessment. J. Funct. Morphol. Kinesiol. 2024, 9, 185. https://doi.org/10.3390/jfmk9040185
Di Giminiani R, La Greca S, Marinelli S, Attanasio M, Masedu F, Mazza M, Valenti M. Locomotion and Postural Control in Young Adults with Autism Spectrum Disorders: A Novel Kinesiological Assessment. Journal of Functional Morphology and Kinesiology. 2024; 9(4):185. https://doi.org/10.3390/jfmk9040185
Chicago/Turabian StyleDi Giminiani, Riccardo, Stefano La Greca, Stefano Marinelli, Margherita Attanasio, Francesco Masedu, Monica Mazza, and Marco Valenti. 2024. "Locomotion and Postural Control in Young Adults with Autism Spectrum Disorders: A Novel Kinesiological Assessment" Journal of Functional Morphology and Kinesiology 9, no. 4: 185. https://doi.org/10.3390/jfmk9040185
APA StyleDi Giminiani, R., La Greca, S., Marinelli, S., Attanasio, M., Masedu, F., Mazza, M., & Valenti, M. (2024). Locomotion and Postural Control in Young Adults with Autism Spectrum Disorders: A Novel Kinesiological Assessment. Journal of Functional Morphology and Kinesiology, 9(4), 185. https://doi.org/10.3390/jfmk9040185






