A Comparison of Bioelectric and Biomechanical EMG Normalization Techniques in Healthy Older and Young Adults during Walking Gait
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.2.1. Walking Trials
2.2.2. Submaximal Isometric Contraction Trials and Load–EMG Relationship Determination
2.3. EMG Collection and Conditioning
2.4. Normalization of EMG Data
2.5. Formulae
2.6. Statistical Analysis
3. Results
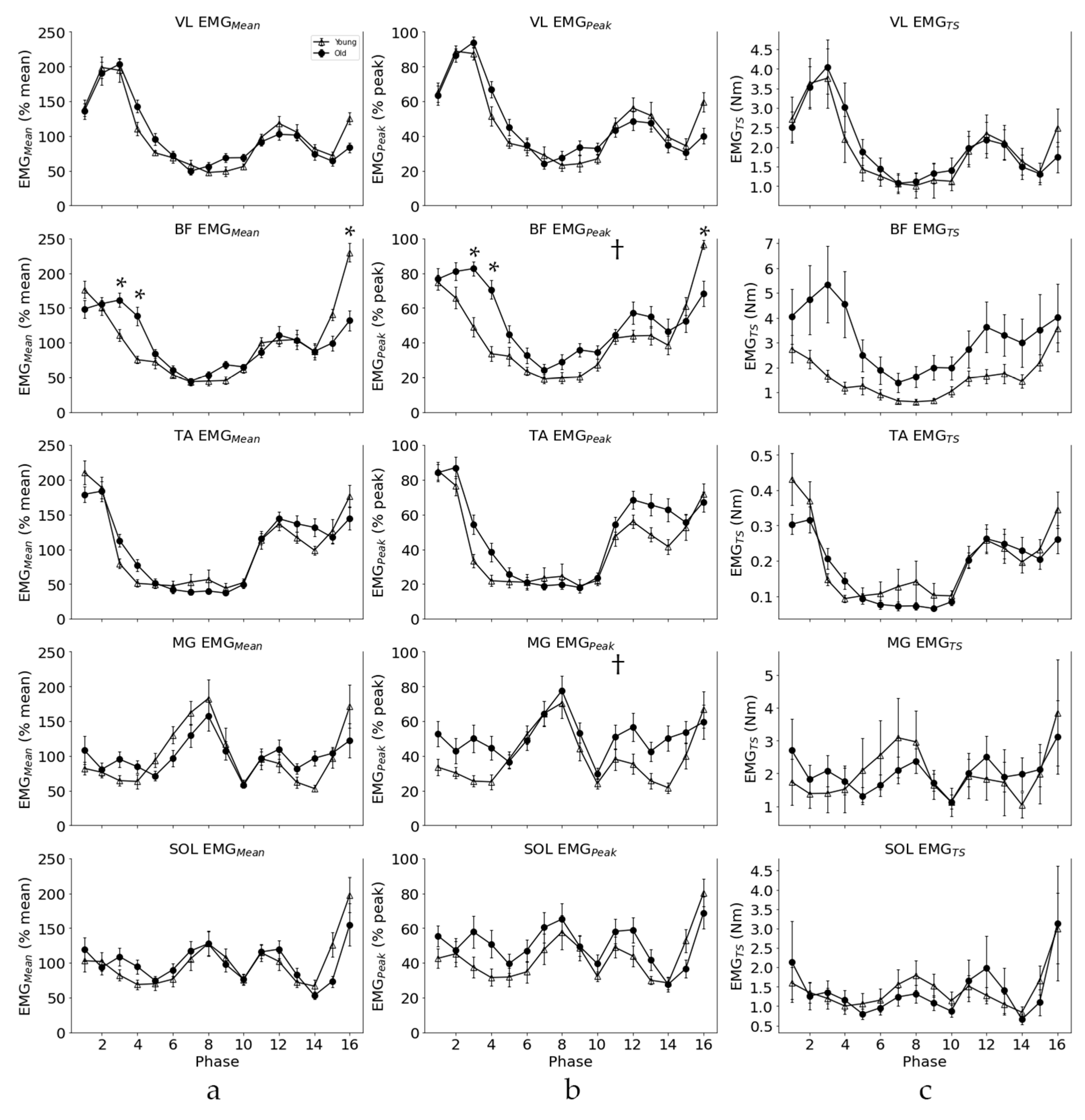
3.1. Amplitude Differences
3.2. EMG Pattern
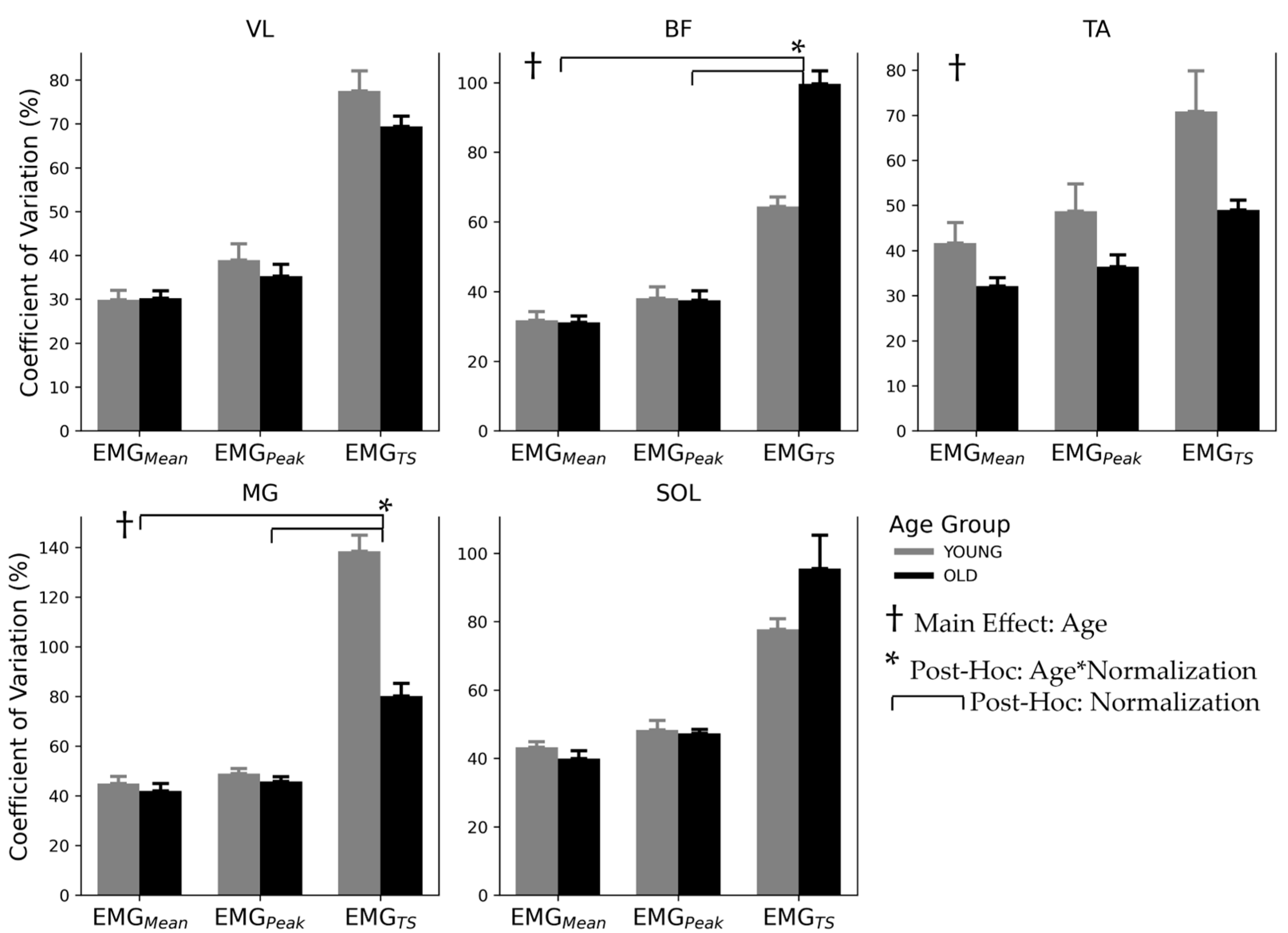
3.3. Inter-Subject Variability
4. Discussion
4.1. Rationale for Normalization Protocols
4.2. EMG Activation Pattern and Amplitude
4.3. Inter-Subject Variability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hemingway, M.A.; Biedermann, H.J.; Inglis, J. Electromyographic Recordings of Paraspinal Muscles: Variations Related to Subcutaneous Tissue Thickness. Biofeedback Self. Regul. 1995, 20, 39–49. [Google Scholar] [CrossRef]
- Beck, T.W.; Housh, T.J.; Mielke, M.; Cramer, J.T.; Weir, J.P.; Malek, M.H.; Johnson, G.O. The Influence of Electrode Placement over the Innervation Zone on Electromyographic Amplitude and Mean Power Frequency versus Isokinetic Torque Relationships. J. Neurosci. Methods 2007, 162, 72–83. [Google Scholar] [CrossRef]
- Basmajian, J.V.; DeLuca, C.J. Muscles Alive: Their Functions Revealed by Electromyography, 5th ed.; Williams & Wilkins: Baltimore, MD, USA, 1985. [Google Scholar]
- Koh, T.J.; Grabiner, M.D. Evaluation of Methods to Minimize Cross Talk in Surface Electromyography. J. Biomech. 1993, 26 (Suppl. S1), 151–157. [Google Scholar] [CrossRef]
- Burden, A. How Should We Normalize Electromyograms Obtained from Healthy Participants? What We Have Learned from over 25 Years of Research. J. Electromyogr. Kinesiol. 2010, 20, 1023–1035. [Google Scholar] [CrossRef]
- Burden, A.M.; Trew, M.; Baltzopoulos, V. Normalisation of Gait EMGs: A Re-Examination. J. Electromyogr. Kinesiol. 2003, 13, 519–532. [Google Scholar] [CrossRef]
- Yang, J.F.; Winter, D.A. Electromyographic Amplitude Normalization Methods: Improving Their Sensitivity as Diagnostic Tools in Gait Analysis. Arch. Phys. Med. Rehabil. 1984, 65, 517–521. [Google Scholar]
- Marras, W.S.; Davis, K.G.; Maronitis, A.B. A Non-MVC EMG Normalization Technique for the Trunk Musculature: Part 2. Validation and Use to Predict Spinal Loads. J. Electromyogr. Kinesiol. 2001, 11, 11–18. [Google Scholar] [CrossRef]
- Cholewicki, J.; van Dieën, J.; Lee, A.S.; Reeves, N.P. A Comparison of a Maximum Exertion Method and a Model-Based, Sub-Maximum Exertion Method for Normalizing Trunk EMG. J. Electromyogr. Kinesiol. 2011, 21, 767–773. [Google Scholar] [CrossRef]
- Knauer, S.R.; Freburger, J.K.; Carey, T.S. Chronic Low Back Pain among Older Adults: A Population-Based Perspective. J. Aging Health 2010, 22, 1213–1234. [Google Scholar] [CrossRef]
- Covinsky, K. Aging, Arthritis, and Disability. Arthritis Rheum. 2006, 55, 175–176. [Google Scholar] [CrossRef]
- Lehman, G.J.; Mcgill, S.M. The Importance of Normalization in the Interpretation of Surface Electromyography: A proof of principle. J. Manip. Physiol. Ther. 1999, 22, 444–446. [Google Scholar] [CrossRef]
- Zehr, E.P.; Loadman, P.M.; Hundza, S.R. Neural Control of Rhythmic Arm Cycling after Stroke. J. Neurophysiol. 2012, 108, 891–905. [Google Scholar] [CrossRef]
- Mathiassen, S.E.; Winkel, J.; Hägg, G.M. Normalization of Surface EMG Amplitude from the Upper Trapezius Muscle in Ergonomic Studies—A Review. J. Electromyogr. Kinesiol. 1995, 5, 197–226. [Google Scholar] [CrossRef]
- Ng, J.K.F.; Richardson, C.A.; Parnianpour, M.; Kippers, V. EMG Activity of Trunk Muscles and Torque Output during Isometric Axial Rotation Exertion: A Comparison between Back Pain Patients and Matched Controls. J. Orthop. Res. 2002, 20, 112–121. [Google Scholar] [CrossRef]
- Allison, G.T.; Marshall, R.N.; Singer, K.P. EMG Signal Amplitude Normalization Technique in Stretch-Shortening Cycle Movements. J. Electromyogr. Kinesiol. 1993, 3, 236–244. [Google Scholar] [CrossRef]
- Hundza, S.R.; Gaur, A.; Brodie, R.; Commandeur, D.; Klimstra, M.D. Age-Related Erosion of Obstacle Avoidance Reflexes Evoked with Electrical Stimulation of Tibial Nerve during Walking. J. Neurophysiol. 2018, 1528–1537. [Google Scholar] [CrossRef]
- Schober, P.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Bakeman, R. Recommended Effect Size Statistic. Behav. Res. Methods 2005, 37, 379–384. [Google Scholar] [CrossRef]
- Marras, W.S.; Davis, K.G. A Non-MVC EMG Normalization Technique for the Trunk Musculature: Part 1. Method Development. J. Electromyogr. Kinesiol. 2001, 11, 1–9. [Google Scholar] [CrossRef]
- Hansson, G.Å.; Nordander, C.; Asterland, P.; Ohlsson, K.; Strömberg, U.; Skerfving, S.; Rempel, D. Sensitivity of Trapezius Electromyography to Differences between Work Tasks—Influence of Gap Definition and Normalisation Methods. J. Electromyogr. Kinesiol. 2000, 10, 103–115. [Google Scholar] [CrossRef]
- Woods, J.J.; Bigland Ritchie, B. Linear and Non-Linear Surface EMG/Force Relationships in Human Muscles. An Anatomical/Functional Argument for the Existence of Both. Am. J. Phys. Med. 1983, 62, 287–299. [Google Scholar] [PubMed]
- Alkner, B.A.; Tesch, P.A.; Berg, H.E. Quadriceps EMG/Force Relationship in Knee Extension and Leg Press. Med. Sci. Sports Exerc. 2000, 32, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.H.; DeLuca, C.J. Myoelectric Signal versus Force Relationship in Different Human Muscles Myoelectric in Different Signal versus Force Relationship Human Muscles. J. Appl. Physiol. 1983, 54, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Earp, J.E.; Newton, R.U.; Cormie, P.; Blazevich, A.J. Knee Angle-Specific EMG Normalization: The Use of Polynomial Based EMG-Angle Relationships. J. Electromyogr. Kinesiol. 2013, 23, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Shiavi, R.; Bourne, J.; Holland, A. Automated Extraction of Activity Features in Linear Envelopes of Locomotor Electromyographic Patterns. IEEE Trans. Biomed. Eng. 1986, 33, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Larsson, L.; Newell, K.M. Effects of Aging on Force Variability, Single Motor Unit Discharge Patterns, and the Structure of 10, 20, and 40 Hz EMG Activity. Neurobiol. Aging 2003, 24, 25–35. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Krishnamoorthy, V.; Scholz, J.P. An Alternative Test of Electromyographic Normalization in Patients. Muscle Nerve 2006, 33, 232–241. [Google Scholar] [CrossRef]

| Muscle | Normalization | df | p | F | np2 |
|---|---|---|---|---|---|
| VL | EMGMean | 15, 330 | 0.036 | 1.781 | 0.08 |
| VL | EMGPeak | 15, 330 | 0.003 | 2.334 | 0.10 |
| BF | EMGMean | 15, 330 | 0.000 | 7.333 | 0.25 |
| BF | EMGPeak | 15, 330 | 0.000 | 6.088 | 0.22 |
| BF | EMGTS | 15, 330 | 0.001 | 2.592 | 0.11 |
| TA | EMGMean | 15, 330 | 0.046 | 1.721 | 0.07 |
| TA | EMGPeak | 15, 330 | 0.007 | 2.170 | 0.09 |
| TA | EMGTS | 15, 330 | 0.032 | 1.814 | 0.08 |
| Age | Correlation | VL | BF | TA | MG | SOL |
|---|---|---|---|---|---|---|
| OLD | EMGPeak vs. EMGMean | 1.00 | 1.00 | 1.00 | 0.99 | 0.98 |
| OLD | EMGPeak vs. EMGTS | 0.99 | 0.98 | 1.00 | 0.70 | 0.72 |
| OLD | EMGMean vs. EMGTS | 0.99 | 0.98 | 1.00 | 0.70 | 0.84 |
| YOUNG | EMGPeak vs. EMGMean | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 |
| YOUNG | EMGPeak vs. EMGTS | 1.00 | 1.00 | 0.99 | 0.93 | 0.97 |
| YOUNG | EMGMean vs. EMGTS | 1.00 | 1.00 | 0.99 | 0.92 | 0.98 |
| Effect | Muscle | df | p | F | np2 |
|---|---|---|---|---|---|
| Age | BF | 1, 90 | 0.000 | 23.667 | 0.21 |
| Age | TA | 1, 90 | 0.001 | 12.470 | 0.12 |
| Age | MG | 1, 90 | 0.000 | 44.749 | 0.33 |
| Normalization | VL | 2, 90 | 0.000 | 119.460 | 0.73 |
| Normalization | BF | 2, 90 | 0.000 | 185.955 | 0.81 |
| Normalization | TA | 2, 90 | 0.000 | 11.348 | 0.20 |
| Normalization | MG | 2, 90 | 0.000 | 176.677 | 0.80 |
| Normalization | SOL | 2, 90 | 0.000 | 57.755 | 0.56 |
| Age*Normalization | BF | 2, 90 | 0.000 | 26.188 | 0.37 |
| Age*Normalization | MG | 2, 90 | 0.000 | 32.780 | 0.42 |
| Age*Normalization | SOL | 2, 90 | 0.043 | 0.821 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Commandeur, D.; Klimstra, M.; Brodie, R.; Hundza, S. A Comparison of Bioelectric and Biomechanical EMG Normalization Techniques in Healthy Older and Young Adults during Walking Gait. J. Funct. Morphol. Kinesiol. 2024, 9, 90. https://doi.org/10.3390/jfmk9020090
Commandeur D, Klimstra M, Brodie R, Hundza S. A Comparison of Bioelectric and Biomechanical EMG Normalization Techniques in Healthy Older and Young Adults during Walking Gait. Journal of Functional Morphology and Kinesiology. 2024; 9(2):90. https://doi.org/10.3390/jfmk9020090
Chicago/Turabian StyleCommandeur, Drew, Marc Klimstra, Ryan Brodie, and Sandra Hundza. 2024. "A Comparison of Bioelectric and Biomechanical EMG Normalization Techniques in Healthy Older and Young Adults during Walking Gait" Journal of Functional Morphology and Kinesiology 9, no. 2: 90. https://doi.org/10.3390/jfmk9020090
APA StyleCommandeur, D., Klimstra, M., Brodie, R., & Hundza, S. (2024). A Comparison of Bioelectric and Biomechanical EMG Normalization Techniques in Healthy Older and Young Adults during Walking Gait. Journal of Functional Morphology and Kinesiology, 9(2), 90. https://doi.org/10.3390/jfmk9020090






