Abstract
Several studies agree that mechanical vibration can induce physiological changes at different levels, improving neuromuscular function through postural control strategies, muscle tuning mechanisms and tonic vibration reflexes. Whole-body vibration has also been reported to increase bone mineral density and muscle mass and strength, as well as to relieve pain and modulate proprioceptive function in patients with osteoarthritis or lower back pain. Furthermore, vibratory training was found to be an effective strategy for improving the physical performance of healthy athletes in terms of muscle strength, agility, flexibility, and vertical jump height. Notably, several benefits have also been observed at the brain level, proving to be an important factor in protecting and/or preventing the development of age-related cognitive disorders. Although research in this field is still debated, certain molecular mechanisms responsible for the response to whole-body vibration also appear to be involved in physiological adaptations to exercise, suggesting the possibility of using it as an alternative or reinforcing strategy to canonical training. Understanding these mechanisms is crucial for the development of whole body vibration protocols appropriately designed based on individual needs to optimize these effects. Therefore, we performed a narrative review of the literature, consulting the bibliographic databases MEDLINE and Google Scholar, to i) summarize the most recent scientific evidence on the effects of whole-body vibration and the molecular mechanisms proposed so far to provide a useful state of the art and ii) assess the potential of whole-body vibration as a form of passive training in place of or in association with exercise.
1. Introduction
Scientific research on the effects of mechanical vibrations has clearly highlighted the danger that this stimulus can pose to the state of health, emphasizing how the risk from vibrations is generated when using specific tools, working instruments and machinery that induce continuous stresses in the body of the worker using them, compromising apparatuses, joints, or even internal organs [1,2]. Furthermore, the study of vibrations’ effect on the human body has led, in recent years, to a greater understanding of degenerative phenomena leading to a broad spectrum of occupational diseases. However, there are still poorly documented aspects of both foot-transmitted vibration (FTV) and hand–arm vibration (HAV) [3,4].
A further condition is the whole-body vibration (WBV) to which many workers are exposed while driving trucks and agricultural machinery, or through the use of tools that produce high-amplitude vibrations that are transferred to the entire body, damaging various organs and apparatuses [5,6]. In this context, prolonged exposure to vibration in the construction work environment has been reported to be significantly associated with musculoskeletal disorders, predominantly in the neck, shoulder, and arm [7,8]. Notably, such disorders induced by WBV exposure are manifested by musculoskeletal pain, the chronicity of which, due to the persistence of the stimulus, leads to reduced hours of work activity, impaired emotional well-being and, in general, a worsening of the individual’s quality of life [9,10].
Although a negative impact of vibrations has also been documented in the peripheral nervous system, the digestive system, the female reproductive system, and the vestibular system [11,12,13,14], an increasing part of the literature reports scientific evidence in favor of the use of WBV as a form of alternative training in patients unable to exercise [15]. In this context, the beneficial effects of WBV appear to be numerous, including the prevention of chronic and degenerative diseases. Vibratory stimulation has also been suggested as an effective tool to prevent and/or counteract age-related cognitive decline and to mitigate the physiological changes that characterize aging [16]. Importantly, the utility of WBV does not appear to be limited to the prevention of disease and aging, but is also often applied for rehabilitation purposes in athletes with various conditions, including to improve balance in individuals with ankle instability [17], to increase muscle strength in individuals with anterior cruciate ligament reconstruction [18], and to reduce patellofemoral pain by optimizing sports performance [19].
Although WBV represents a powerful stimulus for the entire organism, the underlying biological mechanisms have not yet been fully elucidated, due in part to the extreme variability of its effects, which are strongly dependent upon the parameters that characterize mechanical vibration, such as the frequency and amplitude of the vibration as well as the duration of vibration exposure [20]. Nevertheless, a complete understanding of the physiological adaptations to WBV and the consequent application of protocols customized to individual needs should be the primary goal of research in this field, providing a valid strategy to counteract the progression of various degenerative diseases, as well as age-related physiological decline in individuals unable to exercise. Furthermore, depending on the needs of individual athletes, specific WBV protocols, sometimes administered at the same time as exercise, could prove extremely useful in the rehabilitation of musculoskeletal disorders as well as for improving sports performance.
Therefore, the aim of our review was to (i) summarize the scientific information and experimental data on physiological adaptations to WBV with a focus on the effects on cognitive function, musculoskeletal health, and pain perception, and (ii) gather evidence on the clinical efficacy of WBV in order to consider its use as an alternative strategy to exercise.
2. Literature Search Strategy
A non-systematic search strategy was adopted for the writing of this narrative review, selecting 100 scientific articles concerning the effects of vibratory training on the entire organism, with a focus on nervous and musculoskeletal tissues. The bibliographic databases MEDLINE and Google Scholar were used to select peer-reviewed articles of interest published between 1945 (start date) and 2022. The search strategy was based on the use of the following combinations of medical subject headings (MeHS) and keywords: (whole body vibration) AND (benefits); (whole body vibration) AND (nervous system); (whole body vibration) AND (cognitive function); (whole body vibration) AND (neurodegeneration); (whole body vibration) AND (brain); (whole body vibration) AND (musculoskeletal system); (whole body vibration) AND (bone); (whole body vibration) AND (muscle); (whole body vibration) AND (osteoporosis); (whole body vibration) AND (osteoarthritis); (whole body vibration) AND (sarcopenia); (whole body vibration) AND (pain); (whole body vibration) AND (low back pain); (whole body vibration) AND (fibromyalgia); (whole body vibration) OR (prevention); (whole body vibration) AND (exercise); (whole body vibration) OR (alternative strategy). For each combination listed, the keyword “whole body vibration” was replaced with the terms “vibratory training”, “mechanical vibration” and “WBV”. The results included in vitro and in vivo experimental studies, systematic reviews and meta-analyses, narrative reviews, randomized controlled trials and clinical trials to provide a comprehensive overview.
All search results were analyzed by two researchers who defined their relevance to the topic. Any disagreements during the article selection process were resolved through discussion with a third researcher. Finally, a further check of the reference lists was conducted by two other authors who confirmed the validity of the search performed and clarified any doubts. The search process was performed on a worldwide basis, without excluding specific geographical areas or different ethnic groups. Language and species filters were applied to the list of results to eliminate non-English language articles.
3. Physiological Adaptations to WBV
Most of the knowledge regarding the molecular mechanisms underlying physiological adaptations to WBV is provided by studies conducted on rodents, which have shown that the positive effects of vibratory training depend on specific parameters, such as vibration frequency, and vibration exposure time. In particular, most studies agree that the main benefits are associated with low-intensity WBV protocols, with variable effects mainly affecting nervous and musculoskeletal tissue [21,22,23] (Figure 1).
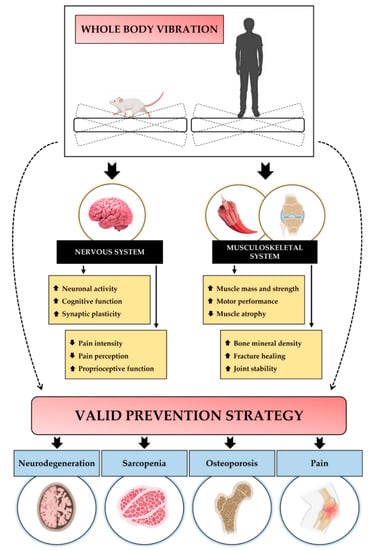
Figure 1.
A schematic representation of the WBV effects on the nervous and musculoskeletal systems. WBV improves brain health by increasing neuronal activity, cognitive function, and synaptic plasticity. At the nervous system level, WBV also promotes proprioceptive function, reducing the intensity and perception of pain. At the level of the musculoskeletal system, WBV increases muscle mass and strength, as well as motor performance, while reducing muscle atrophy. In addition, vibratory training increases bone mineral density and promotes fracture healing and joint stability. Taken together, these effects make WBV a valuable preventive strategy for neurodegenerative diseases, musculoskeletal disorders such as sarcopenia and osteoporosis, and pain-associated diseases.
3.1. WBV Improves Cognitive Function and Counteracts Neurodegeneration
The transmission of vibrations and oscillations to the body is known to stimulate skin receptors, muscle spindles and the vestibular system, inducing numerous changes in brain activity, such as those in the somatosensory cortex, thalamus, hippocampus, and amygdala, and altering the concentrations of important neurotransmitters, such as dopamine and serotonin, which act as messengers for nervous cells [24,25,26]. The positive effects of WBV on brain function were mainly observed through studies in animal models, which showed that 5-week WBV training increased the activity of the cholinergic system in the somatosensory cortex and amygdala of C57Bl/6J mice [27], and preliminary studies found a WBV-induced enhancement of immediate and early c-fos gene expression, indicative of increased neuronal activity [28]. In agreement with this, a significant improvement in neurological and motor abilities was found in female rats exposed to a low-frequency WBV protocol for 30 days, in association with a reduction in post-stroke inflammation and frailty that prevented post-ischemic cognitive decline. [29]. More recently, Peng and colleagues studied the effects of 8 weeks of vibration training on neuronal loss, synaptic protein expression and neurotrophic factor levels in a rat model with chronic restraint stress-induced depression (CRS). Interestingly, a significant improvement in cognitive function was observed, in addition to neuroprotection and reduction of neuronal damage and death, suggesting WBV as a powerful therapeutic strategy for major depressive disorder [30]. Similarly, Cariati et al. investigated changes in synaptic plasticity by means of electrophysiological recordings of the hippocampus in 4-month-old and 24-month-old young mice exposed to different WBV protocols, identifying the protocol characterized by a lower vibration frequency and longer recovery time as the only one capable of positively modulating hippocampal synaptic plasticity and influencing the higher cognitive processes of learning and memory. In contrast, the other vibration training protocols, which differed in vibration frequency, vibration exposure time and recovery time, were found to be too stressful, inducing the appearance of an epileptic tendency and possibly damaging the hippocampus and other brain structures related to memory functions [31]. These results were later confirmed by the same authors through histological and morphometric analyses, showing that reduced synaptic function was associated with the presence of structural alterations, including a reduction in the number of Purkinje cells in the cerebellum, as well as reduced or even absent pyramidal neurons in the hippocampus, indicating WBV-induced sensory stimulation as an essential part of the mechanism underlying the improved cognitive performance in mice [32].
Brain adaptations to WBV could also involve multiple brain regions in humans, given the close communication between the sensory systems of vibration perception and the areas that oversee cognitive functions [33]. In this regard, Regterschot et al. investigated the acute effects of passive WBV on executive functions in 133 healthy young adults subjected to six training sessions (frequency 30 Hz, amplitude approximately 0.5 mm) alternating with six rest sessions of two minutes each. The use of the Color–Word Interference Test (CWIT) and Stroop Difference Score (SDS) showed a significant improvement in executive functions, specifically attention and inhibition, in the trained group compared to the control group, suggesting passive WBV as a therapeutic strategy for the elderly and other populations with reduced attention and inhibition, including persons with attention deficit hyperactivity disorder [34].
Importantly, increased neuronal activity would appear to be directly associated with the acute increase in glucose metabolism, as reduced basal glucose levels have been suggested as indicative of brain pathology, as well as being considered an early biomarker for Alzheimer’s disease [35,36]. However, no significant data are available on possible changes in glucose metabolism in the brain after WBV stimulation. In this context, Boerema et al. investigated the impact of a 5-week WBV intervention on brain activity by assessing glucose metabolism in the murine brain and testing executive functioning and memory in elderly subjects without cognitive deficits. Positron emission tomography (PET) scans performed in the mice revealed that glucose uptake was not altered by WBV exposure, although WBV improved cognition and motor performance and reduced arousal-induced home cage activity. Interestingly, cognitive tests in humans showed a selective improvement in the Stroop Color–Word test, known to be positively correlated with cholinergic activity, disconfirming WBV as a safe intervention to improve brain functioning, albeit with variable effects depending on the protocol used [37]. In agreement with this, Alashram and colleagues highlighted in a systematic review that included twenty randomized controlled trials and pseudo-randomized controlled trials how short-term WBV training represents a valid strategy to reduce lower limb spasticity and improve mobility and balance in patients with neurological disorders, although the optimal parameters of an appropriate WBV protocol remain unclear and current evidence is limited by heterogeneity and a scarcity of research [38].
3.2. WBV Promotes Musculoskeletal Health
The effects of WBV on the musculoskeletal system have been extensively documented in both animal and human models, suggesting, among the main benefits, an increase in muscle mass and strength and bone mineral density, as well as improved motor performance and general health [39,40]. Not surprisingly, vibratory training is currently used as a valid strategy to prevent various diseases, including sarcopenia, osteoporosis, and arthrosis, as well as to improve musculoskeletal function and joint stability [41,42]. In this context, Matsumoto and Goto investigated the effects of low-intensity WBV in 13-week-old mice undergoing tibial perforation, finding an improvement in bone healing induced by an increase in vascular growth [43]. In agreement with this, Keijser and colleagues observed that exposure to a 5-week WBV protocol (30 Hz, 5 or 30 min per day for 5 weeks) in CD1 mice improved motor performance and object recognition in a dose-dependent manner, confirming the duration of the training session as another key parameter to consider when designing appropriate protocols [44]. Similarly, Cariati et al. observed how a WBV protocol characterized by low frequency, short vibration exposure time and longer recovery period between two consecutive sessions improved musculoskeletal health in a middle-aged mouse model. In contrast, light and electron microscopy analyses showed altered sarcomeric structures, abundant inter-fiber fibrosis and a higher percentage of atrophic fibers when animals were exposed to a more stressful WBV protocol [45]. These results were recently confirmed by the same authors in a study on 4-month-old young mice, showing that animals exposed to the less-stressful WBV protocol had the largest mean muscle fiber diameter and the least amount of inter-fiber connective tissue compared to the other experimental groups. Interestingly, histological and morphometric analysis also showed an improvement in the qualitative characteristics of bone tissue, such as higher bone volume and trabecular thickness and less trabecular separation compared to sedentary animals [32]. Indeed, increased bone mass and improved structural parameters in healthy young rodents are well documented. Specifically, a WBV protocol of 5 days per week for 3 weeks increased femoral cortical thickness, cross-sectional area and femoral trabecular bone cell activity in 7-week-old male mice [46]. In addition, a WBV protocol of 15 min/day increased the rate of bone formation in the endocortical surface of the tibial metaphysis and reduced osteoclastic activity in the tibial trabecular bone in 8-week-old female mice [47]; additionally, an increase in tibial trabecular bone fraction was found in 1-week-old mice with osteogenesis imperfecta [48]. Finally, an increase in bone mass and improvement in structural parameters after WBV exposure were also found in young rodents with spinal cord injury, unloading or oophorectomy, confirming it as a valid strategy in the management of bone disorders [49,50,51].
WBV has been shown to produce osteogenic effects, counteracting age-related changes in bone mass. However, contradictory results have been provided regarding the effects of WBV on bone mass in postmenopausal and elderly women. Ruan et al. found a 4.3% increase in the BMD of the lumbar spine and a 3.2% improvement in BMD of the femoral neck in postmenopausal women with osteoporosis exposed to 6 months of WBV (duration 10 min, 5 times per week, frequency of 30 Hz and amplitude of 5 mm) [52]. In agreement with this, ElDeeb and colleagues recently showed how exposure to WBV twice a week for 24 weeks improved leg muscle work and lumbar and femoral BMD in 43 postmenopausal women with low BMD [53]. In contrast, Slatkovska et al. found no increase in calcaneal BMD in postmenopausal women exposed to vibration training for 12 months (frequency of 90 or 30 Hz, with a peak acceleration of 0.3 g) and treated with calcium and vitamin D supplements [54]. Similarly, Rubin et al. found no changes in the bone mineral content (BMC) of the spine, hip, and distal radius in postmenopausal women after WBV (frequency of 30 Hz and size of 0.2 g) [55]. More recently, Marín-Cascales and colleagues conducted a systematic review and meta-analysis evaluating previously published randomized controlled trials investigating the effects of WBV on total, femoral neck, and lumbar spine BMD in postmenopausal women, in order to identify potential moderating factors explaining the adaptations to this type of exercise. Interestingly, vibratory training has been observed to improve the BMD of the lumbar spine in postmenopausal women, especially in those under the age of 65, confirming it as a potential non-pharmacological intervention to improve bone mass in postmenopausal and elderly women, particularly on the lumbar spine, which has been shown to be the most sensitive area [56].
Finally, the improved performance of the balance bundle, object recognition and increased activity of the cholinergic system in the somatosensory cortex and amygdala of experimental mouse models has directed the use of WBV in the management of patients with neurodegenerative diseases such as Parkinson’s disease (PD), especially in the treatment of motor symptoms such as bradykinesia, tremor, muscle rigidity and postural instability. Indeed, the association between PD and significant sensorimotor deficits suggested WBV as a potential strategy to improve sensorimotor function [57]. In this context, Li et al. recently demonstrated how vibratory training represented a passive and safe clinical intervention for patients with moderate PD, especially in cases of motor impairment or poor balance function, with effects comparable to those of conventional therapy [58].
3.3. WBV Favours Pain Relief
WBV, known to reduce pain intensity and improve function and quality of life, is currently used to treat patients with low back pain, which is a major cause of disability and remains a major public health concern in many developed countries [59,60]. Several sources have explained the WBV effects on lower back pain treatment, reporting positive correlations between core muscle inactivity, pain intensity and function in patients with lower back pain. In this regard, vibratory training has been suggested to activate muscle fibres and strengthen core stability muscles, improving back function in lower back pain patients [61,62]. Interestingly, the improvement in muscle activity and strength has been attributed to the enhancement of neural factors, such as recruitment and synchronization, as well as inter- and intramuscular coordination and proprioceptor responses [63]. In this regard, Rittweger et al., in a 6-month follow-up randomized controlled trial, suggested that both lumbar extension and WBV could alleviate pain and improve the associated limitation in everyday life [64], as well as direct evidence of significant effects in patients with chronic lower back pain after a 12-week WBV therapy compared to no treatment was provided [65]. WBV has also been proposed to increase muscle spindle activity and cause a stretch-reflex response of the trunk muscles, thus activating and strengthening the muscles in patients with lower back pain [66]. In fact, a tonic vibration reflex (TVR) has previously been shown to be provoked by direct mechanical vibrations applied to the muscle belly, just as vibration-induced neuromuscular activation leading to TVR would appear to be primarily determined by muscle spindle reflexes [67]. Finally, proprioception deficits in the lumbosacral region often cause dysfunction and spinal instability in lower back pain patients, suggesting WBV as an alternative method to improve proprioceptive function by activating the proprioceptors of lower back pain patients [68,69]. Due to the presence of pain, many pathologies prevent affected individuals from performing exercises on their own, thus hindering rehabilitation. Fortunately, the use of external mechanical vibratory stimuli, which reduces the deficit in voluntary muscle activation by the neuromuscular stretch reflex, has proven to be an effective method for people with severe disabilities [70]. Furthermore, an alteration and attenuation of pain perception has been reported as a consequence of mechanical vibration in large-diameter fiber systems [71]. As reported by Gate in the pain control theory, the interpretation of pain occurs in the higher centers that receive afferent neural signals from the dorsal horn of the spinal cord. Therefore, the synchronous activation of more Aα/Aβ fibers stimulating the dorsal horn of the spinal cord could be responsible for the reduction of pain caused by vibrations [72]. In this context, Sonza et al. recently observed a significant reduction in sensitivity to Aβ fibers mediated by mechanical allodynia and C fibers already mediated by thermal stimulation after the third WBV session in a mouse model of chronic pain. Notably, a strong influence of vibratory training was found on tactile pressure mechanoreceptors, confirming its efficacy in rehabilitation programs based on sensitivity and pain reduction [73].
In summary, the main evidence reported on the effects of WBV on the brain and musculoskeletal system is presented in Table 1.

Table 1.
A schematic representation of the main scientific evidence on the WBV effects.
4. Molecular Mediators Involved in WBV Effects
Although the effects of WBV are widely known, the underlying biological mechanisms have yet to be elucidated, as has the identification of a protocol tailored to the individual’s characteristics for a specific personalized intervention [74,75]. However, in recent years, several studies have been conducted in this field, and some key molecules have been proposed to be responsible for the effects of WBV on the whole organism.
In particular, improved cognitive function has been suggested to depend upon increased production of neurotrophins, known mediators of neuronal development, survival, and function [76]. In this context, brain-derived neurotrophic factor (BDNF) appears to be the most susceptible to exercise-induced regulation and may be among the main contributors to the cascade of molecular and cellular events that support brain plasticity. Indeed, BDNF is considered the main neurotrophin linked to neuronal plasticity, playing a key role in neuronal differentiation and survival [77]. Numerous studies have shown a close correlation between increased BDNF levels and aerobic exercise, while it is unclear how mechanical vibrations may influence the expression of this neurotrophin. In this regard, Simão et al. proposed that the combination of vibratory training with squat exercises improves lower limb muscle performance in elderly women with knee osteoarthritis, probably through an increase in plasma BDNF levels, suggesting a role in the modulation of neuromuscular plasticity [78]. Similarly, Ribeiro and colleagues demonstrated that exposure to WBV for 6 weeks promotes an increase in plasma BDNF levels in association with an improvement in lower limb muscle strength, aerobic capacity, clinical symptoms and quality of life in patients with fibromyalgia syndrome [79]. Interestingly, under pathological conditions in rodent models of cerebral ischemia or stroke, WBV has been observed to stimulate the expression of several mediators involved in neurogenesis, including BDNF, insulin-like growth factor (IGF-1) and doublecortin (DCX) [80]. In this regard, Oberste et al. conducted a double-blind randomized controlled trial to study the effects of a 6-week WBV protocol in adolescent patients hospitalized for major depressive disorder. Notably, antidepressant effects of vibratory training were found in association with increased serum levels of BDNF, IGF-1 and inflammatory markers [81].
Irisin, a polypeptide generated by the cleavage of fibronectin type III domain-containing protein 5 (FNDC5), is also undoubtedly involved in physiological adaptations to exercise, as its expression is known to increase abundantly during exercise in musculoskeletal tissue and nerve tissue. Importantly, BDNF-mediated effects in brain tissue could be enhanced by FNDC5, as its exercise-induced up-regulation is known to result in the up-regulation of BDNF [82]. Furthermore, the increased production of irisin through exercise could influence the bone–muscle crosstalk, promoting an increase in the proliferative and mineralizing capacity of osteoblasts [83], as well as promoting muscle growth through a signaling pathway that reduces the expression of myostatin, the main negative regulator of muscle growth [84]. In this regard, Yang and colleagues observed an up-regulation of osteogenic markers, such as osterix, RUNX2 and osteopontin, in MC3T3-E1 murine osteoblasts in response to irisin, suggesting its involvement in bone formation and mineralization processes [85]. Similarly, Shan and colleagues showed that knockout of the myostatin gene produces an increase in the muscle mass and browning of adipose tissue, effects known to be attributed to irisin [86]. In agreement with this, Cariati et al. recently studied the potential effects of WBV on the expression of FNDC5 and tissue-specific markers such as BDNF in brain, myostatin in muscle, and collagen I (COL-1) in the bone of 4-month-old young mice [32]. Interestingly, increased expression of FNDC5, improved tissue structural organization and increased BDNF expression were detected after exposure to a WBV protocol with shorter vibration exposure times and longer recovery times. Furthermore, increased FNDC5 expression was found in the muscles and bones of trained mice, as well as reduced myostatin expression and increased COL-1, confirming WBV as a valid strategy to preserve musculoskeletal health [32].
5. WBV vs. Exercise: Do We Have a Chance?
The effectiveness of adding WBV to conventional training generally remains an open question. On the one hand, several sources have reported the combination of WBV and physical therapy as a valid strategy to significantly increase muscle strength and power, flexibility and BMD, as well as to reduce abdominal fat [87,88,89,90]. Specifically, a significant increase in isometric and dynamic knee extensor strength and countermovement jump height was observed in healthy women undergoing 12 weeks of WBV and resistance training [91]. Similarly, Osawa and colleagues observed significant improvements in knee extensor strength and countermovement jump height in young and older adults undergoing WBV combined with routine exercises [92]. Furthermore, Berschin et al. investigated whether WBV could be considered a practical alternative to a standard exercise programme in 40 patients undergoing anterior cruciate ligament (ACL) reconstruction. Notably, exposure to WBV resulted in better neuromuscular performance, in terms of strength and co-ordination, as well as improved postural control in a short period of time, making it a good alternative to a standard exercise programme in ACL rehabilitation [93]. In contrast, Cochrane et al. found no improvement in countermovement jump height, sprint speed and agility performance in non-elite athletes subjected to 9 sessions of WBV training [94]. Similarly, Rogan et al. concluded that adding WBV to physical therapy did not improve muscle strength in healthy elderly people [95], and Anwer and colleagues found no beneficial effects of WBV on quadriceps muscle strength in patients with knee osteoarthritis [96]. More recently, Rasti and colleagues conducted a randomized clinical trial to compare the effects of physical training with and without WBV on flexibility, vertical jump height, agility, and pain in 24 athletes with patellofemoral pain (PFP), a well-known musculoskeletal condition prevalent among active young adults [97]. Interestingly, physical therapy with and without WBV was observed to significantly reduce pain and increase agility, vertical jump height and flexibility in athletes with PFP. However, WBV implementation to routine physical therapy increased the latter’s effects on flexibility, confirming vibratory training as a valuable additional strategy to increase the effectiveness of conventional physical therapy and optimize athletic performance [97]. In agreement with this, Gloeckl et al. demonstrated that balance training performed on a WBV platform induced greater benefits in terms of balance performance and muscle power than conventional training in patients with severe chronic obstructive pulmonary disease (COPD) and functional impairment [98]. Finally, Guadarrama-Molina and colleagues found a significant improvement in functional balance status in 45 PD patients after exposure to conventional therapy and WBV, suggesting their combination as a viable therapeutic alternative to improve quality of life in PD patients compared to conventional therapy alone [99].
6. Conclusions
WBV is undoubtedly an expanding area of research with clear potential for medical applications. However, this field could benefit from more standardized and customized protocols. In this regard, the identification of a WBV protocol suitable for a specific age group could pave the way for intervention studies targeting subjects forced to a sedentary lifestyle. Indeed, the future goal of research in this field should be to tailor appropriate training protocols to the individual’s characteristics, as each subject possesses unique and nuanced characteristics at the molecular, physiological, environmental exposure and behavioral levels, thus requiring specific interventions. The use of translational research can facilitate this by considering vibration amplitude, vibration frequency, method of application, session duration/frequency and total duration of intervention as key parameters of WBV. In this regard, studies performed with significantly different protocols in terms of frequency, amplitude, acceleration, and duration were compared in our manuscript, posing difficulties in comparing the evidence analyzed. Notably, such information was not always found to be complete, and this represents a major limitation for both the studies examined and our narrative review. Importantly, van Heuvelen et al. have recently published guidelines for the correct and complete drafting of in vitro and in vivo studies, in animal and human models, relating to vibratory training, indicating all the variables that must necessarily be specified in scientific papers [100].
Nevertheless, the study of physiological adaptations induced by specific WBV protocols is crucial for the development of preventive and/or therapeutic strategies. In this context, WBV training represents a real potential application for improving dose–response effects and counteracting multi-organ decay related to age and/or degenerative diseases, foreseeing an improvement in quality of life and a potential reduction in public health costs. Therefore, substantial investigations into the underlying molecular mechanisms are needed in order to identify the role of potential key mediators involved in physiological adaptations to WBV that could be used as markers of efficacy of the protocol used.
Author Contributions
Conceptualization, R.B. and I.C.; investigation, R.B., I.C. and C.R.; data curation, R.B., I.C. and C.R.; writing—original draft preparation, R.B. and I.C.; writing—review and editing, G.D., G.A. and V.T.; supervision, V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors acknowledge the Centre of Space Bio-medicine, “Tor Vergata” University of Rome for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fethke, N.B.; Schall, M.C.; Merlino, L.A.; Chen, H.; Branch, C.A.; Ramaswamy, M. Whole-Body Vibration and Trunk Posture During Operation of Agricultural Machinery. Ann. Work Expo. Health 2018, 62, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Chadefaux, D.; Moorhead, A.P.; Marzaroli, P.; Marelli, S.; Marchetti, E.; Tarabini, M. Vibration transmissibility and apparent mass changes from vertical whole-body vibration exposure during stationary and propelled walking. Appl. Ergon. 2021, 90, 103283. [Google Scholar] [CrossRef] [PubMed]
- Krajnak, K. Health effects associated with occupational exposure to hand-arm or whole body vibration. J. Toxicol. Environ. Health B Crit. Rev. 2018, 21, 320–334. [Google Scholar] [CrossRef]
- Eger, T.; Thompson, A.; Leduc, M.; Krajnak, K.; Goggins, K.; Godwin, A.; House, R. Vibration induced white-feet: Overview and field study of vibration exposure and reported symptoms in workers. Work 2014, 47, 101–110. [Google Scholar] [CrossRef]
- Bovenzi, M. A prospective cohort study of neck and shoulder pain in professional drivers. Ergonomics 2015, 58, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.J. Predicting and controlling risks from human exposures to vibration and mechanical shock: Flag waving and flag weaving. Ergonomics 2015, 58, 1063–1070. [Google Scholar] [CrossRef][Green Version]
- Charles, L.E.; Ma, C.C.; Burchfiel, C.M.; Dong, R.G. Vibration and Ergonomic Exposures Associated with Musculoskeletal Disorders of the Shoulder and Neck. Saf. Health Work 2018, 9, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, M.; Burström, L.; Ekman, A.; Vilhelmsson, R. The association between whole body vibration exposure and musculoskeletal disorders in the Swedish work force is confounded by lifting and posture. J. Sound Vib. 2006, 298, 492–498. [Google Scholar] [CrossRef]
- Patterson, F.; Miralami, R.; Tansey, K.E.; Prabhu, R.K.; Priddy, L.B. Deleterious effects of whole-body vibration on the spine: A review of in vivo, ex vivo, and in vitro models. Anim. Model. Exp. Med. 2021, 4, 77–86. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; Tarantino, U. Chronic Pain in Musculoskeletal Diseases: Do You Know Your Enemy? J. Clin. Med. 2022, 11, 2609. [Google Scholar] [CrossRef]
- Jalilian, H.; Zamanian, Z.; Gorjizadeh, O.; Riaei, S.; Monazzam, M.R.; Abdoli-Eramaki, M. Autonomic Nervous System Responses to Whole-Body Vibration and Mental Workload: A Pilot Study. Int. J. Occup. Environ. Med. 2019, 10, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y. Adverse effects of whole-body vibration on gastric motility. Kurume Med. J. 2000, 47, 79–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zarei, S.; Dehghan, S.F.; Vaziri, M.H.; Gilani, M.A.S.; Ardakani, S.K. Assessment of semen quality of taxi drivers exposed to whole body vibration. J. Occup. Med. Toxicol. 2022, 17, 16. [Google Scholar] [CrossRef]
- Yilmaz, N.; Ila, K. Effect of vibration on the vestibular system in noisy and noise-free environments in heavy industry. Acta Otolaryngol. 2019, 139, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. The use of vibration as physical exercise and therapy. J. Funct. Morphol. Kinesiol. 2017, 2, 17. [Google Scholar] [CrossRef]
- Odano, I.; Maeyatsu, F.; Asari, M.; Yamaguchi, S.; Miura, T.; Taki, Y. Whole-body vibration exercise and training increase regional CBF in mild cognitive impairment with enhanced cognitive function. Ann. Nucl. Med. 2022, 36, 82–94. [Google Scholar] [CrossRef]
- Sierra-Guzmán, R.; Jiménez-Diaz, F.; Ramírez, C.; Esteban, P.; Abián-Vicén, J. Whole-Body-Vibration Training and Balance in Recreational Athletes with Chronic Ankle Instability. J. Athl. Train. 2018, 53, 355–363. [Google Scholar] [CrossRef]
- Costantino, C.; Bertuletti, S.; Romiti, D. Efficacy of Whole-Body Vibration Board Training on Strength in Athletes After Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Study. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2018, 28, 339–349. [Google Scholar] [CrossRef]
- Shadloo, N.; Kamali, F.; Salehi Dehno, N. A comparison between whole-body vibration and conventional training on pain and performance in athletes with patellofemoral pain. J. Bodyw. Mov. Ther. 2021, 27, 661–666. [Google Scholar] [CrossRef]
- Costantino, C.; Gimigliano, R.; Olvirri, S.; Gimigliano, F. Whole body vibration in sport: A critical review. J. Sports Med. Phys. Fitness 2014, 54, 757–764. [Google Scholar]
- Roberts, R.E.; Bilgen, O.; Kineman, R.D.; Koh, T.J. Parameter-Dependency of Low-Intensity Vibration for Wound Healing in Diabetic Mice. Front. Bioeng. Biotechnol. 2021, 9, 654920. [Google Scholar] [CrossRef] [PubMed]
- Corbiere, T.F.; Weinheimer-Haus, E.M.; Judex, S.; Koh, T.J. Low-Intensity Vibration Improves Muscle Healing in a Mouse Model of Laceration Injury. J. Funct. Morphol. Kinesiol. 2018, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Oroszi, T.; Geerts, E.; de Boer, S.F.; Schoemaker, R.G.; van der Zee, E.A.; Nyakas, C. Whole Body Vibration Improves Spatial Memory, Anxiety-Like Behavior, and Motor Performance in Aged Male and Female Rats. Front. Aging Neurosci. 2021, 13, 801828. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.-S.; Huang, L.; Chen, X.-H.; Wang, H.-B.; Sun, W.-S.; Huo, S.-C.; Li, Z.-Q.; Deng, W.-M. Effect of whole body vibration therapy on circulating serotonin levels in an ovariectomized rat model of osteoporosis. Iran. J. Basic Med. Sci. 2014, 17, 62–68. [Google Scholar] [PubMed]
- Nakamura, H.; Moroji, T.; Nohara, S.; Nakamura, H.; Okada, A. Activation of cerebral dopaminergic systems by noise and whole-body vibration. Environ. Res. 1992, 57, 10–18. [Google Scholar] [CrossRef]
- Vizzi, L.; Padua, E.; D’Amico, A.G.; Tancredi, V.; D’Arcangelo, G.; Cariati, I.; Scimeca, M.; Maugeri, G.; D’Agata, V.; Montorsi, M. Beneficial Effects of Physical Activity on Subjects with Neurodegenerative Disease. J. Funct. Morphol. Kinesiol. 2020, 5, 94. [Google Scholar] [CrossRef]
- Heesterbeek, M.; Jentsch, M.; Roemers, P.; Keijser, J.N.; Toth, K.; Nyakas, C.; Schoemaker, R.G.; van Heuvelen, M.J.G.; Van der Zee, E.A. Whole body vibration enhances choline acetyltransferase-immunoreactivity in cortex and amygdale. J. Neurol. Transl. Neurosci. 2017, 5, 1079. [Google Scholar]
- Van der Zee, E.A.; Riedel, G.; Rutgers, E.H.; De Vries, C.; Postema, F.; Venema, B.J. Enhanced neuronal activity in selective brain regions of mice induced by whole body stimulation. Fed. Eur. Neurosci. Soc. Abstr. 2010, 5, R2. [Google Scholar]
- Raval, A.P.; Schatz, M.; Bhattacharya, P.; d’Adesky, N.; Rundek, T.; Dietrich, W.D.; Bramlett, H.M. Whole Body Vibration Therapy after Ischemia Reduces Brain Damage in Reproductively Senescent Female Rats. Int. J. Mol. Sci. 2018, 19, 2749. [Google Scholar] [CrossRef]
- Peng, G.; Yang, L.; Wu, C.Y.; Zhang, L.L.; Wu, C.Y.; Li, F.; Shi, H.W.; Hou, J.; Zhang, L.M.; Ma, X.; et al. Whole body vibration training improves depression-like behaviors in a rat chronic restraint stress model. Neurochem. Int. 2021, 142, 104926. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Pallone, G.; Annino, G.; Tancredi, V.; D’Arcangelo, G. Modulation of Synaptic Plasticity by Vibratory Training in Young and Old Mice. Brain Sci. 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Pallone, G.; Romagnoli, C.; Rinaldi, A.M.; Annino, G.; D’Arcangelo, G.; Tancredi, V. Whole Body Vibration Improves Brain and Musculoskeletal Health by Modulating the Expression of Tissue-Specific Markers: FNDC5 as a Key Regulator of Vibration Adaptations. Int. J. Mol. Sci. 2022, 23, 388. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, M.; Bonanni, R.; Cariati, I.; Frank, C.; D’Arcangelo, G. Amyloid Prefibrillar Oligomers: The Surprising Commonalities in Their Structure and Activity. Int. J. Mol. Sci. 2021, 22, 6435. [Google Scholar] [CrossRef] [PubMed]
- Regterschot, G.R.H.; Van Heuvelen, M.J.G.; Zeinstra, E.B.; Fuermaier, A.B.M.; Tucha, L.; Koerts, J.; Tucha, O.; Van Der Zee, E.A. Whole body vibration improves cognition in healthy young adults. PLoS ONE 2014, 9, e100506. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Cariati, I.; Masuelli, L.; Bei, R.; Tancredi, V.; Frank, C.; D’Arcangelo, G. Neurodegeneration in Niemann-Pick Type C Disease: An Updated Review on Pharmacological and Non-Pharmacological Approaches to Counteract Brain and Cognitive Impairment. Int. J. Mol. Sci. 2021, 22, 6600. [Google Scholar] [CrossRef]
- Boerema, A.S.; Heesterbeek, M.; Boersma, S.A.; Schoemaker, R.; de Vries, E.F.J.; van Heuvelen, M.J.G.; Van der Zee, E.A. Beneficial Effects of Whole Body Vibration on Brain Functions in Mice and Humans. Dose-Response 2018, 16, 1559325818811756. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Annino, G. Effects of Whole-Body Vibration on Motor Impairments in Patients with Neurological Disorders: A Systematic Review. Am. J. Phys. Med. Rehabil. 2019, 98, 1084–1098. [Google Scholar] [CrossRef]
- Annino, G.; Iellamo, F.; Palazzo, F.; Fusco, A.; Lombardo, M.; Campoli, F.; Padua, E. Acute changes in neuromuscular activity in vertical jump and flexibility after exposure to whole body vibration. Medicine 2017, 96, e7629. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, R.; Zheng, Y.; Xu, J.; Wu, Y.; Wang, X. Effect of Whole-Body Vibration Training on Muscle Activation for Individuals with Knee Osteoarthritis. BioMed Res. Int. 2021, 2021, 6671390. [Google Scholar] [CrossRef]
- Annino, G.; Alashram, A.R.; Alghwiri, A.A.; Romagnoli, C.; Messina, G.; Tancredi, V.; Padua, E.; Mercuri, N.B. Effect of segmental muscle vibration on upper extremity functional ability poststroke: A randomized controlled trial. Medicine 2019, 98, e14444. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Z.; Li, C.; Wang, Q. The effect of whole-body vibration exercise on postmenopausal women with osteoporosis: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e25606. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Goto, D. Effect of low-intensity whole-body vibration on bone defect repair and associated vascularization in mice. Med. Biol. Eng. Comput. 2017, 55, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Keijser, J.N.; van Heuvelen, M.J.G.; Nyakas, C.; Tóth, K.; Schoemaker, R.G.; Zeinstra, E.; van der Zee, E.A. Whole body vibration improves attention and motor performance in mice depending on the duration of the whole-body vibration session. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2017, 14, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Annino, G.; Scimeca, M.; Bonanno, E.; D’Arcangelo, G.; Tancredi, V. Dose-Response Effect of Vibratory Stimulus on Synaptic and Muscle Plasticity in a Middle-Aged Murine Model. Front. Physiol. 2021, 12, 678449. [Google Scholar] [CrossRef]
- Gnyubkin, V.; Guignandon, A.; Laroche, N.; Vanden-Bossche, A.; Malaval, L.; Vico, L. High-acceleration whole body vibration stimulates cortical bone accrual and increases bone mineral content in growing mice. J. Biomech. 2016, 49, 1899–1908. [Google Scholar] [CrossRef]
- Xie, L.; Jacobson, J.M.; Choi, E.S.; Busa, B.; Donahue, L.R.; Miller, L.M.; Rubin, C.T.; Judex, S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone 2006, 39, 1059–1066. [Google Scholar] [CrossRef]
- Vanleene, M.; Shefelbine, S.J. Therapeutic impact of low amplitude high frequency whole body vibrations on the osteogenesis imperfecta mouse bone. Bone 2013, 53, 507–514. [Google Scholar] [CrossRef]
- Minematsu, A.; Nishii, Y.; Imagita, H.; Takeshita, D.; Sakata, S. Whole-body vibration can attenuate the deterioration of bone mass and trabecular bone microstructure in rats with spinal cord injury. Spinal Cord 2016, 54, 597–603. [Google Scholar] [CrossRef]
- Li, Z.; Tan, C.; Wu, Y.; Ding, Y.; Wang, H.; Chen, W.; Zhu, Y.; Ma, H.; Yang, H.; Liang, W.; et al. Whole-body vibration and resistance exercise prevent long-term hindlimb unloading-induced bone loss: Independent and interactive effects. Eur. J. Appl. Physiol. 2012, 112, 3743–3753. [Google Scholar] [CrossRef]
- Chen, G.-X.; Zheng, S.; Qin, S.; Zhong, Z.-M.; Wu, X.-H.; Huang, Z.-P.; Li, W.; Ding, R.-T.; Yu, H.; Chen, J.-T. Effect of low-magnitude whole-body vibration combined with alendronate in ovariectomized rats: A random controlled osteoporosis prevention study. PLoS ONE 2014, 9, e96181. [Google Scholar] [CrossRef]
- Ruan, X.-Y.; Jin, F.-Y.; Liu, Y.-L.; Peng, Z.-L.; Sun, Y.-G. Effects of vibration therapy on bone mineral density in postmenopausal women with osteoporosis. Chin. Med. J. 2008, 121, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- ElDeeb, A.M.; Abdel-Aziem, A.A. Effect of Whole-Body Vibration Exercise on Power Profile and Bone Mineral Density in Postmenopausal Women with Osteoporosis: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Slatkovska, L.; Beyene, J.; Alibhai, S.M.H.; Wong, Q.; Sohail, Q.Z.; Cheung, A.M. Effect of whole-body vibration on calcaneal quantitative ultrasound measurements in postmenopausal women: A randomized controlled trial. Calcif. Tissue Int. 2014, 95, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Recker, R.; Cullen, D.; Ryaby, J.; McCabe, J.; McLeod, K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: A clinical trial assessing compliance, efficacy, and safety. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Marín-Cascales, E.; Alcaraz, P.E.; Ramos-Campo, D.J.; Martinez-Rodriguez, A.; Chung, L.H.; Rubio-Arias, J.Á. Whole-body vibration training and bone health in postmenopausal women: A systematic review and meta-analysis. Medicine 2018, 97, e11918. [Google Scholar] [CrossRef]
- Marazzi, S.; Kiper, P.; Palmer, K.; Agostini, M.; Turolla, A. Effects of vibratory stimulation on balance and gait in Parkinson’s disease: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2021, 57, 254–264. [Google Scholar] [CrossRef]
- Li, K.-Y.; Cho, Y.-J.; Chen, R.-S. The Effect of Whole-Body Vibration on Proprioception and Motor Function for Individuals with Moderate Parkinson Disease: A Single-Blind Randomized Controlled Trial. Occup. Ther. Int. 2021, 2021, 9441366. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Linek, P.; Noormohammadpour, P.; Mansournia, M.A.; Wolny, T.; Sikora, D. Morphological changes of the lateral abdominal muscles in adolescent soccer players with low back pain: A prospective cohort study. J. Sport Health Sci. 2020, 9, 614–619. [Google Scholar] [CrossRef]
- Ye, J.; Ng, G.; Yuen, K. Acute effects of whole-body vibration on trunk muscle functioning in young healthy adults. J. strength Cond. Res. 2014, 28, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.-S.; Wang, X.-D.; Xie, B.; Li, Z.-H.; Chen, B.-L.; Wang, X.-Q.; Zhu, Y. Sling exercise for chronic low back pain: A systematic review and meta-analysis. PLoS ONE 2014, 9, e99307. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.-A.; Abboud, J.; Dubois, J.-D.; Legault, E.; Descarreaux, M.; Henchoz, Y. Trunk neuromuscular responses to a single whole-body vibration session in patients with chronic low back pain: A cross-sectional study. J. Manip. Physiol. Ther. 2013, 36, 564–571. [Google Scholar] [CrossRef]
- Rittweger, J.; Just, K.; Kautzsch, K.; Reeg, P.; Felsenberg, D. Treatment of chronic lower back pain with lumbar extension and whole-body vibration exercise: A randomized controlled trial. Spine (Phila Pa 1976). 2002, 27, 1829–1834. [Google Scholar] [CrossRef]
- del Pozo-Cruz, B.; Hernández Mocholí, M.A.; Adsuar, J.C.; Parraca, J.A.; Muro, I.; Gusi, N. Effects of whole body vibration therapy on main outcome measures for chronic non-specific low back pain: A single-blind randomized controlled trial. J. Rehabil. Med. 2011, 43, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Bosco, C. The use of vibration as an exercise intervention. Exerc. Sport Sci. Rev. 2003, 31, 3–7. [Google Scholar] [CrossRef]
- Burke, D.; Gandevia, S.C. The human muscle spindle and its fusimotor control. In Neural Control Of Movement; Springer: Boston, MA, USA, 1995; pp. 19–25. [Google Scholar]
- Belavý, D.L.; Armbrecht, G.; Gast, U.; Richardson, C.A.; Hides, J.A.; Felsenberg, D. Countermeasures against lumbar spine deconditioning in prolonged bed rest: Resistive exercise with and without whole body vibration. J. Appl. Physiol. 2010, 109, 1801–1811. [Google Scholar] [CrossRef]
- Stewart, V.H.; Saunders, D.H.; Greig, C.A. Responsiveness of muscle size and strength to physical training in very elderly people: A systematic review. Scand. J. Med. Sci. Sports 2014, 24, e1–e10. [Google Scholar] [CrossRef]
- Sonza, A. Human cutaneous mechanoreceptive afferents response after Whole Body Vibration: A literature review. Rev. Hosp. Univ. Pedro Ernesto 2018, 17, 35–38. [Google Scholar]
- Prager, J.P. What does the mechanism of spinal cord stimulation tell us about complex regional pain syndrome? Pain Med. 2010, 11, 1278–1283. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Sonza, A.; Sanada, L.S.; de Oliveira, L.R.; Bernardo-Filho, M.; Sá-Caputo, D.D.C.D.; Zaro, M.A.; Achaval, M. Whole-body vibration mediates mechanical hypersensitivity through Aβ-fiber and C-fiber thermal sensation in a chronic pain model. Exp. Biol. Med. 2021, 246, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Pallone, G.; Palmieri, M.; Cariati, I.; Bei, R.; Masuelli, L.; D’Arcangelo, G.; Tancredi, V. Different continuous training modalities result in distinctive effects on muscle structure, plasticity and function. Biomed. Rep. 2020, 12, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Cariati, I.; Scimeca, M.; Pallone, G.; Bonanno, E.; Tancredi, V.; D’Arcangelo, G.; Frank, C. Effects of short-term aerobic exercise in a mouse model of Niemann-Pick type C disease on synaptic and muscle plasticity. Ann. Dell’istituto Super. Sanità 2019, 55, 330–337. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’Arcangelo, G.; Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. [Google Scholar] [CrossRef]
- von Bohlen Und Halbach, O.; von Bohlen Und Halbach, V. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res. 2018, 373, 729–741. [Google Scholar] [CrossRef]
- Simão, A.P.; Mendonça, V.A.; Avelar, N.C.P.; da Fonseca, S.F.; Santos, J.M.; de Oliveira, A.C.C.; Tossige-Gomes, R.; Ribeiro, V.G.C.; Neves, C.D.C.; Balthazar, C.H.; et al. Whole Body Vibration Training on Muscle Strength and Brain-Derived Neurotrophic Factor Levels in Elderly Woman with Knee Osteoarthritis: A Randomized Clinical Trial Study. Front. Physiol. 2019, 10, 756. [Google Scholar] [CrossRef]
- Ribeiro, V.G.C.; Lacerda, A.C.R.; Santos, J.M.; Coelho-Oliveira, A.C.; Fonseca, S.F.; Prates, A.C.N.; Flor, J.; Garcia, B.C.C.; Tossige-Gomes, R.; Leite, H.R.; et al. Efficacy of Whole-Body Vibration Training on Brain-Derived Neurotrophic Factor, Clinical and Functional Outcomes, and Quality of Life in Women with Fibromyalgia Syndrome: A Randomized Controlled Trial. J. Healthc. Eng. 2021, 2021, 7593802. [Google Scholar] [CrossRef]
- Huang, D.; Yang, Z.; Wang, Z.; Wang, P.; Qu, Y. The macroscopic and microscopic effect of low-frequency whole-body vibration after cerebral ischemia in rats. Metab. Brain Dis. 2018, 33, 15–25. [Google Scholar] [CrossRef]
- Oberste, M.; Großheinrich, N.; Wunram, H.-L.; Graf, J.L.; Ziemendorff, A.; Meinhardt, A.; Fricke, O.; Mahabir, E.; Bender, S. Effects of a 6-week, whole-body vibration strength-training on depression symptoms, endocrinological and neurobiological parameters in adolescent inpatients experiencing a major depressive episode (the “Balancing Vibrations Study”): Study protocol for a randomized placebo-controlled trial. Trials 2018, 19, 347. [Google Scholar] [CrossRef]
- Zsuga, J.; Tajti, G.; Papp, C.; Juhasz, B.; Gesztelyi, R. FNDC5/irisin, a molecular target for boosting reward-related learning and motivation. Med. Hypotheses 2016, 90, 23–28. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Scimeca, M.; Rinaldi, A.M.; Marini, M.; Tarantino, U.; Tancredi, V. Exposure to Random Positioning Machine Alters the Mineralization Process and PTX3 Expression in the SAOS-2 Cell Line. Life 2022, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- LeBrasseur, N.K.; Schelhorn, T.M.; Bernardo, B.L.; Cosgrove, P.G.; Loria, P.M.; Brown, T.A. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, K.; Liu, D.; Yang, J.; Tan, L.; Zhang, D. Irisin enhances osteogenic differentiation of mouse MC3T3-E1 cells via upregulating osteogenic genes. Exp. Ther. Med. 2021, 21, 580. [Google Scholar] [CrossRef]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Fagnani, F.; Giombini, A.; Di Cesare, A.; Pigozzi, F.; Di Salvo, V. The effects of a whole-body vibration program on muscle performance and flexibility in female athletes. Am. J. Phys. Med. Rehabil. 2006, 85, 956–962. [Google Scholar] [CrossRef]
- Machado, A.; García-López, D.; González-Gallego, J.; Garatachea, N. Whole-body vibration training increases muscle strength and mass in older women: A randomized-controlled trial. Scand. J. Med. Sci. Sports 2010, 20, 200–207. [Google Scholar] [CrossRef]
- Verschueren, S.M.P.; Roelants, M.; Delecluse, C.; Swinnen, S.; Vanderschueren, D.; Boonen, S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 352–359. [Google Scholar] [CrossRef]
- Vissers, D.; Verrijken, A.; Mertens, I.; Van Gils, C.; Van de Sompel, A.; Truijen, S.; Van Gaal, L. Effect of long-term whole body vibration training on visceral adipose tissue: A preliminary report. Obes. Facts 2010, 3, 93–100. [Google Scholar] [CrossRef]
- Delecluse, C.; Roelants, M.; Verschueren, S. Strength increase after whole-body vibration compared with resistance training. Med. Sci. Sports Exerc. 2003, 35, 1033–1041. [Google Scholar] [CrossRef]
- Osawa, Y.; Oguma, Y.; Ishii, N. The effects of whole-body vibration on muscle strength and power: A meta-analysis. J. Musculoskelet. Neuronal Interact. 2013, 13, 380–390. [Google Scholar]
- Berschin, G.; Sommer, B.; Behrens, A.; Sommer, H.-M. Whole Body Vibration Exercise Protocol versus a Standard Exercise Protocol after ACL Reconstruction: A Clinical Randomized Controlled Trial with Short Term Follow-Up. J. Sports Sci. Med. 2014, 13, 580–589. [Google Scholar] [PubMed]
- Cochrane, D.J.; Legg, S.J.; Hooker, M.J. The short-term effect of whole-body vibration training on vertical jump, sprint, and agility performance. J. Strength Cond. Res. 2004, 18, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Rogan, S.; de Bruin, E.D.; Radlinger, L.; Joehr, C.; Wyss, C.; Stuck, N.-J.; Bruelhart, Y.; de Bie, R.A.; Hilfiker, R. Effects of whole-body vibration on proxies of muscle strength in old adults: A systematic review and meta-analysis on the role of physical capacity level. Eur. Rev. Aging Phys. Act. 2015, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Anwer, S.; Alghadir, A.; Zafar, H.; Al-Eisa, E. Effect of whole body vibration training on quadriceps muscle strength in individuals with knee osteoarthritis: A systematic review and meta-analysis. Physiotherapy 2016, 102, 145–151. [Google Scholar] [CrossRef]
- Rasti, E.; Rojhani-Shirazi, Z.; Ebrahimi, N.; Sobhan, M.R. Effects of whole body vibration with exercise therapy versus exercise therapy alone on flexibility, vertical jump height, agility and pain in athletes with patellofemoral pain: A randomized clinical trial. BMC Musculoskelet. Disord. 2020, 21, 705. [Google Scholar] [CrossRef]
- Gloeckl, R.; Schneeberger, T.; Leitl, D.; Reinold, T.; Nell, C.; Jarosch, I.; Kenn, K.; Koczulla, A.R. Whole-body vibration training versus conventional balance training in patients with severe COPD-a randomized, controlled trial. Respir. Res. 2021, 22, 138. [Google Scholar] [CrossRef]
- Guadarrama-Molina, E.; Barrón-Gámez, C.E.; Estrada-Bellmann, I.; Meléndez-Flores, J.D.; Ramírez-Castañeda, P.; Hernández-Suárez, R.M.G.; Menchaca-Pérez, M.; Salas-Fraire, O. Comparison of the effect of whole-body vibration therapy versus conventional therapy on functional balance of patients with Parkinson’s disease: Adding a mixed group. Acta Neurol. Belg. 2021, 121, 721–728. [Google Scholar] [CrossRef]
- van Heuvelen, M.J.G.; Rittweger, J.; Judex, S.; Sañudo, B.; Seixas, A.; Fuermaier, A.B.M.; Tucha, O.; Nyakas, C.; Marín, P.J.; Taiar, R.; et al. Reporting Guidelines for Whole-Body Vibration Studies in Humans, Animals and Cell Cultures: A Consensus Statement from an International Group of Experts. Biology 2021, 10, 965. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).