Preliminary Study of the Effects of Eccentric-Overload Resistance Exercise on Physical Function and Torque Capacity in Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Characteristics and Ethical Approval
2.2. Eccentric-Overload Resistance Exercise (ERE)
2.3. Physical Performance Measures
2.4. Maximal Voluntary Isometric Contraction (MVIC) and Isokinetic Testing
2.5. Performance Fatigability
2.6. Statistical Analysis
3. Results
3.1. Participant Demographics
3.2. Physical Function and Torque Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Plantinga, L.C.; Johansen, K.; Crews, D.C.; Shahinian, V.B.; Robinson, B.M.; Saran, R.; Burrows, N.R.; Williams, D.E.; Powe, N.R. Association of CKD With Disability in the United States. Am. J. Kidney Dis. 2011, 57, 212–227. [Google Scholar] [CrossRef]
- Painter, P.; Marcus, R.L. Assessing Physical Function and Physical Activity in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic Kidney Disease and Mortality Risk: A Systematic Review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The systemic nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Gollie, J.M.; Harris-Love, M.O.; Patel, S.S.; Argani, S. Chronic kidney disease: Considerations for monitoring skeletal muscle health and prescribing resistance exercise. Clin. Kidney J. 2018, 11, 822–831. [Google Scholar] [CrossRef]
- Carrero, J.J.; Johansen, K.L.; Lindholm, B.; Stenvinkel, P.; Cuppari, L.; Avesani, C.M. Screening For Muscle Wasting and Dysfunction in Patients with Chronic Kidney Disease. Kidney Int. 2016, 90, 53–66. [Google Scholar] [CrossRef]
- Campistol, J.M. Uremic myopathy. Kidney Int. 2002, 62, 1901–1913. [Google Scholar] [CrossRef]
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transpl. 2014, 29, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Bolton, C.F.; Remtulla, H.; Toth, B.; Bernardi, L.; Lindsay, R.M.; Maryniak, O.; Burton, S.R. Distinctive electrophysiological features of denervated muscle in uremic patients. J. Clin. Neurophysiol. 1997, 14, 539–542. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Kiernan, M.C. Uremic neuropathy: Clinical features and new pathophysiological insights. Muscle Nerve 2007, 35, 273–290. [Google Scholar] [CrossRef]
- Cotton, J.R.; Woodard, T.; Carter, N.W.; Knochel, J.P. Resting Skeletal Muscle Membrane Potential as an Index of Uremic Toxicity. J. Clin. Investig. 1979, 63, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Mitch, W.E. Mechanisms of Muscle Wasting in Chronic Kidney Disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Thome, T.; Salyers, Z.R.; Kumar, R.A.; Hahn, D.; Berru, F.N.; Ferreira, L.F.; Scali, S.T.; Ryan, T.E. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am. J. Physiol. Cell Physiol. 2019, 317, C701–C713. [Google Scholar] [CrossRef]
- Arnold, R.; Pussell, B.A.; Howells, J.; Grinius, V.; Kiernan, M.C.; Lin, C.S.-Y.; Krishnan, A.V. Evidence for a causal relationship between hyperkalaemia and axonal dysfunction in end-stage kidney disease. Clin. Neurophysiol. 2014, 125, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Phoon, R.K.S.; Pussell, B.A.; Charlesworth, J.A.; Bostock, H.; Kiernan, M.C. Altered motor nerve excitability in end-stage kidney disease. Brain 2005, 128, 2164–2174. [Google Scholar] [CrossRef]
- Petersen, A.C.; Leikis, M.J.; McMahon, L.P.; Kent, A.B.; Murphy, K.T.; Gong, X.; McKenna, M.J. Impaired exercise performance and muscle Na+,K+-pump activity in renal transplantation and haemodialysis patients. Nephrol. Dial. Transplant. 2012, 27, 2036–2043. [Google Scholar] [CrossRef]
- Tesch, P.A.; Fernandez-Gonzalo, R.; Lundberg, T.R. Clinical Applications of Iso-Inertial, Eccentric-Overload (YoYoTM) Resistance Exercise. Front. Physiol. 2017, 8, 241. [Google Scholar] [CrossRef]
- Fisher, J.P.; Ravalli, S.; Carlson, L.; Bridgeman, L.A.; Roggio, F.; Scuderi, S.; Maniaci, M.; Cortis, C.; Fusco, A.; Musumeci, G. The “Journal of Functional Morphology and Kinesiology” Journal Club Series: Utility and Advantages of the Eccentric Training through the Isoinertial System. J. Funct. Morphol. Kinesiol. 2020, 5, 6. [Google Scholar] [CrossRef]
- Vicens-Bordas, J.; Esteve, E.; Fort-Vanmeerhaeghe, A.; Bandholm, T.; Thorborg, K. Is inertial flywheel resistance training superior to gravity-dependent resistance training in improving muscle strength? A systematic review with meta-analyses. J. Sci. Med. Sport 2018, 21, 75–83. [Google Scholar] [CrossRef]
- Maroto-Izquierdo, S.; García-López, D.; Fernandez-Gonzalo, R.; Moreira, O.C.; González-Gallego, J.; de Paz, J.A. Skeletal Muscle Functional and Structural Adaptations After Eccentric Overload Flywheel Resistance Training: A Systematic Review and Meta-Analysis. J. Sci. Med. Sport 2017, 20, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, F.J.N.; de Villarreal, E.S. Does Flywheel Paradigm Training Improve Muscle Volume and Force? A Meta-Analysis. J. Strength Cond. Res. 2017, 31, 3177–3186. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalo, R.; Irimia, J.M.; Cusso, R.; Gustafsson, T.; Linné, A.; Tesch, P.A. Flywheel Resistance Exercise to Maintain Muscle Oxidative Potential During Unloading. Aviat. Space Environ. Med. 2014, 85, 694–699. [Google Scholar] [CrossRef] [PubMed]
- KDIGO. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Suchomel, T.J.; Wagle, J.P.; Douglas, J.; Taber, C.B.; Harden, M.; Haff, G.G.; Stone, M.H. Implementing Eccentric Resistance Training—Part 1: A Brief Review of Existing Methods. J. Funct. Morphol. Kinesiol. 2019, 4, 38. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–561. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Studenski, S. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Liu, C.K.; Lyass, A.; Massaro, J.M.; D’Agostino, R.B.; Fox, C.S.; Murabito, J.M. Chronic Kidney Disease Defined by Cystatin C Predicts Mobility Disability and Changes in Gait Speed: The Framingham Offspring Study. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2014, 69A, 301–307. [Google Scholar] [CrossRef]
- Roshanravan, B.; Robinson-Cohen, C.; Patel, K.V.; Ayers, E.; Littman, A.J.; de Boer, I.H.; Ikizler, T.A.; Himmelfarb, J.; Katzel, L.I.; Kestenbaum, B.; et al. Association between Physical Performance and All-Cause Mortality in CKD. J. Am. Soc. Nephrol. 2013, 24, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Painter, P.; Marcus, R. Physical function and gait speed in patients with chronic kidney disease. Nephrol. Nurs. J. 2013, 40, 529–538. [Google Scholar] [PubMed]
- Joo, Y.S.; Jhee, J.H.; Kim, H.-W.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; Park, J.T. Physical performance and chronic kidney disease development in elderly adults: Results from a nationwide cohort study. Aging 2020, 12, 17393–17417. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C.; et al. Added Value of Physical Performance Measures in Predicting Adverse Health-Related Events: Results from the Health, Aging and Body Composition Study: Physical performance and prediction of events. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Painter, P.; Roshanravan, B. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 615–623. [Google Scholar] [CrossRef]
- Lattanzio, F.; Corsonello, A.; Abbatecola, A.M.; Volpato, S.; Pedone, C.; Pranno, L.; Laino, I.; Garasto, S.; Corica, F.; Passarino, G.; et al. Relationship Between Renal Function and Physical Performance in Elderly Hospitalized Patients. Rejuvenation Res. 2012, 15, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Vasunilashorn, S.; Coppin, A.K.; Patel, K.V.; Lauretani, F.; Ferrucci, L.; Bandinelli, S.; Guralnik, J.M. Use of the Short Physical Performance Battery Score to Predict Loss of Ability to Walk 400 Meters: Analysis From the InCHIANTI Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64A, 223–229. [Google Scholar] [CrossRef]
- Van Kan, G.A.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette-Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Bohannon, R.W. Comfortable and maximum walking speed of adults aged 20—79 years: Reference values and determinants. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef]
- Bohannon, R.W. Reference Values for the Five-Repetition Sit-to-Stand Test: A Descriptive Meta-Analysis of Data from Elders. Percept Mot. Ski. 2006, 103, 215–222. [Google Scholar] [CrossRef]
- Onambélé, G.L.; Maganaris, C.N.; Mian, O.S.; Tam, E.; Rejc, E.; McEwan, I.M.; Narici, M.V. Neuromuscular and balance responses to flywheel inertial versus weight training in older persons. J. Biomech. 2008, 41, 3133–3138. [Google Scholar] [CrossRef] [PubMed]
- Sañudo, B.; de Hoyo, M.; McVeigh, J.G. Improved Muscle Strength, Muscle Power, and Physical Function after Flywheel Resistance Training in Healthy Older Adults: A Randomized Controlled Trial. J. Strength Cond. Res. 2020. [Google Scholar] [CrossRef]
- Kowalchuk, K.; Butcher, S. Eccentric Overload Flywheel Training in Older Adults. J. Funct. Morphol. Kinesiol. 2019, 4, 61. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults: Meaningful Change and Performance. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Izquierdo, M.; Ibañez, J.; Calbet, J.; González-Izal, M.; Navarro-Amézqueta, I.; Granados, C.; Malanda, A.; Idoate, F.; González-Badillo, J.; Häkkinen, K.; et al. Neuromuscular Fatigue after Resistance Training. Int. J. Sports Med. 2009, 30, 614–623. [Google Scholar] [CrossRef]
- Behm, D.G.; St-Pierre, D.M.M. Effects of fatigue duration and muscle type on voluntary and evoked contractile properties. J. Appl. Physiol. 1997, 82, 1654–1661. [Google Scholar] [CrossRef]
- Enoka, R.M.; Stuart, D.G. Neurobiology of muscle fatigue. J. Appl. Physiol. 1992, 72, 1631–1648. [Google Scholar] [CrossRef]
- Leikis, M.J. Exercise Performance Falls over Time in Patients with Chronic Kidney Disease Despite Maintenance of Hemoglobin Concentration. Clin. J. Am. Soc. Nephrol. 2006, 1, 488–495. [Google Scholar] [CrossRef]
- Krüger, R.L.; Aboodarda, S.J.; Samozino, P.; Rice, C.L.; Millet, G.Y. Isometric versus Dynamic Measurements of Fatigue: Does Age Matter? A Meta-analysis. Med. Sci. Sports Exerc. 2018, 50, 2132–2144. [Google Scholar] [CrossRef]
- Thorstensson, A.; Karlsson, J. Fatiguability and Fibre Composition of Human Skeletal Muscle. Acta Physiol. Scand. 1976, 98, 318–322. [Google Scholar] [CrossRef]
- Martinez-Aranda, L.M.; Fernandez-Gonzalo, R. Effects of inertial setting on power, force, work and eccentric overload during flywheel resistance exercise in women and men. J. Strength Cond. Res. 2016, 31, 1653–1661. [Google Scholar] [CrossRef]
- Carroll, K.M.; Wagle, J.P.; Sato, K.; Taber, C.B.; Yoshida, N.; Bingham, G.E.; Stone, M.H. Characterising overload in inertial flywheel devices for use in exercise training. Sports Biomech. 2019, 18, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Sabido, R.; Hernández-Davó, J.L.; Pereyra-Gerber, G.T. Influence of Different Inertial Loads on Basic Training Variables During the Flywheel Squat Exercise. In.t J. Sports Physiol. Perform. 2018, 13, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Painter, P. Exercise in Individuals with CKD. Am. J. Kidney Dis. 2012, 59, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Heiwe, S.; Jacobson, S.H. Exercise Training in Adults with CKD: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2014, 64, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Cheema, B.S.; Chan, D.; Fahey, P.; Atlantis, E. Effect of Progressive Resistance Training on Measures of Skeletal Muscle Hypertrophy, Muscular Strength and Health-Related Quality of Life in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Sports Med. 2014, 44, 1125–1138. [Google Scholar] [CrossRef]
- Chan, D.; Cheema, B.S. Progressive Resistance Training in End-Stage Renal Disease: Systematic Review. Am. J. Nephrol. 2016, 44, 32–45. [Google Scholar] [CrossRef]
- Smart, N.A.; Williams, A.D.; Levinger, I.; Selig, S.; Howden, E.; Coombes, J.S.; Fassett, R.G. Exercise & Sports Science Australia (ESSA) Position Statement on Exercise and Chronic Kidney Disease. J. Sci. Med. Sport 2013, 16, 406–411. [Google Scholar] [CrossRef]
- Barcellos, F.C.; Santos, I.S.; Umpierre, D.; Bohlke, M.; Hallal, P.C. Effects of Exercise in the Whole Spectrum of Chronic Kidney Disease: A Systematic Review. Clin. Kidney J. 2015, 8, 753–765. [Google Scholar] [CrossRef]
- Gould, D.W.; Graham-Brown, M.P.; Watson, E.L.; Viana, J.L.; Smith, A.C. Physiological Benefits of Exercise in Pre-Dialysis Chronic Kidney Disease: Exercise in Chronic Kidney Disease. Nephrology 2014, 19, 519–527. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Keith, D.S.; Nichols, G.A.; Gullion, C.M.; Brown, J.B.; Smith, D.H. Longitudinal Follow-up and Outcomes Among a Population With Chronic Kidney Disease in a Large Managed Care Organization. Arch. Intern. Med. 2004, 164, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Farrington, K.; Covic, A.; Nistor, I.; Aucella, F.; Clyne, N.; De Vos, L.; Findlay, A.; Fouque, D.; Grodzicki, T.; Iyasere, O.; et al. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR < 45 mL/min/1.73 m2): A summary document from the European Renal Best Practice Group. Nephrol. Dial. Transplant. 2017, 32, 9–16. [Google Scholar] [CrossRef] [PubMed]
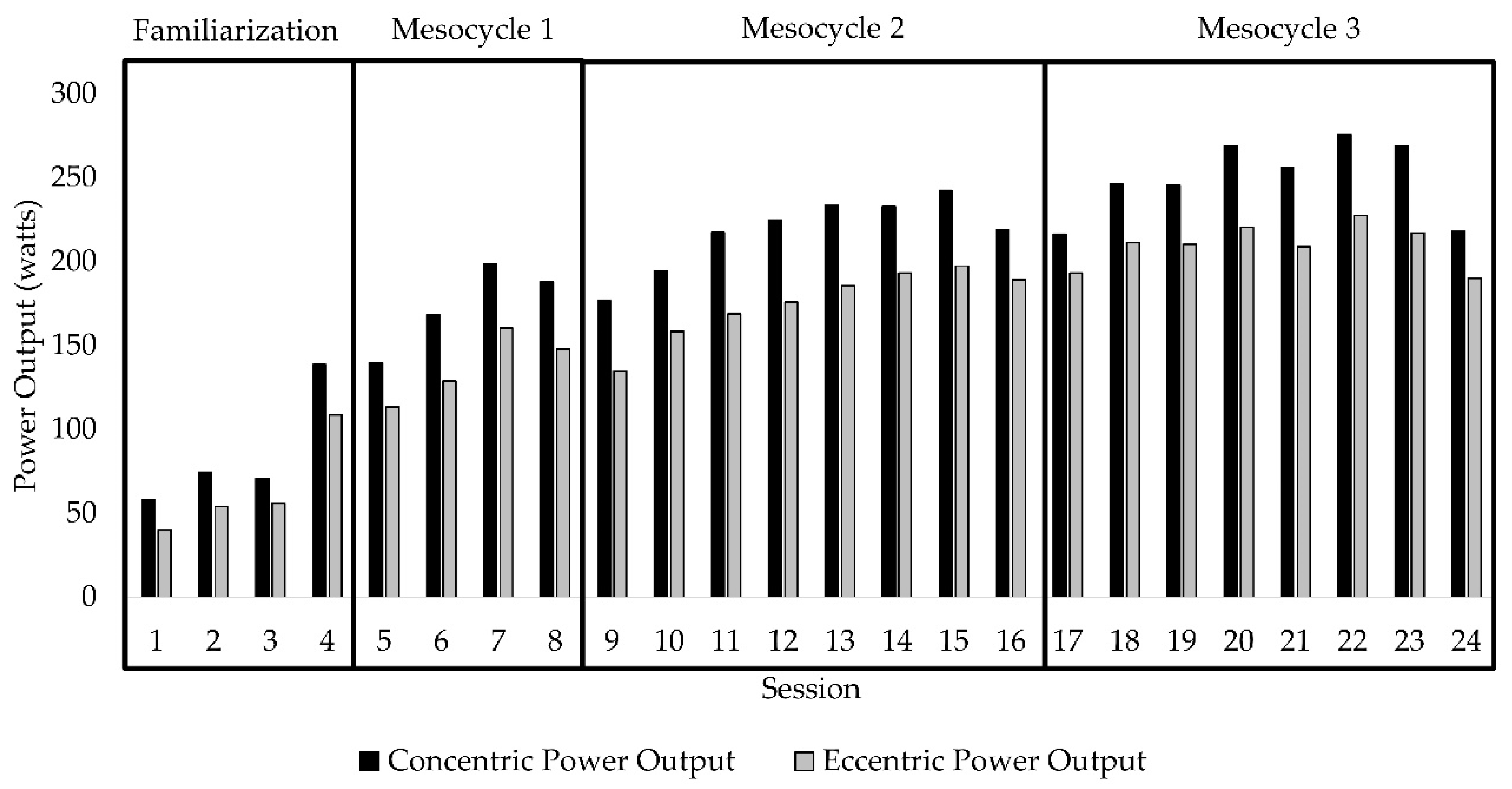

| Familiarization | Mesocycle 1 | Mesocycle 2 | Mesocycle 3 | |
|---|---|---|---|---|
| Sessions | 1−4 | 5−8 | 9−16 | 17−24 |
| Volume (sets × reps) | 2 × 12 | 3 × 12 | 3 × 12 | 3 × 12 |
| Mean Intensity (inertial load kg⋅m−2) | N/A | 0.028 | 0.036 | 0.045 |
| Rest between sets | 60 s | 60 s | 60 s | 60 s |
| Pre-Test | Post-Test | Reference Range | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 68.8 (57−78) | ||
| Sex (n) | Male (n = 4) | ||
| Body Mass Index (BMI) (kg/m2) | 34.1 (24.5−40.8) | 34.3 (23.6−40.3) | |
| Blood Pressure Medication (n) | 2.8 (2.0−4.0) | 2.8 (2.0−4.0) | |
| Laboratory Values | |||
| eGFR (mL/kg/1.73 m2 BSA) | 37.6 (13.3−55.2) | 38.0 (14.3−59.8) | >60.0 |
| Hemoglobin (Hgb) (g/dL) | 12.1 (10.7−13.6) | 11.9 (10.3−13.6) | 13.2−17.3 |
| Potassium (K) (mEq/L) | 4.4 (4.0−4.7) | 4.6 (4.1−5.1) | 3.5−5.3 |
| Bicarbonate (HCO3) (mEq/L) | 26.0 (24.0−29.0) | 26.3 (21.0−31.0) | 21.0−31.0 |
| Blood Urea Nitrogen (BUN) (mg/dL) | 33.3 (10.0−69.0) | 35.8 (10.0−61.0) | 6.0−23.0 |
| Creatinine (mg/dL) | 2.8 (1.5−5.1) | 2.8 (1.4−4.8) | 0.7−1.5 |
| Calcium (Ca) (mg/dL) | 9.0 (7.8−9.8) | 9.1 (7.9−10.1) | 8.9−10.5 |
| Albumin (Alb) (g/dL) | 4.2 (3.6−4.7) | 4.1 (3.6−4.6) | 3.7−5.0 |
| Pre-Test | Post-Test | % Change | |
|---|---|---|---|
| Functional Outcomes | |||
| Short Physical Performance Battery (SPPB) | 9.5 (8−12) | 9.8 (9−11) | +2.6 |
| Five repetitions of Sit-to-Stand (STS) (s) | 16.1 (10.3−21.8) | 13.6 (11.5−15.5) | −15.8 |
| Gait speed (m/s) | 1.0 (0.8−1.5) | 0.90 (0.7−1.2) | −10.1 |
| Timed Up and Go (TUG) (s) | 11.5 (9.6−13.8) | 10.8 (8.6−13.3) | −6.1 |
| Strength Outcomes | |||
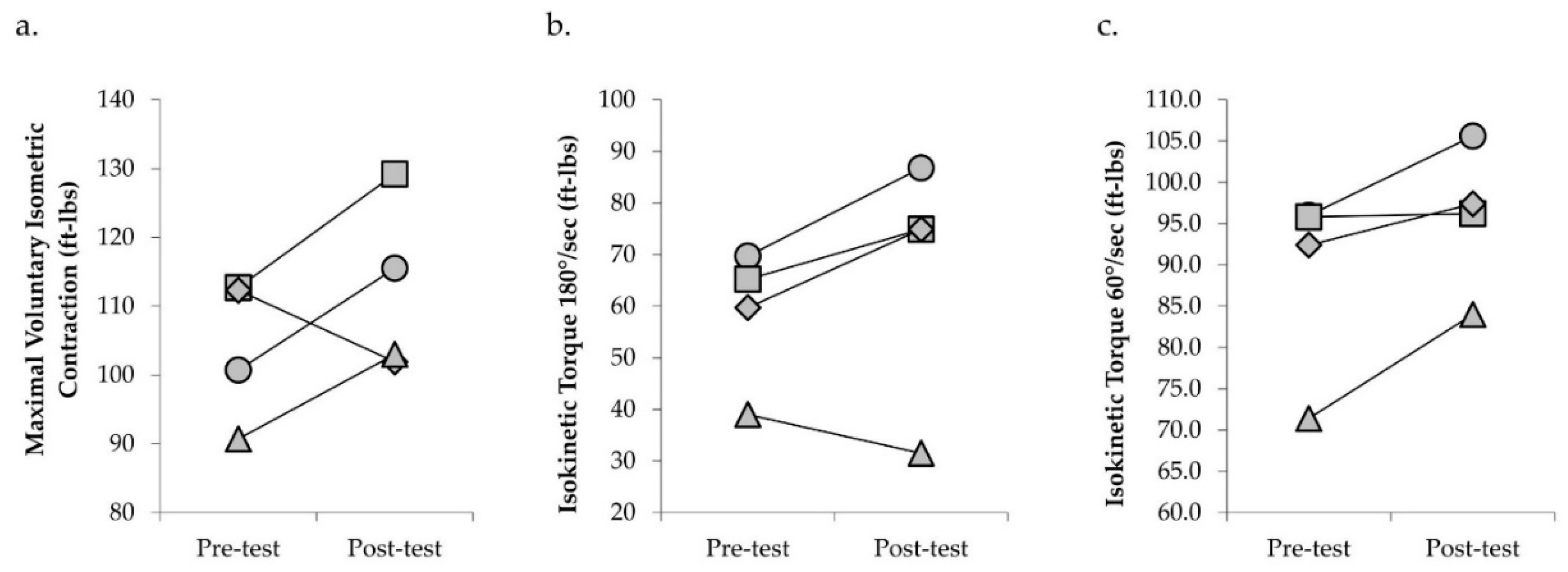
| Knee Extensor Isometric Strength (ft-lbs) | 104.1 (90.7−112.7) | 112.4 (101.9−129.2) | +8.0 |
| Knee Extensor Isokinetic Strength (180°/s) (ft-lbs) | 58.4 (39.0−69.7) | 67.0 (31.5−86.8) | +14.8 |
| Knee Extensor Isokinetic Strength (60°/s) (ft-lbs) | 88.9 (71.4−96.0) | 95.8 (84.0−105.6) | +7.8 |
| Performance Fatigability Outcomes | |||
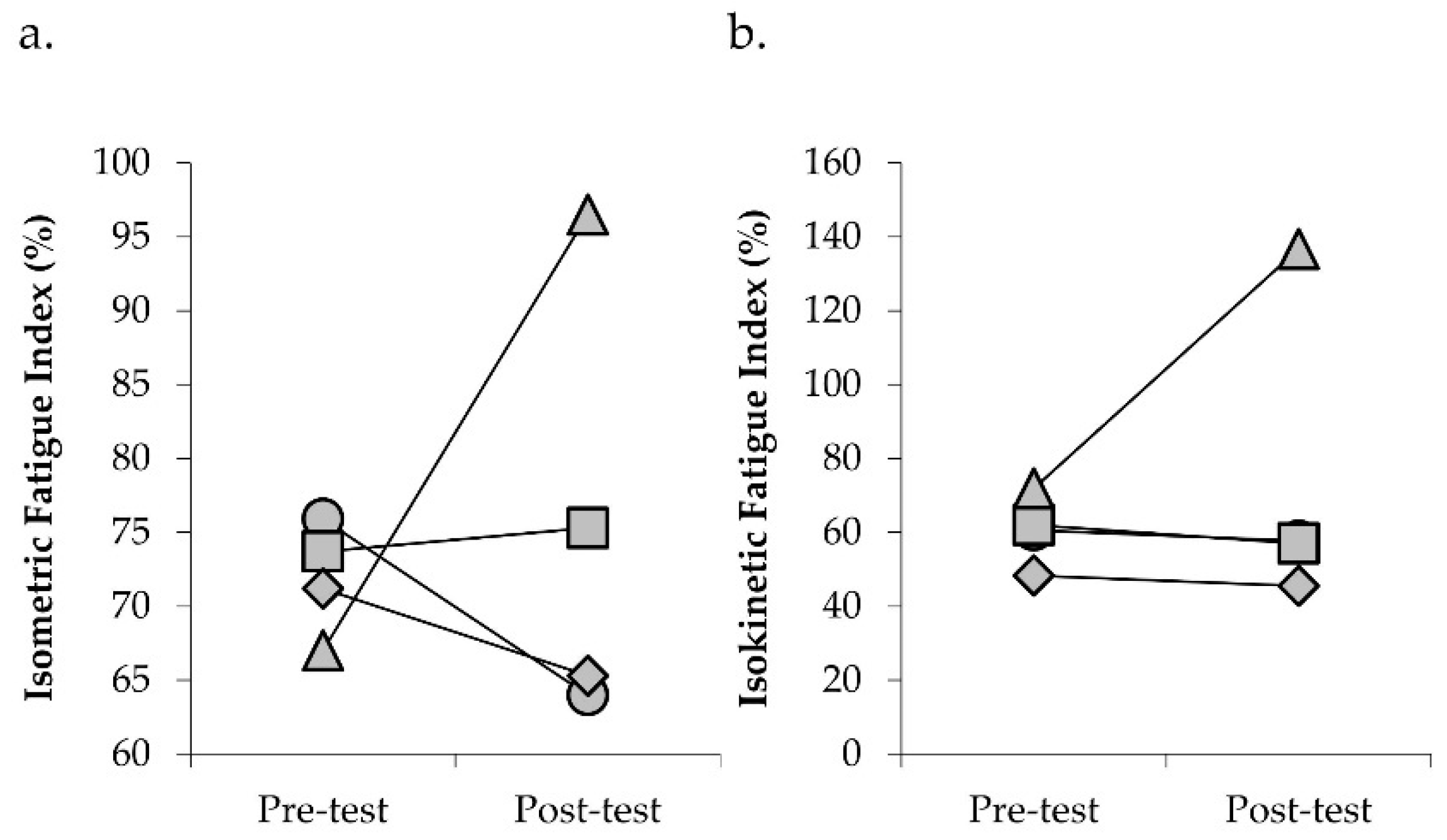
| Isometric FI (%) | 72.2 (67.0−75.9) | 75.9 (64−96.5) | +4.6 |
| Isokinetic FI (%) | 69.2 (48.3−71.9) | 74.8 (45.5−136.7) | +22.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gollie, J.M.; Patel, S.S.; Scholten, J.D.; Harris-Love, M.O. Preliminary Study of the Effects of Eccentric-Overload Resistance Exercise on Physical Function and Torque Capacity in Chronic Kidney Disease. J. Funct. Morphol. Kinesiol. 2020, 5, 97. https://doi.org/10.3390/jfmk5040097
Gollie JM, Patel SS, Scholten JD, Harris-Love MO. Preliminary Study of the Effects of Eccentric-Overload Resistance Exercise on Physical Function and Torque Capacity in Chronic Kidney Disease. Journal of Functional Morphology and Kinesiology. 2020; 5(4):97. https://doi.org/10.3390/jfmk5040097
Chicago/Turabian StyleGollie, Jared M., Samir S. Patel, Joel D. Scholten, and Michael O. Harris-Love. 2020. "Preliminary Study of the Effects of Eccentric-Overload Resistance Exercise on Physical Function and Torque Capacity in Chronic Kidney Disease" Journal of Functional Morphology and Kinesiology 5, no. 4: 97. https://doi.org/10.3390/jfmk5040097
APA StyleGollie, J. M., Patel, S. S., Scholten, J. D., & Harris-Love, M. O. (2020). Preliminary Study of the Effects of Eccentric-Overload Resistance Exercise on Physical Function and Torque Capacity in Chronic Kidney Disease. Journal of Functional Morphology and Kinesiology, 5(4), 97. https://doi.org/10.3390/jfmk5040097







