Research and Publication Ethics in Journal of Functional Morphology and Kinesiology
Abstract
1. Introduction
- Any facts that may represent a possible conflict of interest of the author(s) must be disclosed in the document prior to submission;
- Authors should critically show their research findings and propose an objective discussion of the significance of their results;
- To ensure the reproducibility of results, data and methods performed in the research must be presented in detail;
- Place your power analyses in the manuscript to obtain an "n sample" adapted to the study;
- The results must be clear, intuitive, and not duplicated in table/s and vice versa;
- Raw data have to be available for presentation to referees and/or editors of the journal if requested. Authors need to preserve raw data for a reasonable time after publication;
- A concise and explanatory cover letter must be included with each manuscript submission. It should describe the aim and content of the document according to the scope of the journal.
- Original Articles: These provide novel scientific information, whose quality and impact will be considered during the peer review process. Authors are encouraged to not divide their study in different related manuscripts, although Short Communications of preliminary, but significant, results will be taken into consideration.
- Reviews: These provide concise and precise updates on the latest progress made in a given area of research e.g., narrative review. Systematic reviews should follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [3].
- Observational study: This provides nonexperimental data and it should follow Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. See strobe-statement.org for statements and/or explanations.
- Meta-analyses: These should be performed according to PRISMA, Quality of Reporting of Meta-analyses (QUOROM), and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.
- Case reports: These should comprise an accurate report of the symptoms, signs, diagnosis, treatment, and outcomes of patients. Case reports usually show findings that shed new light on the possible pathogenesis of a disease or an adverse effect, as well as new therapeutic approaches [4].
1.1. Research Involving Human Subjects
1.2. Research Involving Animals
1.3. Research Involving Cell Lines
1.4. Research Involving Plants
1.5. Research Involving the Use of Bio-Banks
1.6. Research Involving Sports Medicine and Movement Sciences
1.7. Authorship
- -
- the planning and contribution of some component of the study (conception, experimental design, conduct, analysis, or findings interpretation) which led to the paper;
- -
- writing a draft of the paper or revising it for intellectual content;
- -
- final approval of the version to be published. All authors should review and approve the article before it is submitted for publication.
1.8. Conflicts of Interest
1.9. Clinical Trials Registration
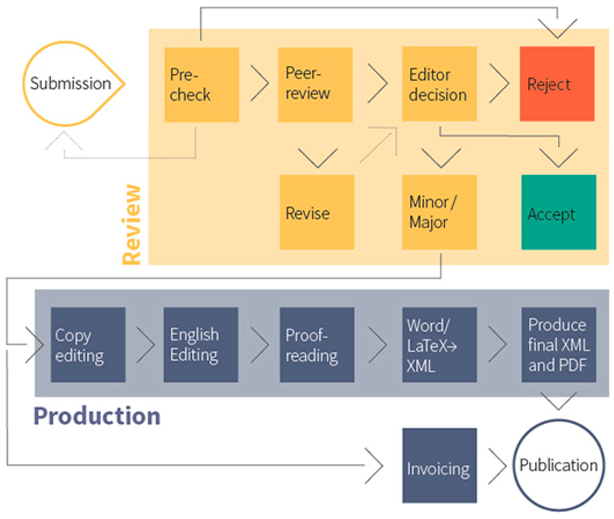
2. Summary
Funding
Conflicts of Interest
References
- Promoting Integrity in Scholarly Research and Its Publication. Available online: https://publicationethics.org (accessed on 12 May 2020).
- iThenticate: Plagiarism Detection Software. Available online: http://www.ithenticate.com (accessed on 12 May 2020).
- Chisari, E.; Pavone, V.; Sessa, G.; Ravalli, S.; Musumeci, G. Electromyostimulation and whole-body vibration effects in elder sarcopenic patients. MLTJ 2019, 9, 433–441. [Google Scholar] [CrossRef]
- Guglielmino, C.; Musumeci, G. Early Elbow Osteoarthritis in Competitive Enduro Motorcyclist. Scand. J. Med. Sci. Sports 2020. [Google Scholar] [CrossRef] [PubMed]
- Declaration of Helsinki Medical Research Involving Human Subjects. Available online: https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/ (accessed on 12 May 2020).
- Musumeci, G.; Magro, G.; Cardile, V.; Coco, M.; Marzagalli, R.; Castrogiovanni, P.; Imbesi, R.; Graziano, A.C.; Barone, F.; Di Rosa, M.; et al. Characterization of matrix metalloproteinase-2 and -9, ADAM-10 and N-cadherin expression in human glioblastoma multiforme. Cell Tissue Res. 2015, 362, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Home Office. Animals (Scientific Procedures) Act 1986. Code of Practice for the Housing and Care of Animals Used in Scientific Procedures. Available online: http://www.official-documents.gov.uk/document/hc8889/hc01/0107/0107.pdf (accessed on 12 May 2020).
- Di Rosa, M.; Szychlinska, M.A.; Tibullo, D.; Malaguarnera, L.; Musumeci, G. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur. J. Histochem. 2014, 58, 2423. [Google Scholar] [PubMed]
- Szychlinska, M.A.; Trovato, F.M.; Di Rosa, M.; Malaguarnera, L.; Puzzo, L.; Leonardi, R.; Castrogiovanni, P.; Musumeci, G. Co-Expression and Co-Localization of Cartilage Glycoproteins CHI3L1 and Lubricin in Osteoarthritic Cartilage: Morphological, Immunohistochemical and Gene Expression Profiles. Int. J. Mol. Sci. 2016, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- ARRIVE Guidelines. Available online: https://www.nc3rs.org.uk/arrive-guidelines (accessed on 12 May 2020).
- Castrogiovanni, P.; Di Rosa, M.; Ravalli, S.; Castorina, A.; Guglielmino, C.; Imbesi, R.; Vecchio, M.; Drago, F.; Szychlinska, M.A.; Musumeci, G. Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key. Int. J. Mol. Sci. 2019, 20, 511. [Google Scholar] [CrossRef] [PubMed]
- D′Amico, A.G.; Maugeri, G.; Rasà, D.M.; La Cognata, V.; Saccone, S.; Federico, C.; Cavallaro, S.; D′Agata, V. NAP counteracts hyperglycemia/hypoxia induced retinal pigment epithelial barrier breakdown through modulation of HIFs and VEGF expression. J. Cell Physiol. 2018, 233, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Calabrese, G.; Ravalli, S.; Parrinello, N.L.; Forte, S.; Castrogiovanni, P.; Pricoco, E.; Imbesi, R.; Castorina, S.; Leonardi, R.; et al. Cycloastragenol as an Exogenous Enhancer of Chondrogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. A Morphological Study. Cells 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D′Amico, A.G.; Rasà, D.M.; Federico, C.; Saccone, S.; Morello, G.; La Cognata, V.; Cavallaro, S.; D′Agata, V. Molecular mechanisms involved in the protective effect of pituitary adenylate cyclase-activating polypeptide in an in vitro model of amyotrophic lateral sclerosis. J. Cell Physiol. 2019, 234, 5203–5214. [Google Scholar] [CrossRef] [PubMed]
- Convention on Biological Diversity. Available online: https://www.cbd.int (accessed on 12 May 2020).
- Convention on International Trade in Endangered Species of Wild Fauna and Flora. Available online: https://www.cites.org/eng/disc/what.php (accessed on 12 May 2020).
- Szychlinska, M.A.; Castrogiovanni, P.; Trovato, F.M.; Nsir, H.; Zarrouk, M.; Lo Furno, D.; Di Rosa, M.; Imbesi, R.; Musumeci, G. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 2019, 58, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, M.J. Ethical Legal and Social Issues of Biobanking: Past, Present, and Future. Biopreserv. Biobank. 2017, 15, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Padulo, J.; Oliva, F.; Frizziero, A.; Maffulli, N. Muscles, Ligaments and Tendons Journal—Basic principles and recommendations in clinical and field Science Research: 2016 Update. MLTJ 2016, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Padulo, J.; Oliva, F.; Frizziero, A.; Maffulli, N. Muscle, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research. MLTJ 2014, 3, 250–252. [Google Scholar] [PubMed]
- Musumeci, G. Physical Activity for Health—An Overview and an Update of the Physical Activity Guidelines of the Italian Ministry of Health. J. Funct. Morphol. Kinesiol. 2016, 1, 269–275. [Google Scholar] [CrossRef]
- International Committee of Medical Journal Editors (ICMJE) Guidelines. Available online: http://www.icmje.org (accessed on 12 May 2020).
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Simera, I.; Moher, D.; Hoey, J.; Schulz, K.F.; Altman, D.G. A catalogue of reporting guidelines for health research. Eur. J. Clin. Investig. 2010, 40, 35–53. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, G.; Musumeci, G. Research and Publication Ethics in Journal of Functional Morphology and Kinesiology. J. Funct. Morphol. Kinesiol. 2020, 5, 42. https://doi.org/10.3390/jfmk5020042
Maugeri G, Musumeci G. Research and Publication Ethics in Journal of Functional Morphology and Kinesiology. Journal of Functional Morphology and Kinesiology. 2020; 5(2):42. https://doi.org/10.3390/jfmk5020042
Chicago/Turabian StyleMaugeri, Grazia, and Giuseppe Musumeci. 2020. "Research and Publication Ethics in Journal of Functional Morphology and Kinesiology" Journal of Functional Morphology and Kinesiology 5, no. 2: 42. https://doi.org/10.3390/jfmk5020042
APA StyleMaugeri, G., & Musumeci, G. (2020). Research and Publication Ethics in Journal of Functional Morphology and Kinesiology. Journal of Functional Morphology and Kinesiology, 5(2), 42. https://doi.org/10.3390/jfmk5020042





