Intermittent Energy Restriction Attenuates the Loss of Fat Free Mass in Resistance Trained Individuals. A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
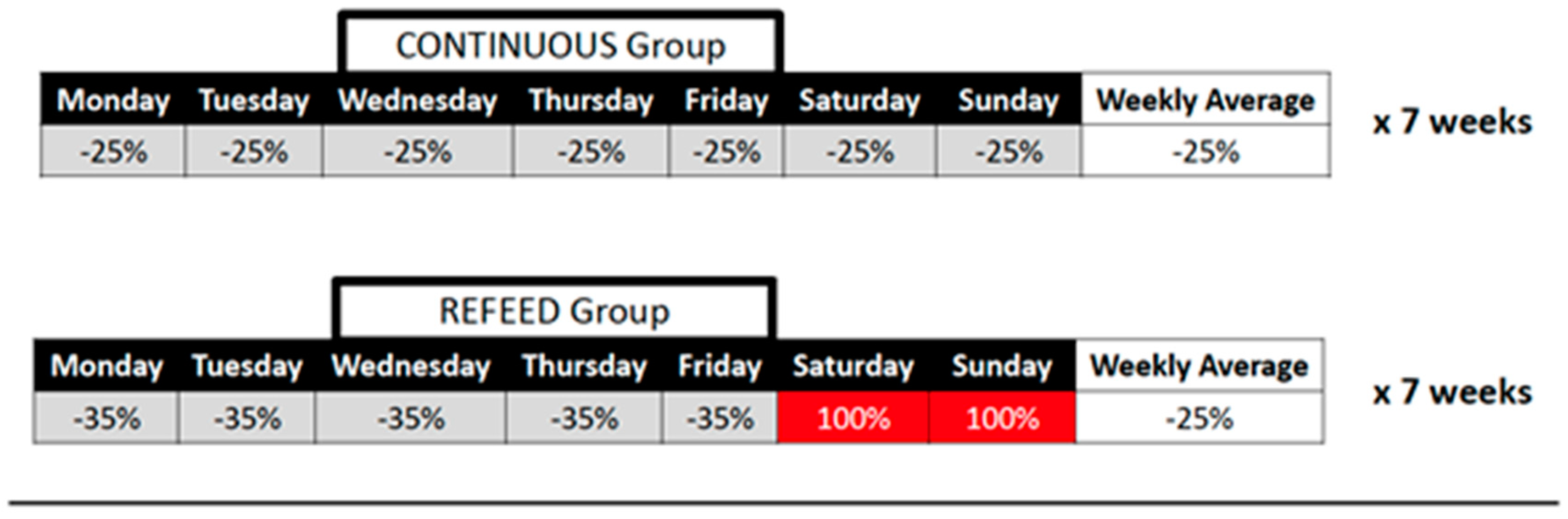
2.1. Study Design
2.2. Participants
2.3. Diet and Exercise Intervention
2.4. Baseline and Post-Intervention Testing
2.5. Total Body Water Assessment
2.6. Resting Metabolic Rate (RMR) Assessment
2.7. Body Composition Assessment
2.8. Fasting Leptin Assessment
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sainsbury, A.; Wood, R.; Seimon, R.V.; Hills, A.; King, N.A.; Gibson, A.A.; Byrne, N.M. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes. Rev. 2018, 19, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Leibel, R.L.; Rosenbaum, M.; Hirsch, J. Changes in Energy Expenditure Resulting from Altered Body Weight. N. Engl. J. Med. 1995, 332, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Smith-Ryan, A.E.; E Norton, L. Metabolic adaptation to weight loss: Implications for the athlete. J. Int. Soc. Sports Nutr. 2014, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Leibel, R.L. Adaptive thermogenesis in humans. Int. J. Obes. 2010, 34, S47–S55. [Google Scholar] [CrossRef]
- Knuth, N.D.; Johannsen, D.L.; Tamboli, R.A.; Marks-Shulman, P.A.; Huizenga, R.; Chen, K.Y.; Abumrad, N.N.; Ravussin, E.; Hall, K.D. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity 2014, 22, 2563–2569. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Byrne, N.; Sainsbury, A.; A King, N.; Hills, A.P.; Wood, R. Intermittent energy restriction improves weight loss efficiency in obese men: The MATADOR study. Int. J. Obes. 2017, 42, 129–138. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Donahoo, W.T. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef]
- LaRose, J.G.; Leahey, T.M.; Hill, J.O.; Wing, R.R. Differences in motivations and weight loss behaviors in young adults and older adults in the National Weight Control Registry. Obesity 2013, 21, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Garthe, I.; Raastad, T.; Refsnes, P.E.; Koivisto, A.E.; Sundgot-Borgen, C. Effect of two different weight-loss rates on body composition and strength and power-related performance in elite athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mettler, S.; Mitchell, N.; Tipton, K. Increased Protein Intake Reduces Lean Body Mass Loss during Weight Loss in Athletes. Med. Sci. Sports Exerc. 2010, 42, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.I.; Aguilar, D.; Conlin, L.; Vargas, A.; Schoenfeld, B.J.; Corson, A.; Gai, C.; Best, S.; Galvan, E.; Couvillion, K. Effects of High Versus Low Protein Intake on Body Composition and Maximal Strength in Aspiring Female Physique Athletes Engaging in an 8-Week Resistance Training Program. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 580–585. [Google Scholar] [CrossRef]
- Helms, E.; Aragon, A.A.; Fitschen, P.J. Evidence-based recommendations for natural bodybuilding contest preparation: Nutrition and supplementation. J. Int. Soc. Sports Nutr. 2014, 11, 20. [Google Scholar] [CrossRef]
- Chappell, A.; Simper, T.; Barker, M. Nutritional strategies of high level natural bodybuilders during competition preparation. J. Int. Soc. Sports Nutr. 2018, 15, 4. [Google Scholar] [CrossRef]
- Pardue, A.; Trexler, E.T.; Sprod, L.K. Case Study: Unfavorable But Transient Physiological Changes During Contest Preparation in a Drug-Free Male Bodybuilder. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 550–559. [Google Scholar] [CrossRef]
- Horner, N.K.; Lampe, J.W.; Patterson, R.E.; Neuhouser, M.L.; Beresford, S.A.; Prentice, R.L. Indirect calorimetry protocol development for measuring resting metabolic rate as a component of total energy expenditure in free-living postmenopausal women. J. Nutr. 2001, 131, 2215–2218. [Google Scholar] [CrossRef][Green Version]
- Vincent, W.J.; Weir, J.P. Statistics in Kinesiology, 4th ed.; Human Kinetics: Champaign, IL, USA, 2012; p. 81. [Google Scholar]
- Weinheimer, E.M.; Sands, L.; Campbell, W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 2010, 68, 375–388. [Google Scholar] [CrossRef]
- Chaston, T.B.; Dixon, J.; E O’Brien, P. Changes in fat-free mass during significant weight loss: A systematic review. Int. J. Obes. 2006, 31, 743–750. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Vislocky, L.M.; Carbone, J.W.; Altieri, N.; Konopelski, K.; Freake, H.C.; Anderson, J.M.; Ferrando, A.A.; Wolfe, R.R.; Rodriguez, N.R. Acute Energy Deprivation Affects Skeletal Muscle Protein Synthesis and Associated Intracellular Signaling Proteins in Physically Active Adults. J. Nutr. 2010, 140, 745–751. [Google Scholar] [CrossRef]
- Carbone, J.W.; Pasiakos, S.M.; Vislocky, L.M.; Anderson, J.M.; Rodriguez, N.R. Effects of short-term energy deficit on muscle protein breakdown and intramuscular proteolysis in normal-weight young adults. Appl. Physiol. Nutr. Metab. 2014, 39, 960–968. [Google Scholar] [CrossRef]
- Kistler, B.M.; Fitschen, P.J.; Ranadive, S.M.; Fernhall, B.; Wilund, K.R. Case Study: Natural Bodybuilding Contest Preparation. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Rossow, L.M.; Fukuda, D.; Fahs, C.A.; Loenneke, J.P.; Stout, J.R. Natural Bodybuilding Competition Preparation and Recovery: A 12-Month Case Study. Int. J. Sports Physiol. Perform. 2013, 8, 582–592. [Google Scholar] [CrossRef]
- Robinson, S.L.; Lambeth-Mansell, A.; Gillibrand, G.; Smith-Ryan, A.E.; Bannock, L. A nutrition and conditioning intervention for natural bodybuilding contest preparation: Case study. J. Int. Soc. Sports Nutr. 2015, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.; Sauter, E.R.; Whigham, L.; McClung, J.P.; Rood, J.; Carbone, J.W.; Combs, G.F.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.J.; McGlory, C.; Damas, F.; Mazara, N.; Baker, S.K.; Phillips, S.M. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2017, 32, 265–275. [Google Scholar] [CrossRef]
- Zachwieja, J.J.; Ezell, D.M.; Cline, A.D.; Ricketts, J.C.; Vicknair, P.C.; Schorle, S.M.; Ryan, D. Short-Term Dietary Energy Restriction Reduces Lean Body Mass but Not Performance in Physically Active Men and Women. Int. J. Sports Med. 2001, 22, 310–316. [Google Scholar] [CrossRef]
- Filaire, E.; Maso, F.; Degoutte, F.; Jouanel, P.; Lac, G. Food Restriction, Performance, Psychological State and Lipid Values in Judo Athletes. Int. J. Sports Med. 2001, 22, 454–459. [Google Scholar] [CrossRef]
- Roy, B.D.; Tarnopolsky, M.A.; MacDougall, J.D.; Fowles, J.; Yarasheski, K. Effect of glucose supplement timing on protein metabolism after resistance training. J. Appl. Physiol. 1997, 82, 1882–1888. [Google Scholar] [CrossRef]
- Børsheim, E.; Cree-Green, M.; Tipton, K.; Elliott, T.A.; Aarsland, A.; Wolfe, R.R. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J. Appl. Physiol. 2004, 96, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.P.; Tarpenning, K.M.; Marino, F.E. Liquid carbohydrate/essential amino acid ingestion during a short-term bout of resistance exercise suppresses myofibrillar protein degradation. Metabolism 2006, 55, 570–577. [Google Scholar] [CrossRef]
- Sparti, A.; Delany, J.; De La Bretonne, J.A.; Sander, G.E.; Bray, G.A. Relationship between resting metabolic rate and the composition of the fat-free mass. Metabolism 1997, 46, 1225–1230. [Google Scholar] [CrossRef]
- Müller, M.J.; Enderle, J.; Pourhassan, M.; Braun, W.; Eggeling, B.; Lagerpusch, M.; Glüer, C.-C.; Kehayias, J.J.; Kiosz, D.; Bosy-Westphal, A. Metabolic adaptation to caloric restriction and subsequent refeeding: The Minnesota Starvation Experiment revisited. Am. J. Clin. Nutr. 2015, 102, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Dirlewanger, M.; Di Vetta, V.; Guenat, E.; Battilana, P.; Seematter, G.; Schneiter, P.; Jéquier, E.; Tappy, L. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int. J. Obes. 2000, 24, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Rohrig, B.J.; Pettitt, R.W.; Pettitt, C.D.; Kanzenbach, T.L. Psychophysiological Tracking of a Female Physique Competitor through Competition Preparation. Int. J. Exerc. Sci. 2017, 10, 301–311. [Google Scholar]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-Term Persistence of Hormonal Adaptations to Weight Loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Seimon, R.; Roekenes, J.A.; Zibellini, J.; Zhu, B.; Gibson, A.A.; Hills, A.; Wood, R.; King, N.A.; Byrne, N.M.; Sainsbury, A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol. Cell. Endocrinol. 2015, 418, 153–172. [Google Scholar] [CrossRef]
- Klempel, M.C.; Bhutani, S.; FitzGibbon, M.; Freels, S.; Varady, K.A. Dietary and physical activity adaptations to alternate day modified fasting: Implications for optimal weight loss. Nutr. J. 2010, 9, 35. [Google Scholar] [CrossRef]
- Harvey, J.; Howell, A.; Morris, J.; Harvie, M. Intermittent energy restriction for weight loss: Spontaneous reduction of energy intake on unrestricted days. Food Sci. Nutr. 2018, 6, 674–680. [Google Scholar] [CrossRef]
- Davoodi, S.H.; Ajami, M.; Ayatollahi, S.A.; Dowlatshahi, K.; Javedan, G.; Pazoki-Toroudi, H.R. Calorie Shifting Diet Versus Calorie Restriction Diet: A Comparative Clinical Trial Study. Int. J. Prev. Med. 2014, 5, 447–456. [Google Scholar] [PubMed]
- Keogh, J.B.; Pedersen, E.; Petersen, K.; Clifton, P.M. Effects of intermittent compared to continuous energy restriction on short-term weight loss and long-term weight loss maintenance. Clin. Obes. 2014, 4, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Arguin, H.; Dionne, I.J.; Sénéchal, M.; Bouchard, D.R.; Carpentier, A.C.; Ardilouze, J.-L.; Tremblay, A.; Leblanc, C.; Brochu, M. Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women. Menopause 2012, 19, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Jeffery, R.W. Prescribed “Breaks” as a Means to Disrupt Weight Control Efforts. Obes. Res. 2003, 11, 287–291. [Google Scholar] [CrossRef]
| Variable | Refeed | Continuous |
|---|---|---|
| Male (#) | n = 6 | n = 8 |
| Female (#) | n = 7 | n = 6 |
| Age (yrs) | 26.3 ± 8.0 | 24.2 ± 3.7 |
| Height (cm) | 166.4 ± 9.9 | 172.2 ± 8.2 |
| Body Mass (kg) | 76.4 ± 15.6 | 83.1 ± 15.4 |
| RT Experience (yrs) | 4.9 ± 6.0 | 5.1 ± 4.7 |
| Refeed (n = 13) | Continuous (n = 14) | |||
|---|---|---|---|---|
| Nutrient Variable | Baseline | Diet | Baseline | Diet |
| Kcals | 2012 ± 452 | 1488 ± 371↓ | 2042 ± 452 | 1617 ± 402↓ |
| CHO (grams) | 217 ± 55 | 123 ± 52↓ | 217 ± 51 | 132 ± 37↓ |
| PRO (grams) | 109 ± 34 | 130 ± 23↑ | 109 ± 32 | 140 ± 26↑ |
| Fat (grams) | 79 ± 21 | 53 ± 15↓ | 82 ± 22 | 59 ± 20↓ |
| Kcal/kg body mass | 27 ± 5.5 | 20 ± 4.6↓ | 25 ± 4.0 | 19 ± 3.5↓ |
| CHO (g/kg/day) | 2.9 ± 0.7 | 1.7 ± 0.6↓ | 2.6 ± 0.5 | 1.6 ± 0.4↓ |
| PRO (g/kg/day) | 1.4 ± 0.4 | 1.8 ± 0.1↑ | 1.3 ± 0.3 | 1.7 ± 0.2↑ |
| Fat (g/kg/day) | 1.1 ± 0.3 | 0.7 ± 0.2↓ | 1.0 ± 0.2 | 0.7 ± 0.2↓ |
| CHO/PRO/Fat (%) | 43-22-35 | 33-35-32 | 43-21-36 | 33-34-33 |
| Refeed | Continuous | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Pre | Post | Change (CI) | ES | Pre | Post | Change (CI) | ES |
| Body weight (kg) | 76.4 ± 15.6 | 73.2 ± 13.8 * | −3.2 (−1.4; −5.1) | 0.22 | 83.1 ± 15.4 | 79.5 ± 15.0 # | −3.6 (−2.6; −4.6) | 0.24 |
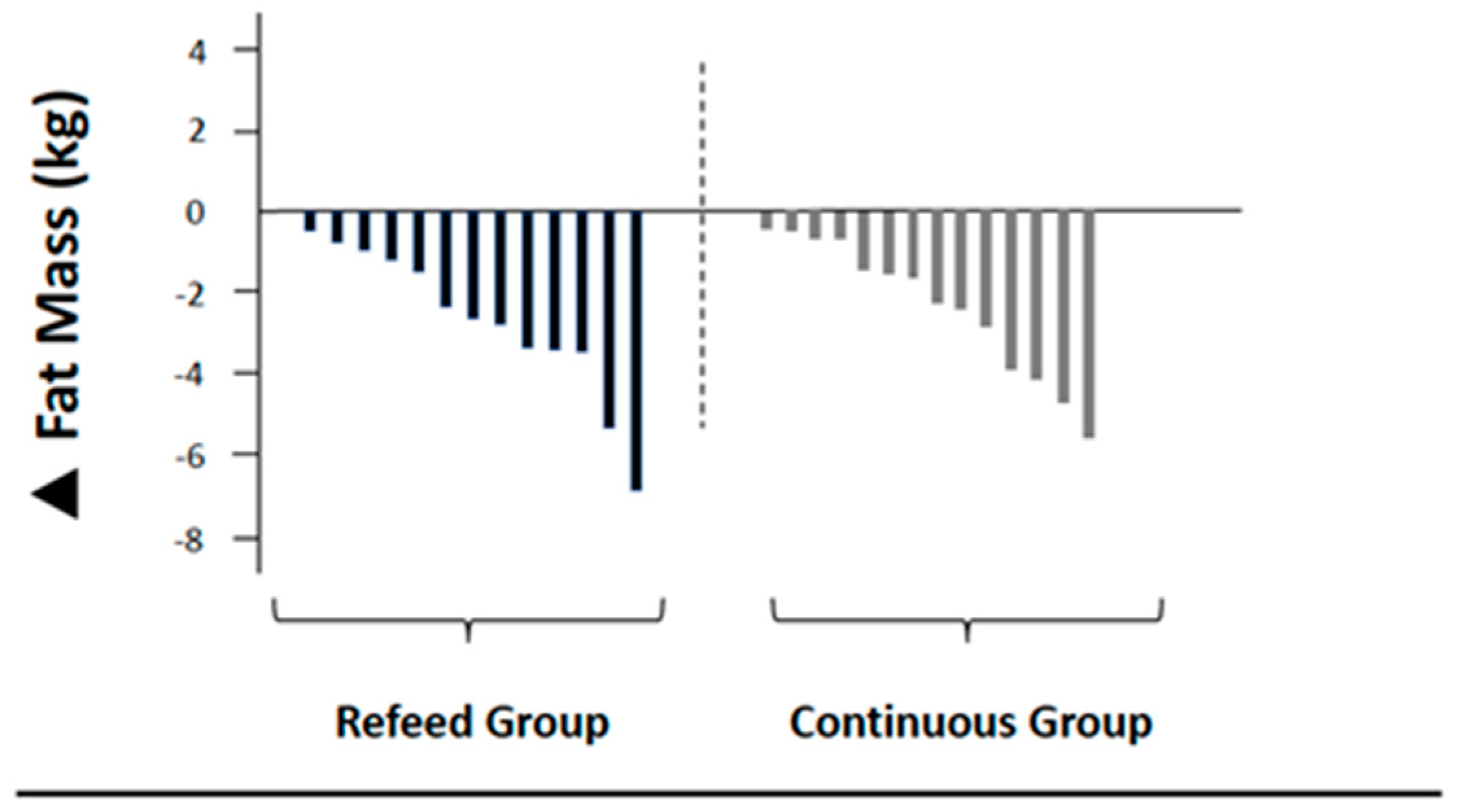
| Fat mass (kg) | 16.3 ± 4.0 | 13.5 ± 3.6 # | −2.8 (−1.7; −3.9) | 0.74 | 16.7 ± 4.5 | 14.4 ± 4.9 # | −2.3 (−1.4; −3.3) | 0.49 |
| Body fat (%) | 21.6 ± 4.6 | 18.8 ± 6.8 # | −2.8 (−1.9; −3.8) | 0.49 | 20.6 ± 6.1 | 18.6 ± 6.8 # | −2.0 (−1.1; −3.0) | 0.31 |
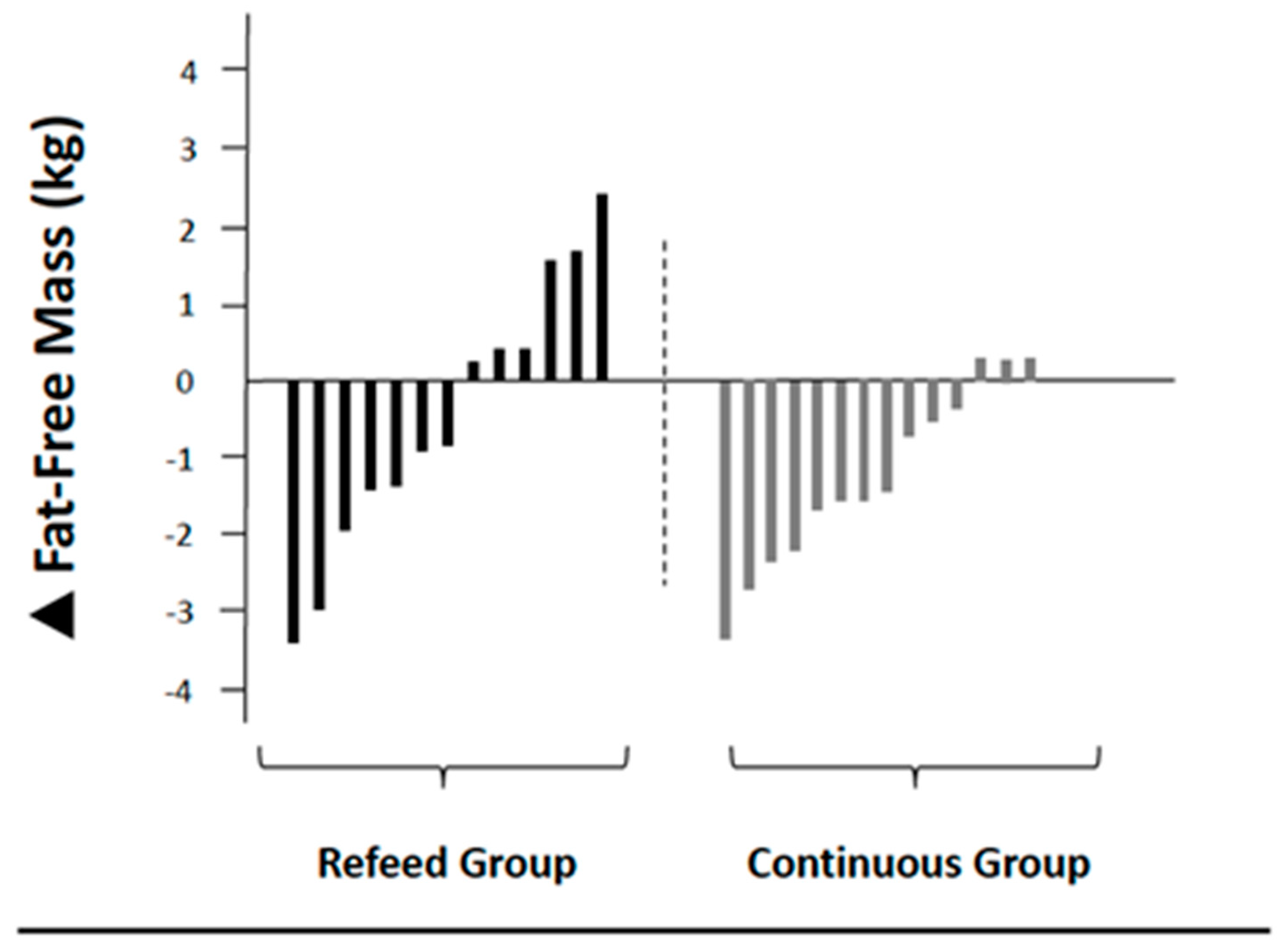
| FFM (kg) | 60.1 ± 13.8 | 59.7 ± 13.0 | −0.4 (−1.5; 0.6) | 0.03 | 66.4 ± 15.2 | 65.1 ± 15.2 * | −1.3 (−1.9; −0.6) | 0.09 |
| Dry FFM (kg) | 18.7 ± 5.0 | 18.5 ± 4.5 ∧ | −0.2 (−0.7; 0.3) | 0.04 | 21.9 ± 5.7 | 20.0 ± 5.7 ∧ | −1.9 (−2.7; −1.2) | 0.33 |
| RMR (kcals) | 1703 ± 294 | 1665 ± 270 | −38 (−141; 62) | 0.13 | 1867 ± 342 | 1789 ± 409 * | −78 (−139; −16) | 0.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, B.I.; Aguilar, D.; Colenso-Semple, L.M.; Hartke, K.; Fleming, A.R.; Fox, C.D.; Longstrom, J.M.; Rogers, G.E.; Mathas, D.B.; Wong, V.; et al. Intermittent Energy Restriction Attenuates the Loss of Fat Free Mass in Resistance Trained Individuals. A Randomized Controlled Trial. J. Funct. Morphol. Kinesiol. 2020, 5, 19. https://doi.org/10.3390/jfmk5010019
Campbell BI, Aguilar D, Colenso-Semple LM, Hartke K, Fleming AR, Fox CD, Longstrom JM, Rogers GE, Mathas DB, Wong V, et al. Intermittent Energy Restriction Attenuates the Loss of Fat Free Mass in Resistance Trained Individuals. A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology. 2020; 5(1):19. https://doi.org/10.3390/jfmk5010019
Chicago/Turabian StyleCampbell, Bill I., Danielle Aguilar, Lauren M. Colenso-Semple, Kevin Hartke, Abby R. Fleming, Carl D. Fox, Jaymes M. Longstrom, Gavin E. Rogers, David B. Mathas, Vickie Wong, and et al. 2020. "Intermittent Energy Restriction Attenuates the Loss of Fat Free Mass in Resistance Trained Individuals. A Randomized Controlled Trial" Journal of Functional Morphology and Kinesiology 5, no. 1: 19. https://doi.org/10.3390/jfmk5010019
APA StyleCampbell, B. I., Aguilar, D., Colenso-Semple, L. M., Hartke, K., Fleming, A. R., Fox, C. D., Longstrom, J. M., Rogers, G. E., Mathas, D. B., Wong, V., Ford, S., & Gorman, J. (2020). Intermittent Energy Restriction Attenuates the Loss of Fat Free Mass in Resistance Trained Individuals. A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology, 5(1), 19. https://doi.org/10.3390/jfmk5010019





