The Influence of Upper and Lower Extremity Strength on Performance-Based Sarcopenia Assessment Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Body Composition Analysis
2.3. Quantitative Strength Testing
2.4. Physical Performance Assessments
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Hand Grip Strength as a Proxy Measure for Lower Extremity Strength
4.2. The Association of Functional Performance with Hand Grip Strength and Lower Extremity Strength
4.3. Potential Uses of Lower Extremity Strength Assessment in the Management of Sarcopenia
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carvalho do Nascimento, P.R.; Poitras, S.; Bilodeau, M. How do we define and measure sarcopenia? Protocol for a systematic review. Syst. Rev. 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. Bethesda Md 1985 2003, 95, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Evans, W.J. Changes in skeletal muscle with aging: Effects of exercise training. Exerc. Sport Sci. Rev. 1993, 21, 65–102. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.P.; Rejeski, W.J.; Espeland, M.A.; Miller, M.E.; Church, T.S.; Fielding, R.A.; Gill, T.M.; Guralnik, J.M.; Newman, A.B.; Pahor, M. LIFE Study Investigators Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders pilot (LIFE-P). J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.-L.; Walston, J.D.; Fried, L.P.; Beamer, B.A. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: The women’s health and aging study. Arch. Intern. Med. 2011, 171, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. Sarcopenia and exercise “The State of the Art”. J. Funct. Morphol. Kinesiol. 2017, 2, 40. [Google Scholar] [CrossRef]
- Nicastro, H.; Zanchi, N.E.; Luz, C.R.D.; Lancha, A.H. Functional and morphological effects of resistance exercise on disuse-induced skeletal muscle atrophy. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2011, 44, 1070–1079. [Google Scholar] [CrossRef]
- Musumeci, G. The Use of vibration as physical exercise and therapy. J. Funct. Morphol. Kinesiol. 2017, 2, 17. [Google Scholar] [CrossRef]
- Dam, T.-T.; Peters, K.W.; Fragala, M.; Cawthon, P.M.; Harris, T.B.; McLean, R.; Shardell, M.; Alley, D.E.; Kenny, A.; Ferrucci, L.; et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Fielding, R.; Visser, M.; Loon, L.J.; Rolland, Y.; Orwoll, E.; Reid, K.; Boonen, S.; Dere, W.; Epstein, S.; et al. Tools in the assessment of sarcopenia. Calcif. Tissue Int. 2013, 93, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli, T.J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Choi, H.; Ha, Y.C. Mean hand grip strength and cut-off value for sarcopenia in korean adults using KNHANES VI. J. Korean Med. Sci. 2017, 32, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Alley, D.E.; Shardell, M.D.; Harris, T.B.; McLean, R.R.; Kiel, D.P.; Cawthon, P.M.; Dam, T.-T.L.; Ferrucci, L.; Guralnik, J.M.; et al. Comparison of Handgrip to Leg Extension Strength for Predicting Slow Gait Speed in Older Adults. J. Am. Geriatr. Soc. 2016, 64, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.S.; Oldaker, S.; Schrager, M.; Talbot, L.A.; Fozard, J.L.; Metter, E.J. Relationship between muscle strength and the time taken to complete a standardized walk-turn-walk test. J. Gerontol. Ser. Biol. Sci. Med. Sci. 2001, 56, B398–B404. [Google Scholar] [CrossRef]
- Martien, S.; Delecluse, C.; Boen, F.; Seghers, J.; Pelssers, J.; Van Hoecke, A.-S.; Van Roie, E. Is knee extension strength a better predictor of functional performance than handgrip strength among older adults in three different settings? Arch. Gerontol. Geriatr. 2015, 60, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Sinacore, D.R.; Host, H.H. The relationship of strength to function in the older adult. J. Gerontol. Ser. Biol. Sci. Med. Sci. 1995, 50, 55–59. [Google Scholar]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Avila, N.A.; Adams, B.; Zhou, J.; Seamon, B.; Ismail, C.; Zaidi, S.H.; Kassner, C.A.; Liu, F.; Blackman, M.R. The comparative associations of ultrasound and computed tomography estimates of muscle quality with physical performance and metabolic parameters in older men. J. Clin. Med. 2018, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Herning, M.M.; Morley, J.E. Low appendicular skeletal muscle mass (ASM) with limited mobility and poor health outcomes in middle-aged African Americans. J. Cachexia Sarcopenia Muscle 2013, 4, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.M.; Bürger, A.; Rickert, M.; Crispin, A.; Schulz, C.U. Grip strength in healthy Caucasian adults: Reference values. J. Hand Surg. 2008, 33, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O. Safety and efficacy of submaximal eccentric strength training for a subject with polymyositis. Arthritis Rheum. 2005, 53, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.; Lephart, S.; Karunakara, R. Reliability and precision of isokinetic strength and muscular endurance for the quadriceps and hamstrings. Int. J. Sports Med. 1997, 18, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Seamon, B.A.; Gonzales, T.I.; Hernandez, H.J.; Pennington, D.; Hoover, B.M. Eccentric exercise program design: A periodization model for rehabilitation applications. Front. Physiol. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Andrews, A.W.; Thomas, M.W. Walking speed: Reference values and correlates for older adults. J. Orthop. Sports Phys. Ther. 1996, 24, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Catlin, P.A.; Gage, K.; Gurucharri, K.; Robertson, R.; Stephen, K. Establishing the reliability and validity of measurements of walking time using the Emory Functional Ambulation Profile. Phys. Ther. 1999, 79, 1122–1133. [Google Scholar] [PubMed]
- Dos Santos, L.; Cyrino, E.S.; Antunes, M.; Santos, D.A.; Sardinha, L.B. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J. Cachexia Sarcopenia Muscle 2017, 8, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Glenn, J.M.; Binns, A. Predicting sarcopenia from functional measures among community-dwelling older adults. AGE 2016, 38, 22. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Bubela, D.J.; Magasi, S.R.; Wang, Y.-C.; Gershon, R.C. Sit-to-stand test: Performance and determinants across the age-span. Isokinet. Exerc. Sci. 2010, 18, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Reference values for the five-repetition sit-to-stand test: A descriptive meta-analysis of data from elders. Percept. Mot. Skills 2006, 103, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Reuben, D.B.; Siu, A.L. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J. Am. Geriatr. Soc. 1990, 38, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, M.M.; Pellecchia, G.L.; Schulman, M. Functional performance in community living older adults. J. Geriatr. Phys. Ther. 2003, 26, 14–22. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS; Sage: Los Angeles, CA, 2009; ISBN 978-1-84787-906-6. [Google Scholar]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2009; ISBN 978-0-13-171640-7. [Google Scholar]
- Alonso, A.C.; Ribeiro, S.M.; Luna, N.M.S.; Peterson, M.D.; Bocalini, D.S.; Serra, M.M.; Brech, G.C.; Greve, J.M.D.; Garcez-Leme, L.E. Association between handgrip strength, balance, and knee flexion/extension strength in older adults. PLoS ONE 2018, 13, e0198185. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.Y.A.; van Houwelingen, A.H.; Gussekloo, J.; Blom, J.W.; den Elzen, W.P.J. Comparison of quadriceps strength and handgrip strength in their association with health outcomes in older adults in primary care. AGE 2014, 36, 9714. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Trappenburg, M.C.; Hogrel, J.-Y.; McPhee, J.S.; Piasecki, M.; Sipila, S.; Salpakoski, A.; Butler-Browne, G.; Pääsuke, M.; et al. Handgrip Strength Cannot Be Assumed a Proxy for Overall Muscle Strength. J. Am. Med. Dir. Assoc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Felicio, D.C.; Pereira, D.S.; Assumpção, A.M.; de Jesus-Moraleida, F.R.; de Queiroz, B.Z.; da Silva, J.P.; de Brito Rosa, N.M.; Dias, J.M.D.; Pereira, L.S.M. Poor correlation between handgrip strength and isokinetic performance of knee flexor and extensor muscles in community-dwelling elderly women. Geriatr. Gerontol. Int. 2014, 14, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.D.M.; Buckner, S.L.; Bergstrom, H.C.; Cochrane, K.C.; Goldsmith, J.A.; Housh, T.J.; Johnson, G.O.; Schmidt, R.J.; Cramer, J.T. Reliability and relationships among handgrip strength, leg extensor strength and power, and balance in older men. Exp. Gerontol. 2014, 58, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Magasi, S.R.; Bubela, D.J.; Wang, Y.-C.; Gershon, R.C. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 2012, 46, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Rowe, P. An investigation of the association between grip strength and hip and knee joint moments in older adults. Arch. Gerontol. Geriatr. 2012, 54, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, D.; Marco, E.; Miralles, R.; Guillén-Solà, A.; Vázquez-Ibar, O.; Escalada, F.; Muniesa, J.M. Does gait speed contribute to sarcopenia case-finding in a postacute rehabilitation setting? Arch. Gerontol. Geriatr. 2015, 61, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Association of grip and knee extension strength with walking speed of older women receiving home-care physical therapy. J. Frailty Aging 2015, 4, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Davenport, T.E.; Benson, K.; Baker, S.; Gracey, C.; Rakocevic, G.; McElroy, B.; Dalakas, M.; Shrader, J.A.; Harris-Love, M.O. Lower extremity peak force and gait kinematics in individuals with inclusion body myositis. Arthritis Care Res. 2015, 67, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Liu, L.-K.; Peng, L.-N.; Lin, M.-H.; Chen, L.-K.; ILAS Research Group. Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: Results from the I-Lan longitudinal aging study. J. Am. Med. Dir. Assoc. 2013, 14, 528.e1–528.e7. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Murray, S.M.; Chapman, K.; Munro, B.; Tiedemann, A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M539–M543. [Google Scholar] [CrossRef] [PubMed]
- Netz, Y.; Ayalon, M.; Dunsky, A.; Alexander, N. “The multiple-sit-to-stand” field test for older adults: What does it measure? Gerontology 2004, 50, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Jaric, S. Muscle strength testing—Use of normalisation for body size. Sports Med. 2002, 32, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.R.; DeMets, D.L. Surrogate end points in clinical trials: Are we being misled? Ann. Intern. Med. 1996, 125, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Kennedy, E.H.; Roy, J.A.; Stephens-Shields, A.J.; Small, D.S.; Joffe, M.M. Surrogate markers for time-varying treatments and outcomes. Clin. Trials 2015, 12, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Falcon, L.; Harris-Love, M.O. Sarcopenia and the new ICD-10-CM code: Screening, staging, and diagnosis considerations. Fed. Pract. 2017, 34, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Verdijk, L.B.; de Groot, L.C.P.G.M.; van Loon, L.J.C. Handgrip strength does not represent an appropriate measure to evaluate changes in muscle strength during an exercise intervention program in frail older people. Int. J. Sport Nutr. Exerc. MeTable 2015, 25, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Fox, K.M.; Gandra, S.R.; Delmonico, M.J.; Chiou, C.-F.; Anthony, M.S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S.R. For the Health, Aging and Body Composition Study Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J. Am. Geriatr. Soc. 2009, 57, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Czerwinski, S.; Abellan Van Kan, G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef] [PubMed]

| Study Participant Demographics | |
|---|---|
| Age (years) | 62.5 ± 9.2 |
| Body mass index | 26.3 ± 3.8 |
| Height (cm) | 177.1 ± 6.8 |
| Weight (kg) | 82.5 ± 13.1 |
| Lean body mass (kg/m2) | 8.57 ± 1.12 |
| Total adiposity (body fat, %) | 27.8 ± 7.4 |
| Racial/ethnic group | |
| African American | 24 |
| Caucasian | 6 |
| Muscle Strength | ||
| Mean | SD | |
| Peak Grip Force (0°/s) | 0.49 | ±0.98 |
| Peak Knee Extensor Torque (60°/s) | 0.58 | ±0.16 |
| Peak Knee Extensor Torque (180°/s) | 0.37 | ±0.12 |
| Peak Knee Flexor Torque (60°/s) | 0.26 | ±0.09 |
| Peak Knee Flexor Torque (180°/s) | 0.18 | ±0.07 |
| Functional Performance | ||
| Mean | SD | |
| Customary Gait Speed (m/s) | 1.23 | ±0.34 |
| Fast Gait Speed (m/s) | 1.62 | ±0.41 |
| Sit to Stand (s) | 11.75 | ±4.01 |
| PPT-7 | 21.2 | ±3.1 |
| Customary Gait Speed | Fast Gait Speed | Sit to Stand | PPT-7 | ||
|---|---|---|---|---|---|
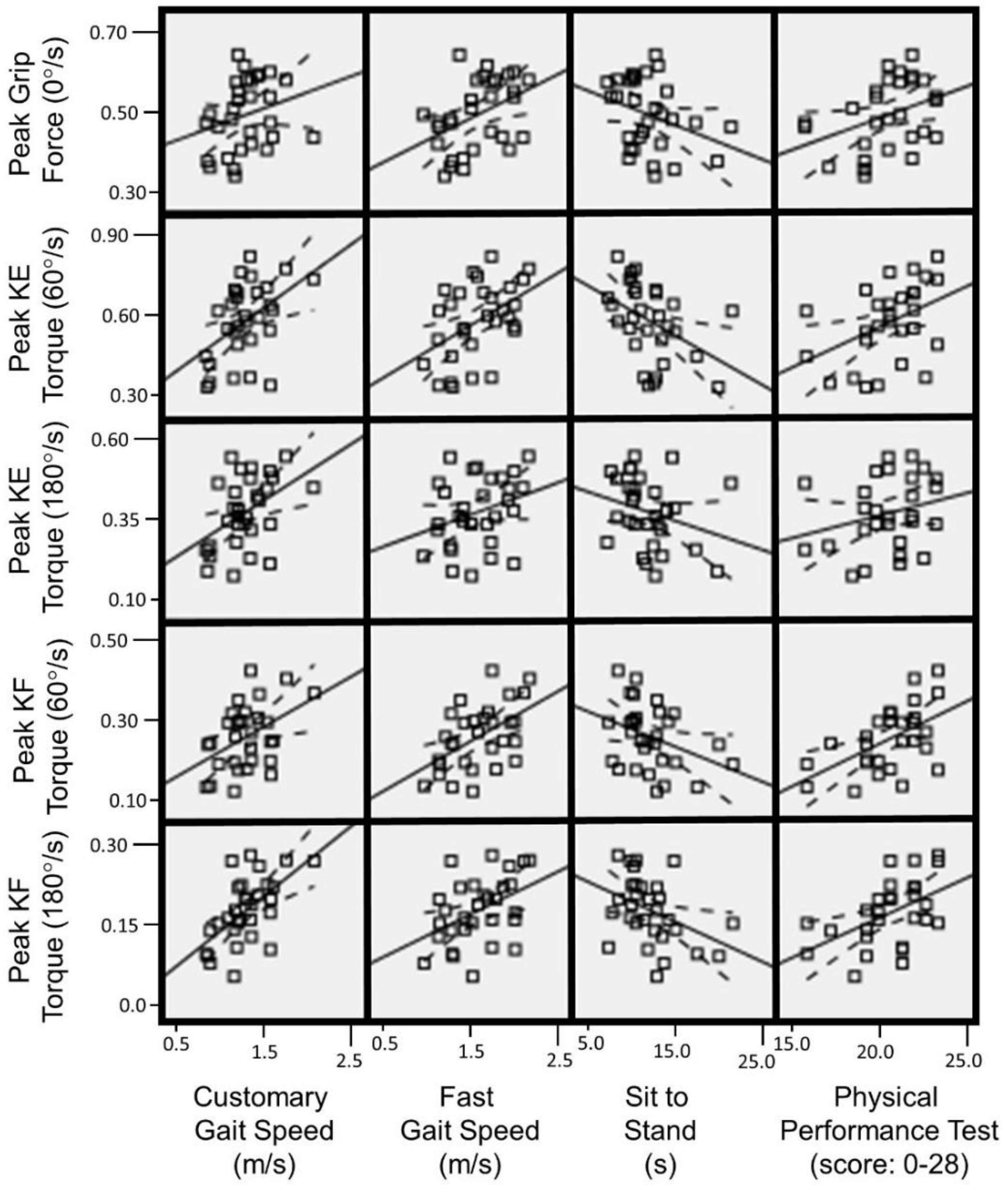
| Peak Grip Force (0°/s) | r | 0.25 | 0.42 | −0.32 | 0.33 |
| p-value | 0.175 | 0.021 | 0.084 | 0.079 | |
| Peak Knee Extensor Torque (60°/s) | r | 0.47 | 0.46 | −0.43 | 0.45 |
| p-value | 0.009 | 0.010 | 0.017 | 0.013 | |
| Peak Knee Extensor Torque (180°/s) | r | 0.45 | 0.31 | −0.27 | 0.29 |
| p-value | 0.013 | 0.101 | 0.146 | 0.124 | |
| Peak Knee Flexor Torque (60°/s) | r | 0.41 | 0.52 | −0.35 | 0.55 |
| p-value | 0.026 | 0.004 | 0.067 | 0.002 | |
| Peak Knee Flexor Torque (180°/s) | r | 0.59 | 0.41 | −0.37 | 0.57 |
| p-value | 0.001 | 0.028 | 0.051 | 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris-Love, M.O.; Benson, K.; Leasure, E.; Adams, B.; McIntosh, V. The Influence of Upper and Lower Extremity Strength on Performance-Based Sarcopenia Assessment Tests. J. Funct. Morphol. Kinesiol. 2018, 3, 53. https://doi.org/10.3390/jfmk3040053
Harris-Love MO, Benson K, Leasure E, Adams B, McIntosh V. The Influence of Upper and Lower Extremity Strength on Performance-Based Sarcopenia Assessment Tests. Journal of Functional Morphology and Kinesiology. 2018; 3(4):53. https://doi.org/10.3390/jfmk3040053
Chicago/Turabian StyleHarris-Love, Michael O., Kimberly Benson, Erin Leasure, Bernadette Adams, and Valerie McIntosh. 2018. "The Influence of Upper and Lower Extremity Strength on Performance-Based Sarcopenia Assessment Tests" Journal of Functional Morphology and Kinesiology 3, no. 4: 53. https://doi.org/10.3390/jfmk3040053
APA StyleHarris-Love, M. O., Benson, K., Leasure, E., Adams, B., & McIntosh, V. (2018). The Influence of Upper and Lower Extremity Strength on Performance-Based Sarcopenia Assessment Tests. Journal of Functional Morphology and Kinesiology, 3(4), 53. https://doi.org/10.3390/jfmk3040053






