Low-Intensity Vibration Improves Muscle Healing in a Mouse Model of Laceration Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Muscle Injury
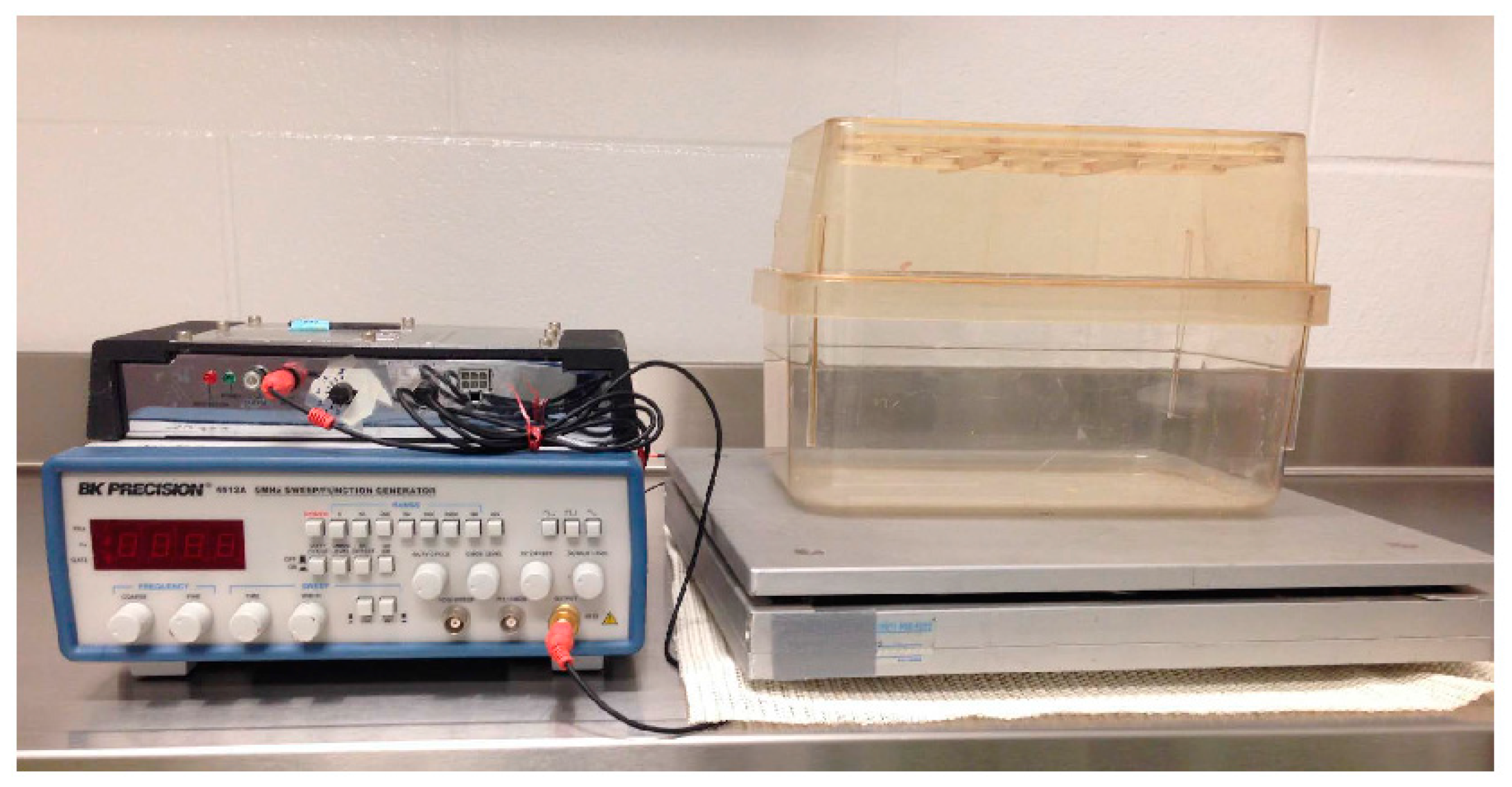
2.3. Whole Body Low-Intensity Vibration
2.4. Histology
2.5. Statistics
3. Results
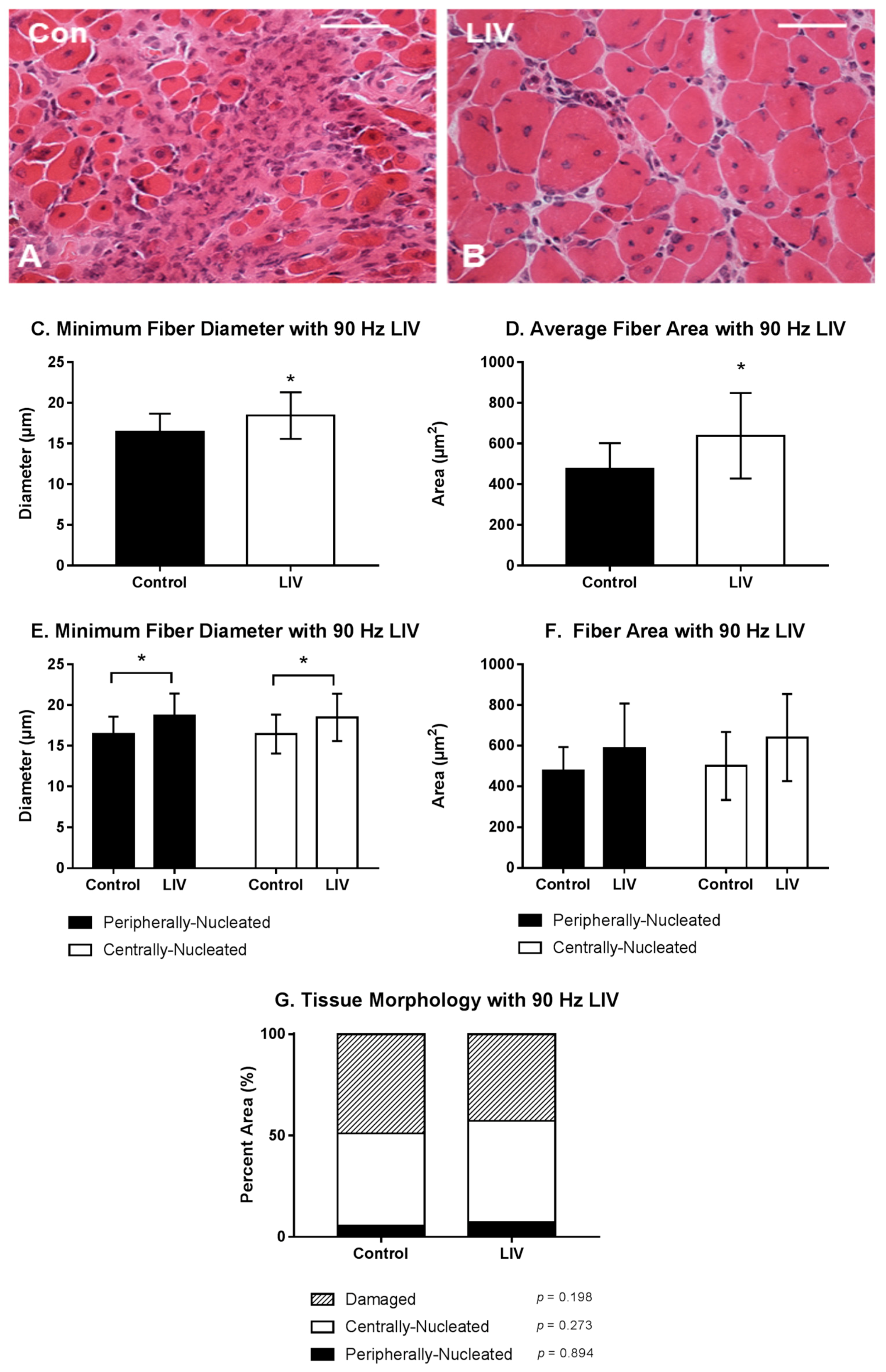
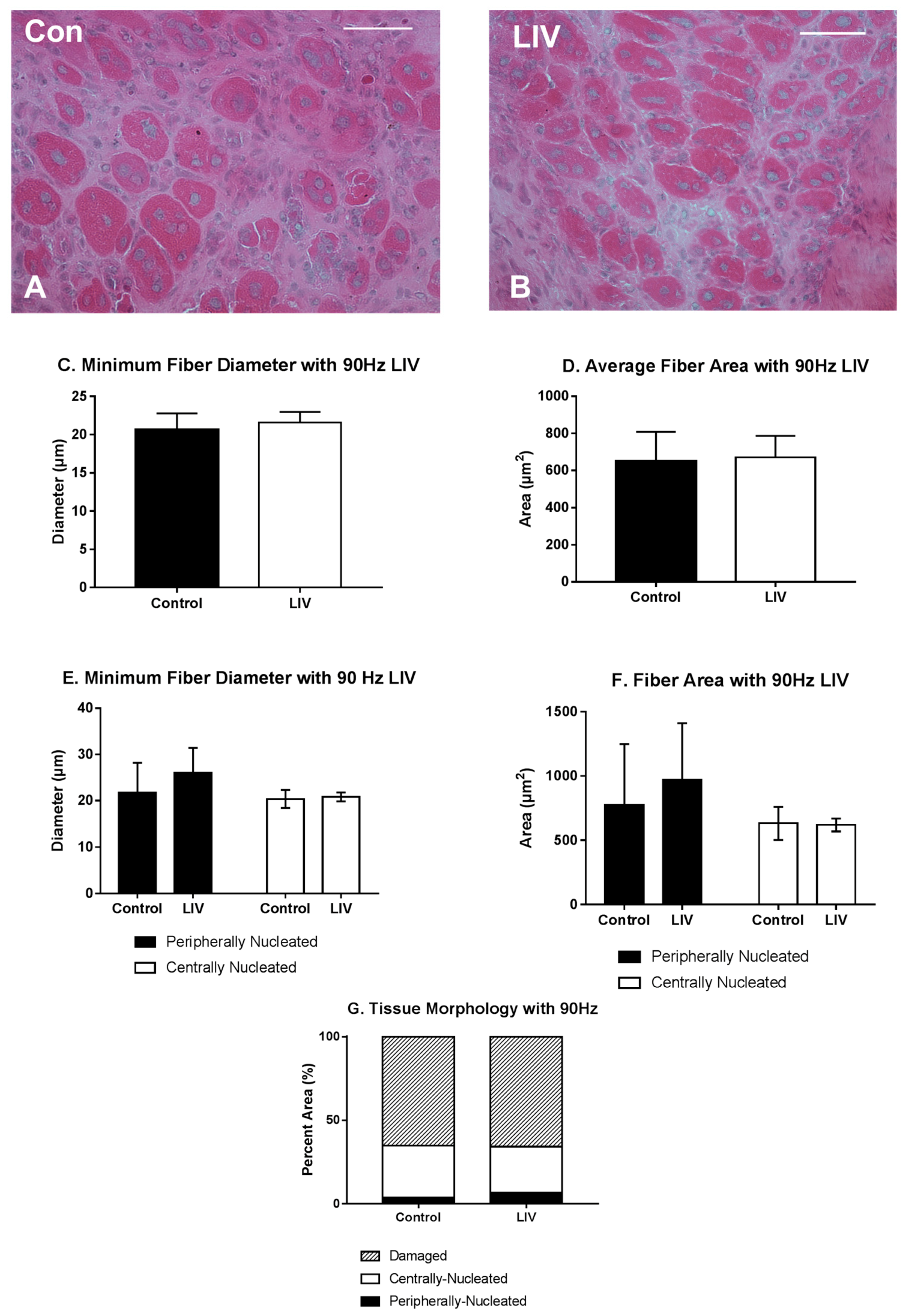
3.1. LIV Protocol Using 90 Hz and 0.2 g for 14 Day Treatment Period
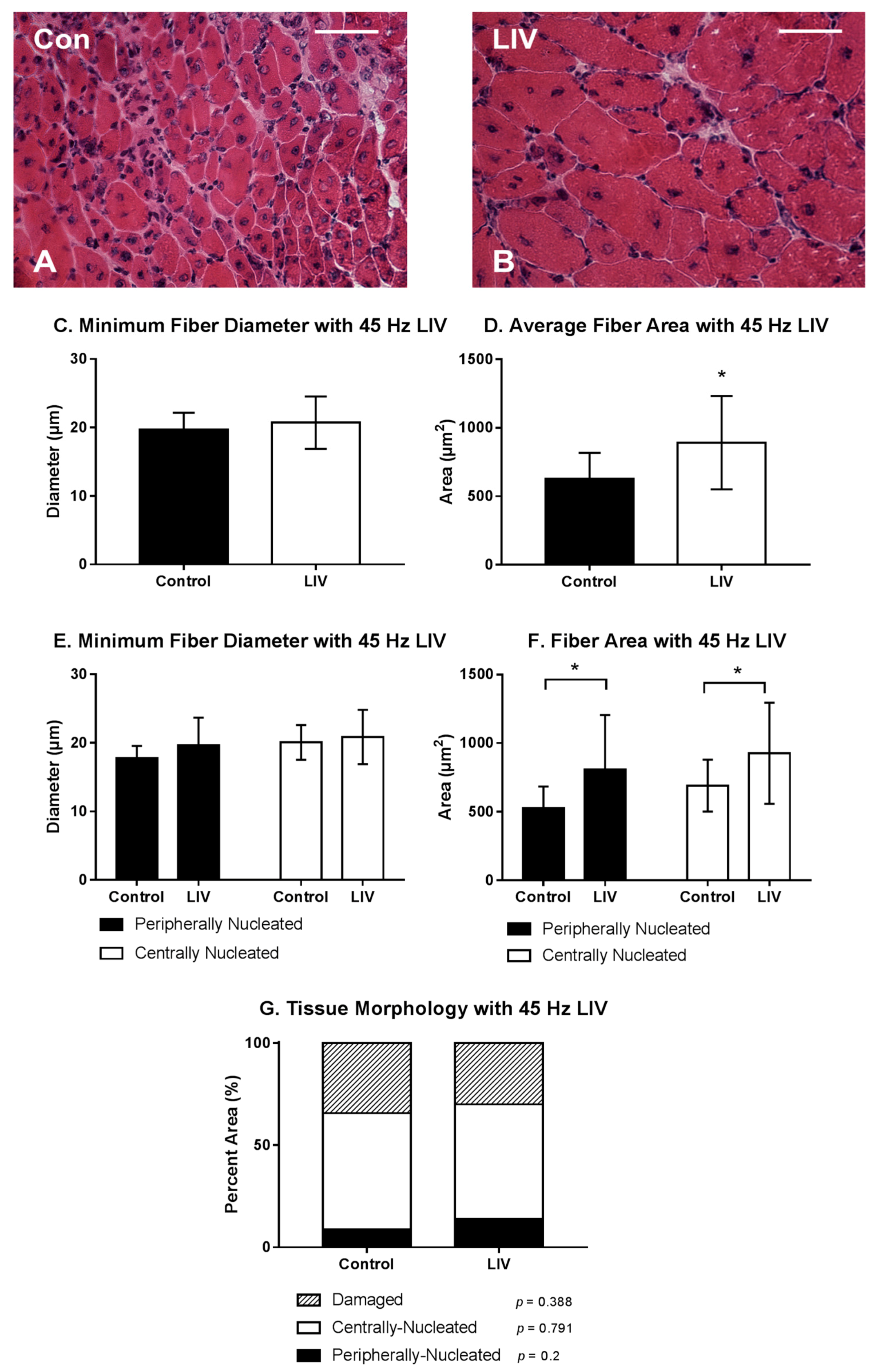
3.2. LIV Protocol Using 45 Hz and 0.4 g for 14 Day Treatment Period
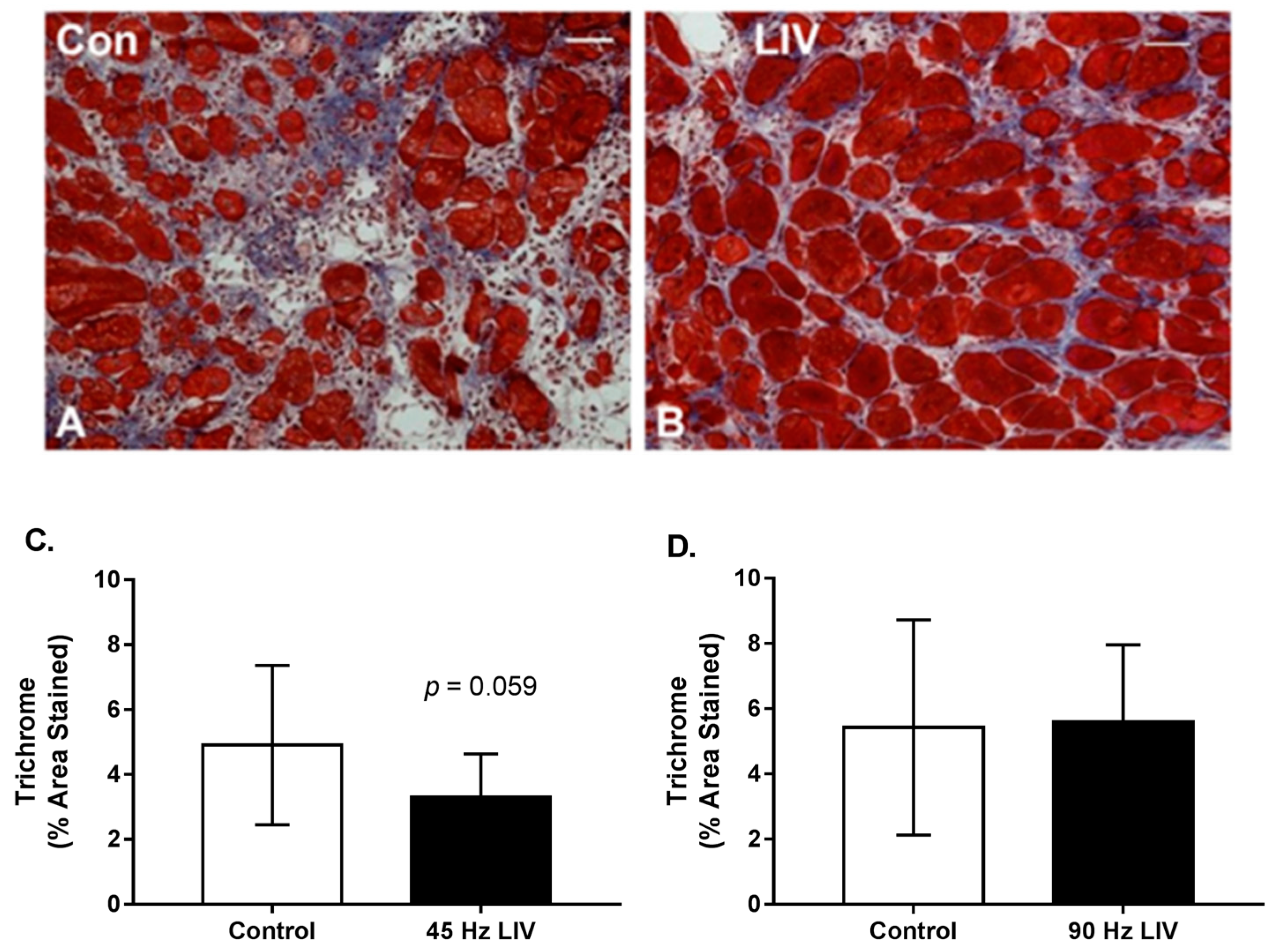
3.3. Effects of LIV on Fibrosis for 14 Day Treatment Period
3.4. LIV Protocol Using 90 Hz and 0.2 g for Seven Day Treatment Period
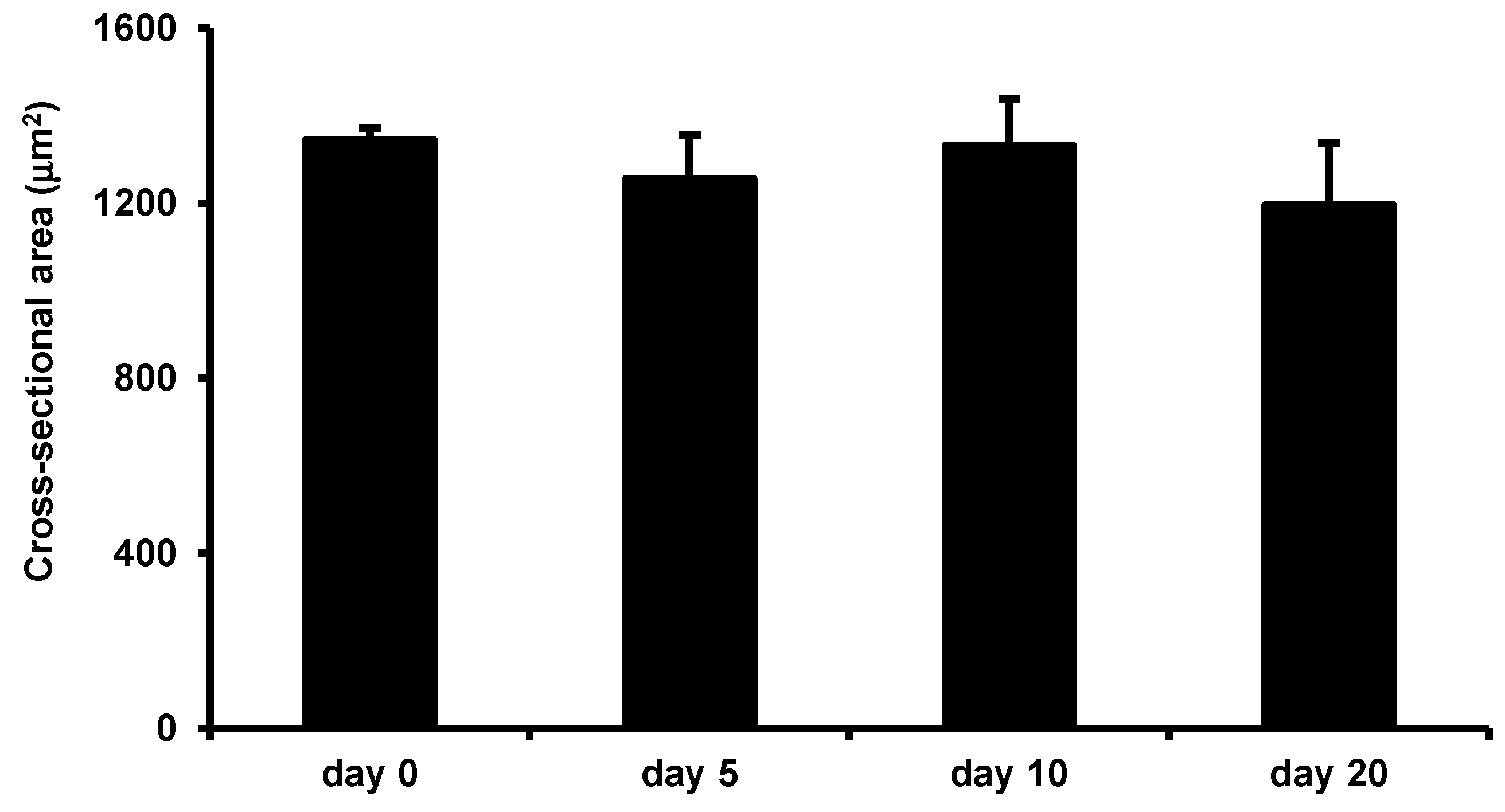
3.5. Effects of LIV on Uninjured Muscle
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Covey, D.C. Combat orthopaedics: A view from the trenches. J. Am. Acad. Orthop. Surg. 2006, 14, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Bedair, H.; Liu, T.T.; Kaar, J.L.; Badlani, S.; Russell, A.J.; Li, Y.; Huard, J. Matrix metalloproteinase-1 therapy improves muscle healing. J. Appl. Physiol. 2007, 102, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Menetrey, J.; Kasemkijwattana, C.; Fu, F.H.; Moreland, M.S.; Huard, J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am. J. Sports Med. 1999, 27, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, Y.; Tang, Y.; Cummins, J.; Huard, J. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am. J. Pathol. 2005, 167, 1105–1117. [Google Scholar] [CrossRef]
- Vaittinen, S.; Hurme, T.; Rantanen, J.; Kalimo, H. Transected myofibres may remain permanently divided in two parts. Neuromuscul. Disord. NMD 2002, 12, 584–587. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Bryer, S.C.; Fantuzzi, G.; van Rooijen, N.; Koh, T.J. Urokinase-type plasminogen activator plays essential roles in macrophage chemotaxis and skeletal muscle regeneration. J. Immunol. 2008, 180, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- DiPasquale, D.M.; Cheng, M.; Billich, W.; Huang, S.A.; van Rooijen, N.; Hornberger, T.A.; Koh, T.J. Urokinase-type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am. J. Physiol. Cell Physiol. 2007, 293, C1278–C1285. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Billich, W.; Smith, S.M.; Sukhija, K.B.; McLoughlin, T.J.; Hornberger, T.A.; Koh, T.J. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1132–R1139. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Bryer, S.C.; Cheng, M.; Nguyen, M.H.; Conley, K.L.; Cunningham, A.K.; Xue, B.; Sisson, T.H.; You, J.S.; Hornberger, T.A.; et al. Macrophage-specific expression of urokinase-type plasminogen activator promotes skeletal muscle regeneration. J. Immunol. 2011, 187, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Summan, M.; Warren, G.L.; Mercer, R.R.; Chapman, R.; Hulderman, T.; Van Rooijen, N.; Simeonova, P.P. Macrophages and skeletal muscle regeneration: A clodronate-containing liposome depletion study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1488–R1495. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.; Garg, K.; Corona, B.T.; Walters, T.J. Physical rehabilitation improves muscle function following volumetric muscle loss injury. BMC Sports Sci. Med. Rehabil. 2014, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, K.; Murton, A.J.; Greenhaff, P.L. Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. J. Appl. Physiol. 2011, 110, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.P. Molecular regulation of skeletal muscle mass. Clin. Exp. Pharmacol. Physiol. 2010, 37, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Spangenburg, E.E. Changes in muscle mass with mechanical load: Possible cellular mechanisms. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2009, 34, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Garman, R.; Gaudette, G.; Donahue, L.R.; Rubin, C.; Judex, S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J. Orthop. Res. 2007, 25, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Gilsanz, V.; Wren, T.A.; Sanchez, M.; Dorey, F.; Judex, S.; Rubin, C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006, 21, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Rubin, C.; Judex, S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J. Appl. Physiol. 2008, 104, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Lublinsky, S.; Seo, Y.K.; Kim, I.S.; Judex, S. Extremely small-magnitude accelerations enhance bone regeneration: A preliminary study. Clin. Orthop. Relat. Res. 2009, 467, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Pollak, A.N.; Powell, E.T.; Fang, R.; Cooper, E.O.; Ficke, J.R.; Flaherty, S.F. Use of negative pressure wound therapy during aeromedical evacuation of patients with combat-related blast injuries. J. Surg. Orthop. Adv. 2010, 19, 44–48. [Google Scholar] [PubMed]
- Weinheimer-Haus, E.M.; Judex, S.; Ennis, W.J.; Koh, T.J. Low-intensity vibration improves angiogenesis and wound healing in diabetic mice. PLoS ONE 2014, 9, e91355. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Weinheimer-Haus, E.M.; Koh, T.J. Macrophage activation and skeletal muscle healing following traumatic injury. J. Pathol. 2014, 232, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Judex, S.; Lei, X.; Han, D.; Rubin, C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J. Biomech. 2007, 40, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D. The need to more precisely define aspects of skeletal muscle regeneration. Int. J. Biochem. Cell Biol. 2014, 56, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.D.; Ficke, J.R.; Hsu, J.R.; Masini, B.D.; Wenke, J.C. Battlefield orthopaedic injuries cause the majority of long-term disabilities. J. Am. Acad. Orthop. Surg. 2011, 19, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.D.; Kragh, J.F., Jr.; Macaitis, J.; Svoboda, S.J.; Wenke, J.C. Characterization of extremity wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J. Orthop. Trauma 2007, 21, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, R.C.; Tredget, E.E. Concise review: Bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 2010, 28, 905–915. [Google Scholar] [PubMed]
- Chan, Y.S.; Li, Y.; Foster, W.; Horaguchi, T.; Somogyi, G.; Fu, F.H.; Huard, J. Antifibrotic effects of suramin in injured skeletal muscle after laceration. J. Appl. Physiol. 2003, 95, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Nguyen, M.H.; Fantuzzi, G.; Koh, T.J. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2008, 294, C1183–C1191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Shen, W.; Qiao, C.; Ambrosio, F.; Lavasani, M.; Nozaki, M.; Branca, M.F.; Huard, J. Relationships between transforming growth factor-beta1, myostatin, and decorin: Implications for skeletal muscle fibrosis. J. Biol. Chem. 2007, 282, 25852–25863. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Corona, B.T.; Walters, T.J. Losartan administration reduces fibrosis but hinders functional recovery after volumetric muscle loss injury. J. Appl. Physiol. 2014, 117, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Ichioka, S.; Yokogawa, H.; Nakagami, G.; Sekiya, N.; Sanada, H. In vivo analysis of skin microcirculation and the role of nitric oxide during vibration. Ostomy Wound Manag. 2011, 57, 40. [Google Scholar]
- Lohman, E.B., 3rd; Petrofsky, J.S.; Maloney-Hinds, C.; Betts-Schwab, H.; Thorpe, D. The effect of whole body vibration on lower extremity skin blood flow in normal subjects. Med. Sci. Monit. 2007, 13, CR71–CR76. [Google Scholar] [PubMed]
- Maloney-Hinds, C.; Petrofsky, J.S.; Zimmerman, G.; Hessinger, D.A. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol. Ther. 2009, 11, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.A.; Mangino, M.J.; Bassuk, J.; Kurlansky, P.; Sackner, M.A. Regional blood flow during per acceleration. Crit. Care Med. 2001, 29, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.A.; Moore, J.J.E.; Moreno, M.R.; Coelho, J.; Bassuk, J.; Wu, D. Effects of Periodic Body Acceleration on the In Vivo Vasoactive Response to N-w-nitro—l-arginine and the In Vitro Nitric Oxide Production. Ann. Biomed. Eng. 2003, 31, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Arashi, M.; Sugama, J.; Sanada, H.; Konya, C.; Okuwa, M.; Nakagami, G.; Inoue, A.; Tabata, K. Vibration therapy accelerates healing of Stage I pressure ulcers in older adult patients. Adv. Skin Wound Care 2010, 23, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y.; Sanada, H.; Minematsu, T.; Nakagami, G.; Nagase, T.; Huang, L.; Noguchi, H.; Mori, T.; Yoshimura, K.; Sugama, J. Vibration inhibits deterioration in rat deep-tissue injury through HIF1-MMP axis. Wound Repair Regen. 2015, 23, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Kiel, D.P.; Hannan, M.T.; Barton, B.A.; Bouxsein, M.L.; Lang, T.F.; Brown, K.M.; Shane, E.; Magaziner, J.; Zimmerman, S.; Rubin, C.T. Insights from the conduct of a device trial in older persons: Low magnitude mechanical stimulation for musculoskeletal health. Clin. Trials 2010, 7, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Recker, R.; Cullen, D.; Ryaby, J.; McCabe, J.; McLeod, K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: A clinical trial assessing compliance, efficacy, and safety. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Turner, A.S.; Bain, S.; Mallinckrodt, C.; McLeod, K. Anabolism. Low mechanical signals strengthen long bones. Nature 2001, 412, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.K.; Capilla, E.; Rosen, C.J.; Gilsanz, V.; Pessin, J.E.; Judex, S.; Rubin, C.T. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2009, 24, 50–61. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbiere, T.F.; Weinheimer-Haus, E.M.; Judex, S.; Koh, T.J. Low-Intensity Vibration Improves Muscle Healing in a Mouse Model of Laceration Injury. J. Funct. Morphol. Kinesiol. 2018, 3, 1. https://doi.org/10.3390/jfmk3010001
Corbiere TF, Weinheimer-Haus EM, Judex S, Koh TJ. Low-Intensity Vibration Improves Muscle Healing in a Mouse Model of Laceration Injury. Journal of Functional Morphology and Kinesiology. 2018; 3(1):1. https://doi.org/10.3390/jfmk3010001
Chicago/Turabian StyleCorbiere, Thomas F., Eileen M. Weinheimer-Haus, Stefan Judex, and Timothy J. Koh. 2018. "Low-Intensity Vibration Improves Muscle Healing in a Mouse Model of Laceration Injury" Journal of Functional Morphology and Kinesiology 3, no. 1: 1. https://doi.org/10.3390/jfmk3010001
APA StyleCorbiere, T. F., Weinheimer-Haus, E. M., Judex, S., & Koh, T. J. (2018). Low-Intensity Vibration Improves Muscle Healing in a Mouse Model of Laceration Injury. Journal of Functional Morphology and Kinesiology, 3(1), 1. https://doi.org/10.3390/jfmk3010001





