Cardiovascular and Thermal Responses to Cold Exposure During Exercise in Iron-Deficient Anemic Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Measurements
2.4. Statistical Analysis
3. Results
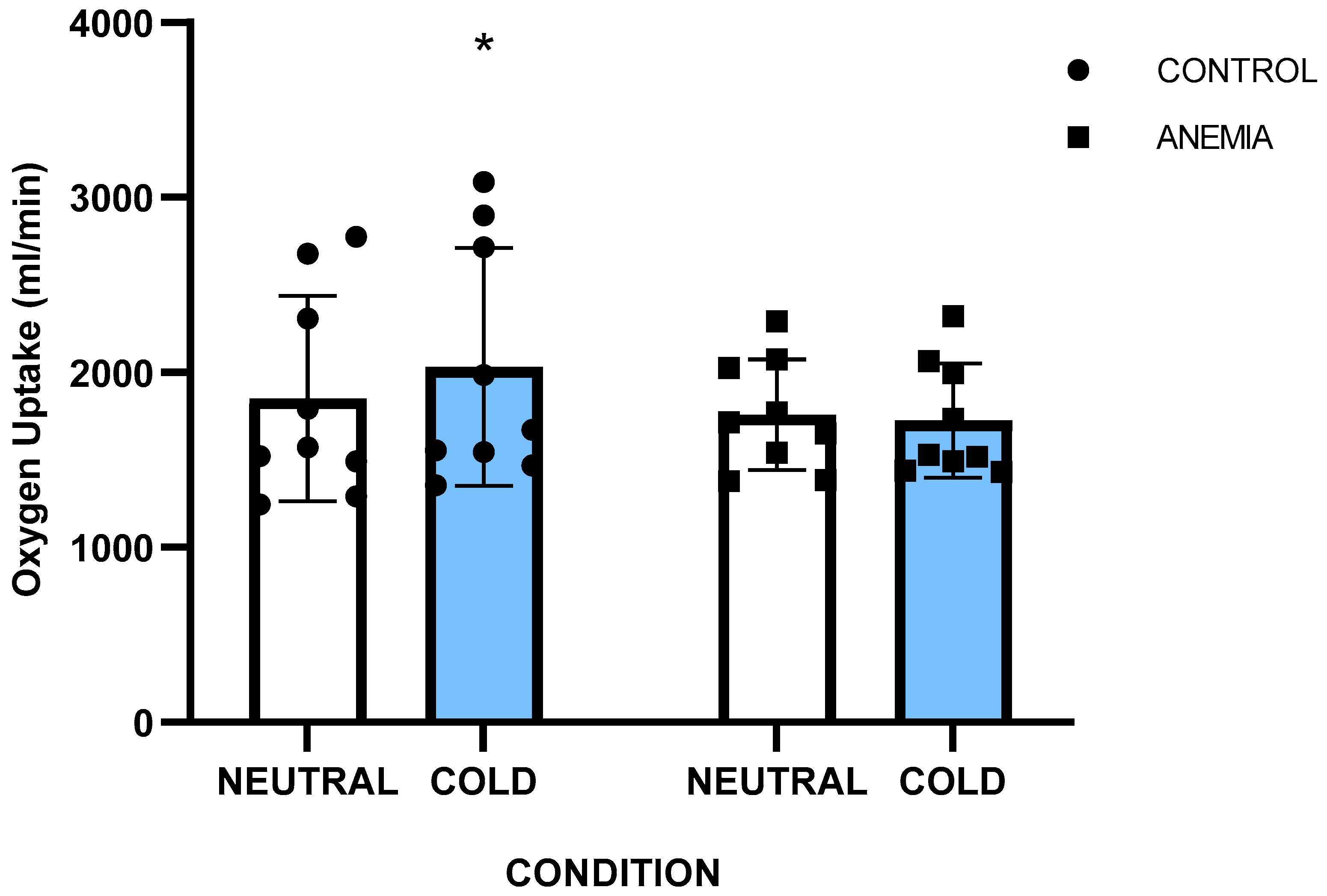
3.1. Sample Characteristics
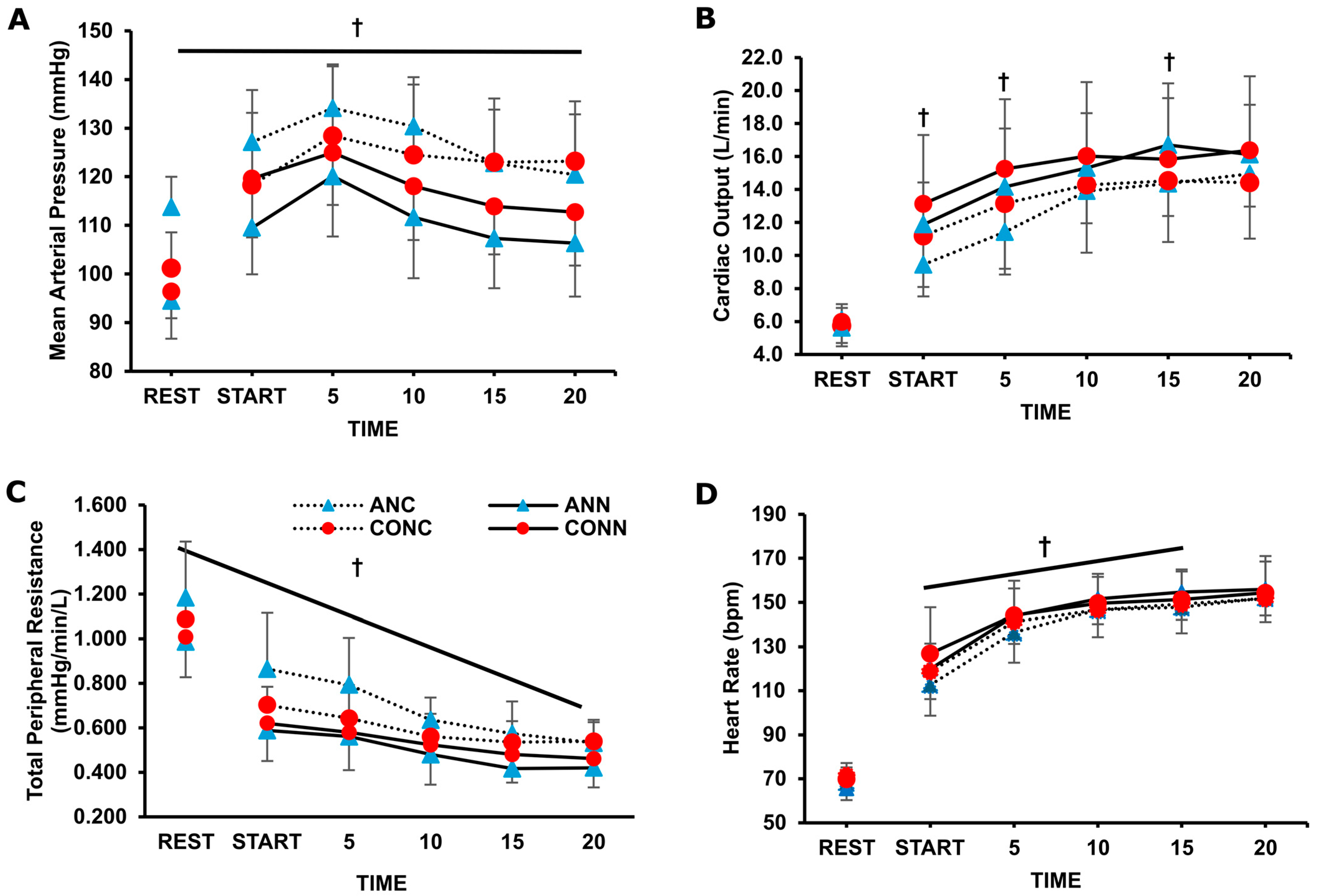
3.2. Cardiovascular Responses
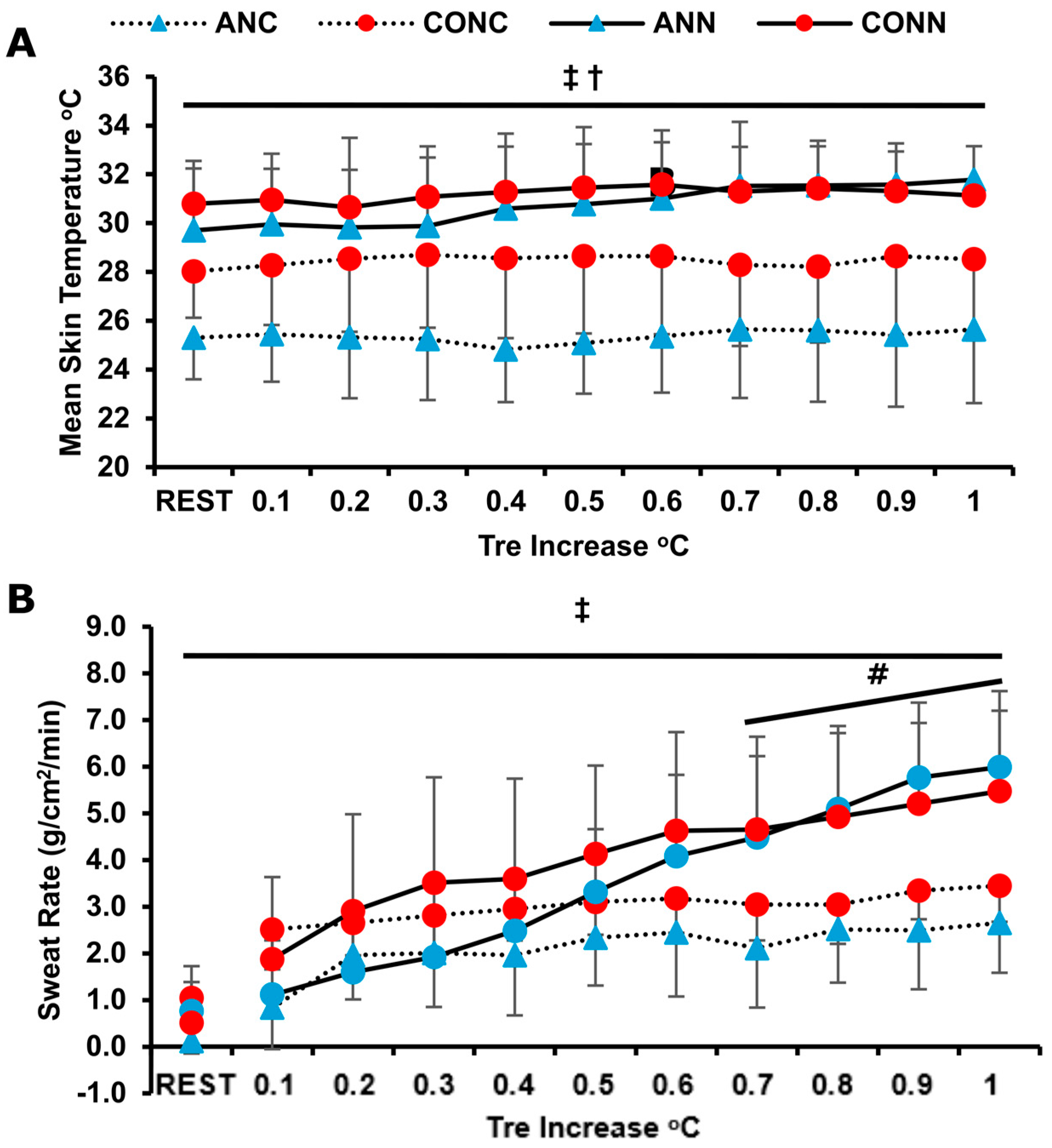
3.3. Thermoregulatory Responses
4. Discussion
4.1. Cardiovascular Responses
4.2. Temperature Regulation Responses
4.3. Methodological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Tsk | Mean skin temperature |
| Tre | Rectal temperature |
| Tf-f | Forearm–fingertip temperature gradient |
| VO2 | Oxygen uptake |
| TPR | Total peripheral resistance |
| MAP | Mean arterial pressure |
| CO | Cardiac output |
| HR | Heart rate |
| RH | Relative humidity |
| SwR | Sweat rate |
| Hprod | Heat production |
| [Hb] | Hemoglobin concentration |
| Hct | Hematocrit |
| AΝΝ | Anemic group in neutral conditions |
| ANC | Anemic group in cold conditions |
| CONN | Control group in neutral conditions |
| CONC | Control group in cold conditions |
References
- Brigham, D.; Beard, J. Iron and thermoregulation: A review. Crit. Rev. Food Sci. Nutr. 1996, 36, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, P.H.; Volpe, S.L. Iron, thermoregulation, and metabolic rate. Crit. Rev. Food Sci. Nutr. 1999, 39, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, 568S–579S; discussion 580S. [Google Scholar] [CrossRef]
- Beard, J.L.; Tobin, B.W.; Smith, S.M. Effects of iron repletion and correction of anemia on norepinephrine turnover and thyroid metabolism in iron deficiency. Proc. Soc. Exp. Biol. Med. 1990, 193, 306–312. [Google Scholar] [CrossRef]
- Beard, J.L.; Borel, M.J.; Derr, J. Impaired thermoregulation and thyroid function in iron-deficiency anemia. Am. J. Clin. Nutr. 1990, 52, 813–819. [Google Scholar] [CrossRef]
- Dillman, E.; Gale, C.; Green, W.; Johnson, D.G.; Mackler, B.; Finch, C. Hypothermia in iron deficiency due to altered triiodothyronine metabolism. Am. J. Physiol. 1980, 239, R377–R381. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Hall, C.B.; Nielsen, F.H. Thermogenesis and thermoregulatory function of iron-deficient women without anemia. Aviat. Space Environ. Med. 1990, 61, 913–920. [Google Scholar]
- Castellani, J.W.; Tipton, M.J. Cold Stress Effects on Exposure Tolerance and Exercise Performance. Compr. Physiol. 2015, 6, 443–469. [Google Scholar] [CrossRef]
- Ikaheimo, T.M. Cardiovascular diseases, cold exposure and exercise. Temperature 2018, 5, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Alba, B.K.; Castellani, J.W.; Charkoudian, N. Cold-induced cutaneous vasoconstriction in humans: Function, dysfunction and the distinctly counterproductive. Exp. Physiol. 2019, 104, 1202–1214. [Google Scholar] [CrossRef]
- Mugele, H.; Marume, K.; Amin, S.B.; Possnig, C.; Kuhn, L.C.; Riehl, L.; Pieper, R.; Schabbehard, E.L.; Oliver, S.J.; Gagnon, D.; et al. Control of blood pressure in the cold: Differentiation of skin and skeletal muscle vascular resistance. Exp. Physiol. 2023, 108, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklov, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Greaney, J.L.; Kenney, W.L.; Alexander, L.M. Sympathetic function during whole body cooling is altered in hypertensive adults. J. Appl. Physiol. (1985) 2017, 123, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Fares, A. Winter Hypertension: Potential mechanisms. Int. J. Health Sci. 2013, 7, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yavar, Z.; Sun, Q. Cardiovascular response to thermoregulatory challenges. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1793–H1812. [Google Scholar] [CrossRef]
- Voorhess, M.L.; Stuart, M.J.; Stockman, J.A.; Oski, F.A. Iron deficiency anemia and increased urinary norepinephrine excretion. J. Pediatr. 1975, 86, 542–547. [Google Scholar] [CrossRef]
- Dillmann, E.; Johnson, D.G.; Martin, J.; Mackler, B.; Finch, C. Catecholamine elevation in iron deficiency. Am. J. Physiol. 1979, 237, R297–R300. [Google Scholar] [CrossRef]
- Martinez-Torres, C.; Cubeddu, L.; Dillmann, E.; Brengelmann, G.L.; Leets, I.; Layrisse, M.; Johnson, D.G.; Finch, C. Effect of exposure to low temperature on normal and iron-deficient subjects. Am. J. Physiol. 1984, 246, R380–R383. [Google Scholar] [CrossRef]
- McClung, J.P.; Karl, J.P.; Cable, S.J.; Williams, K.W.; Young, A.J.; Lieberman, H.R. Longitudinal decrements in iron status during military training in female soldiers. Br. J. Nutr. 2009, 102, 605–609. [Google Scholar] [CrossRef]
- Parks, R.B.; Hetzel, S.J.; Brooks, M.A. Iron Deficiency and Anemia among Collegiate Athletes: A Retrospective Chart Review. Med. Sci. Sports Exerc. 2017, 49, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.R.; Moir-Meyer, G. Measuring the global burden of anaemia. Lancet Haematol. 2023, 10, e696–e697. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Stough, W.G.; Shaw, L.K.; O’Connor, C.M. Anaemia and coronary artery disease severity in patients with heart failure. Eur. J. Heart Fail. 2006, 8, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Shaw, L.K.; Stough, W.G.; O’Connor, C.M. Anemia in patients with heart failure and preserved systolic function. Am. Heart J. 2006, 151, 457–462. [Google Scholar] [CrossRef]
- Caro, J.J.; Salas, M.; Ward, A.; Goss, G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer 2001, 91, 2214–2221. [Google Scholar] [CrossRef]
- Stauffer, M.E.; Fan, T. Prevalence of anemia in chronic kidney disease in the United States. PLoS ONE 2014, 9, e84943. [Google Scholar] [CrossRef]
- Koskolou, M.; Komboura, S.; Zacharakis, E.D.; Georgopoulou, O.; Keramidas, M.; Geladas, N. Physiological Responses of Anemic Women to Exercise under Hypoxic Conditions. Physiologia 2023, 3, 247–258. [Google Scholar] [CrossRef]
- Baker, F.C.; Waner, J.I.; Vieira, E.F.; Taylor, S.R.; Driver, H.S.; Mitchell, D. Sleep and 24 hour body temperatures: A comparison in young men, naturally cycling women and women taking hormonal contraceptives. J. Physiol. 2001, 530, 565–574. [Google Scholar] [CrossRef]
- Calbet, J.A.; Lundby, C.; Koskolou, M.; Boushel, R. Importance of hemoglobin concentration to exercise: Acute manipulations. Respir. Physiol. Neurobiol. 2006, 151, 132–140. [Google Scholar] [CrossRef]
- Miliotis, P.G.; Ntalapera, S.D.; Lakeas, P.; Loukas, I.; Toubekis, A.G.; Geladas, N.D.; Koskolou, M.D. Cardiorespiratory and oxygenation responses in iron-deficient anemic women during whole-body exercise under moderate hypoxia. Eur. J. Appl. Physiol. 2025. [Google Scholar] [CrossRef]
- Jackson, A.S.; Pollock, M.L. Practical Assessment of Body Composition. Phys. Sport. 1985, 13, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, S.A. Assessing hydration status. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 519–524. [Google Scholar] [CrossRef]
- Burton, A.C. A New Technic for the Measurement of Average Skin Temperature over Surfaces of the Body and the Changes of Skin Temperature During Exercise: Six Figures. J. Nutr. 1934, 7, 481–496. [Google Scholar] [CrossRef]
- Mitchell, D.; Wyndham, C.H. Comparison of weighting formulas for calculating mean skin temperature. J. Appl. Physiol. 1969, 26, 616–622. [Google Scholar] [CrossRef]
- Keramidas, M.E.; Geladas, N.D.; Mekjavic, I.B.; Kounalakis, S.N. Forearm-finger skin temperature gradient as an index of cutaneous perfusion during steady-state exercise. Clin. Physiol. Funct. Imaging 2013, 33, 400–404. [Google Scholar] [CrossRef] [PubMed]
- ASHRAE. Thermal Comfort Conditions, ASHRAE 55.66; ASHRAE: New York, NY, USA, 1966. [Google Scholar]
- Bogert, L.W.; van Lieshout, J.J. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp. Physiol. 2005, 90, 437–446. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Fadel, P.J.; Smith, S.A.; Norton, K.H.; Querry, R.G.; Olivencia-Yurvati, A.; Raven, P.B. Increases in intramuscular pressure raise arterial blood pressure during dynamic exercise. J. Appl. Physiol. (1985) 2001, 91, 2351–2358. [Google Scholar] [CrossRef]
- Sproule, B.J.; Mitchell, J.H.; Miller, W.F. Cardiopulmonary physiological responses to heavy exercise in patients with anemia. J. Clin. Investig. 1960, 39, 378–388. [Google Scholar] [CrossRef]
- Koskolou, M.D.; Roach, R.C.; Calbet, J.A.; Radegran, G.; Saltin, B. Cardiovascular responses to dynamic exercise with acute anemia in humans. Am. J. Physiol. 1997, 273, H1787–H1793. [Google Scholar] [CrossRef]
- Greaney, J.L.; Stanhewicz, A.E.; Kenney, W.L.; Alexander, L.M. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J. Appl. Physiol. (1985) 2014, 117, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Fagius, J.; Kay, R. Low ambient temperature increases baroreflex-governed sympathetic outflow to muscle vessels in humans. Acta Physiol. Scand. 1991, 142, 201–209. [Google Scholar] [CrossRef]
- Cui, J.; Durand, S.; Crandall, C.G. Baroreflex control of muscle sympathetic nerve activity during skin surface cooling. J. Appl. Physiol. (1985) 2007, 103, 1284–1289. [Google Scholar] [CrossRef]
- Ogoh, S.; Fisher, J.P.; Dawson, E.A.; White, M.J.; Secher, N.H.; Raven, P.B. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J. Physiol. 2005, 566, 599–611. [Google Scholar] [CrossRef]
- McArdle, W.D.; Magel, J.R.; Lesmes, G.R.; Pechar, G.S. Metabolic and cardiovascular adjustment to work in air and water at 18, 25, and 33 degrees C. J. Appl. Physiol. 1976, 40, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Raven, P.B.; Niki, I.; Dahms, T.E.; Horvath, S.M. Compensatory cardiovascular responses during an environmental cold stress, 5 degrees C. J. Appl. Physiol. 1970, 29, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alonso, J.; Mora-Rodriguez, R.; Coyle, E.F. Stroke volume during exercise: Interaction of environment and hydration. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H321–H330. [Google Scholar] [CrossRef]
- Wilson, T.E.; Crandall, C.G. Effect of thermal stress on cardiac function. Exerc. Sport. Sci. Rev. 2011, 39, 12–17. [Google Scholar] [CrossRef]
- Bianco, A.C.; McAninch, E.A. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol. 2013, 1, 250–258. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Frank, S.M.; Raja, S.N.; Bulcao, C.F.; Goldstein, D.S. Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J. Appl. Physiol. (1985) 1999, 86, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Marchant, B.; Donaldson, G.; Mridha, K.; Scarborough, M.; Timmis, A.D. Mechanisms of cold intolerance in patients with angina. J. Am. Coll. Cardiol. 1994, 23, 630–636. [Google Scholar] [CrossRef]
- Juneau, M.; Johnstone, M.; Dempsey, E.; Waters, D.D. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation 1989, 79, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Sato, K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev. Physiol. Biochem. Pharmacol. 1977, 79, 51–131. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Crandall, C.G. Mechanisms and controllers of eccrine sweating in humans. Front. Biosci. (Schol. Ed.) 2010, 2, 685–696. [Google Scholar] [CrossRef]
- Schafer, E.A.; Chapman, C.L.; Castellani, J.W.; Looney, D.P. Energy expenditure during physical work in cold environments: Physiology and performance considerations for military service members. J. Appl. Physiol. (1985) 2024, 137, 995–1013. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | AN | CON | p Value |
|---|---|---|---|
| N (M/F) | 9 (4/5) | 9 (4/5) | |
| Height (cm) | 169 ± 8 | 171 ± 8 | 0.62 |
| Body mass (kg) | 62 ± 8 | 63 ± 11 | 0.86 |
| Body fat (%) | 17 ± 6 | 16 ± 6 | 0.79 |
| BSA (m2) | 1.72 ± 0.15 | 1.74 ± 0.18 | 0.78 |
| [Hb] (g/dL) | 11.8 ± 0.4 | 14.1 ± 0.9 | 0.0001 |
| Hct (%) | 37.1 ± 1.8 | 42.3 ± 2.7 | 0.0001 |
| Fe (μg/dL) | 53 ± 16 | 93 ± 13 | 0.0001 |
| Ferritin (ng/mL) | 15 ± 9 | 56 ± 12 | 0.0001 |
| VO2max Neutral (mL·kg−1·min−1) | 40.6 ± 4.7 | 46.3 ± 7.1 | 0.06 |
| VO2max Cold (mL·kg−1·min−1) | 41.1 ± 4.9 | 46.7 ± 6.6 | 0.07 |
| Variables | ANN | ANC | CONN | CONC |
|---|---|---|---|---|
| Tre Rest (°C) | 37.02 ± 0.13 | 37.08 ± 0.15 | 37.05 ± 0.13 | 37.06 ± 0.20 |
| Tre End exercise (°C) | 38.01 ± 0.10 | 37.93 ± 0.15 | 38.02 ± 0.16 | 38.03 ± 0.1 |
| Tre increase (°C·min−1), value × 100 | 2.69 ± 0.9 | 1.84 ± 0.9 *# | 2.74 ± 0.4 | 2.88 ± 0.5 |
| Exercise time to increase +1 °C or 1 h duration (min) | 41.3 ± 12.1 | 52.8 ± 9.3 *# | 35.8 ± 5.4 | 38.2 ± 8.8 |
| Hprod (W) | 610 ± 105 | 606 ± 107 | 645 ± 204 | 710 ± 236 * |
| Hprod (W·kg−1) | 9.8 ± 1.4 | 9.7 ± 1.0 | 10.1 ± 1.7 | 11.1 ± 2.2 * |
| Hprod (W·m−2) | 355 ± 48 | 352 ± 42 | 365 ± 78 | 402 ± 94 * |
| Threshold (°C) for SwR increase (g·cm−2·min−1) | 0.15 ± 0.1 | 0.33 ± 0.2 ** | 0.14 ± 0.0 | 0.21 ± 0.1 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miliotis, P.; Ntalapera, S.; Lakeas, P.; Toubekis, A.; Geladas, N.; Koskolou, M. Cardiovascular and Thermal Responses to Cold Exposure During Exercise in Iron-Deficient Anemic Individuals. J. Funct. Morphol. Kinesiol. 2025, 10, 362. https://doi.org/10.3390/jfmk10030362
Miliotis P, Ntalapera S, Lakeas P, Toubekis A, Geladas N, Koskolou M. Cardiovascular and Thermal Responses to Cold Exposure During Exercise in Iron-Deficient Anemic Individuals. Journal of Functional Morphology and Kinesiology. 2025; 10(3):362. https://doi.org/10.3390/jfmk10030362
Chicago/Turabian StyleMiliotis, Panagiotis, Spyridoula Ntalapera, Panagiotis Lakeas, Argyris Toubekis, Nickos Geladas, and Maria Koskolou. 2025. "Cardiovascular and Thermal Responses to Cold Exposure During Exercise in Iron-Deficient Anemic Individuals" Journal of Functional Morphology and Kinesiology 10, no. 3: 362. https://doi.org/10.3390/jfmk10030362
APA StyleMiliotis, P., Ntalapera, S., Lakeas, P., Toubekis, A., Geladas, N., & Koskolou, M. (2025). Cardiovascular and Thermal Responses to Cold Exposure During Exercise in Iron-Deficient Anemic Individuals. Journal of Functional Morphology and Kinesiology, 10(3), 362. https://doi.org/10.3390/jfmk10030362







