Low-Intensity Virtual Reality Exercise for Caregivers of People with Mild Cognitive Impairment: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcome Measures
2.3. Procedures
2.4. Intervention and System Description
2.5. Data Governance and Privacy
2.6. Statistical Analysis
3. Results
4. Discussion
Limitation of the Study and Clinical Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corallo, F.; Maggio, M.G.; Bonanno, L.; De Luca, R.; Cardile, D.; Cappadona, I.; Todaro, A.; Calabrò, R.S. Burden in Caregivers of Patients with Acquired Brain Injury: Influence of Family Role and Gender. NeuroRehabilitation 2024, 55, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.G.; Corallo, F.; De Francesco, M.; De Cola, M.C.; De Luca, R.; Manuli, A.; Quartarone, A.; Rizzo, A.; Calabrò, R.S. Understanding the Family Burden and Caregiver Role in Stroke Rehabilitation: Insights from a Retrospective Study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2024, 45, 5347–5353. [Google Scholar] [CrossRef] [PubMed]
- Adelman, R.D.; Tmanova, L.L.; Delgado, D.; Dion, S.; Lachs, M.S. Caregiver Burden: A Clinical Review. JAMA 2014, 311, 1052–1060. [Google Scholar] [CrossRef]
- Schulz, R.; Sherwood, P.R. Physical and Mental Health Effects of Family Caregiving. Am. J. Nurs. 2008, 108, 23–27. [Google Scholar] [CrossRef]
- Pottie, C.G.; Burch, K.A.; Thomas, L.P.M.; Irwin, S.A. Informal Caregiving of Hospice Patients. J. Palliat. Med. 2014, 17, 845–856. [Google Scholar] [CrossRef]
- Sallim, A.B.; Sayampanathan, A.A.; Cuttilan, A.; Ho, R.C.-M. Prevalence of Mental Health Disorders Among Caregivers of Patients With Alzheimer Disease. J. Am. Med. Dir. Assoc. 2015, 16, 1034–1041. [Google Scholar] [CrossRef]
- Capistrant, B.D.; Moon, J.R.; Glymour, M.M. Spousal Caregiving and Incident Hypertension. Am. J. Hypertens. 2012, 25, 437–443. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Preacher, K.J.; MacCallum, R.C.; Atkinson, C.; Malarkey, W.B.; Glaser, R. Chronic Stress and Age-Related Increases in the Proinflammatory Cytokine IL-6. Proc. Natl. Acad. Sci. USA 2003, 100, 9090–9095. [Google Scholar] [CrossRef]
- von Känel, R.; Dimsdale, J.E.; Mills, P.J.; Ancoli-Israel, S.; Patterson, T.L.; Mausbach, B.T.; Grant, I. Effect of Alzheimer Caregiving Stress and Age on Frailty Markers Interleukin-6, C-Reactive Protein, and D-Dimer. J. Gerontol. Ser. A 2006, 61, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.M.; Taback, R.L.; Bean, J.; Weiner, W.J. Comorbidity of the Nonmotor Symptoms of Parkinson’s Disease. Mov. Disord. 2001, 16, 507–510. [Google Scholar] [CrossRef]
- Lawton, M.; Moss, M.; Duhamel, L. The Quality of Daily-Life Among Elderly Care Receivers. J. Appl. Gerontol. 1995, 14, 150–171. [Google Scholar] [CrossRef]
- Henry, R.S.; Lageman, S.K.; Perrin, P.B. The Relationship between Parkinson’s Disease Symptoms and Caregiver Quality of Life. Rehabil. Psychol. 2020, 65, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.; Travis, S.S. Analysis of the Reliability of the Modified Caregiver Strain Index. J. Gerontol. Ser. B 2003, 58, S127–S132. [Google Scholar] [CrossRef]
- Bastawrous, M. Caregiver Burden—A Critical Discussion. Int. J. Nurs. Stud. 2013, 50, 431–441. [Google Scholar] [CrossRef]
- Greenwood, N.; Mezey, G.; Smith, R. Social Exclusion in Adult Informal Carers: A Systematic Narrative Review of the Experiences of Informal Carers of People with Dementia and Mental Illness. Maturitas 2018, 112, 39–45. [Google Scholar] [CrossRef]
- Lopez-Hartmann, M.; Wens, J.; Verhoeven, V.; Remmen, R. The Effect of Caregiver Support Interventions for Informal Caregivers of Community-Dwelling Frail Elderly: A Systematic Review. Int. J. Integr. Care 2012, 12, e133. [Google Scholar] [CrossRef]
- Lilly, M.B.; Robinson, C.A.; Holtzman, S.; Bottorff, J.L. Can We Move beyond Burden and Burnout to Support the Health and Wellness of Family Caregivers to Persons with Dementia? Evidence from British Columbia, Canada. Health Soc. Care Community 2012, 20, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Northouse, L.L.; Katapodi, M.C.; Song, L.; Zhang, L.; Mood, D.W. Interventions with Family Caregivers of Cancer Patients: Meta-Analysis of Randomized Trials. CA. Cancer J. Clin. 2010, 60, 317–339. [Google Scholar] [CrossRef]
- Geraets, C.N.W.; van der Stouwe, E.C.D.; Pot-Kolder, R.; Veling, W. Advances in Immersive Virtual Reality Interventions for Mental Disorders: A New Reality? Curr. Opin. Psychol. 2021, 41, 40–45. [Google Scholar] [CrossRef]
- Bruno, R.R.; Wolff, G.; Wernly, B.; Masyuk, M.; Piayda, K.; Leaver, S.; Erkens, R.; Oehler, D.; Afzal, S.; Heidari, H.; et al. Virtual and Augmented Reality in Critical Care Medicine: The Patient’s, Clinician’s, and Researcher’s Perspective. Crit. Care 2022, 26, 326. [Google Scholar] [CrossRef]
- Kanschik, D.; Bruno, R.R.; Wolff, G.; Kelm, M.; Jung, C. Virtual and Augmented Reality in Intensive Care Medicine: A Systematic Review. Ann. Intensive Care 2023, 13, 81. [Google Scholar] [CrossRef]
- Jung, C.; Wolff, G.; Wernly, B.; Bruno, R.R.; Franz, M.; Schulze, P.C.; Silva, J.N.A.; Silva, J.R.; Bhatt, D.L.; Kelm, M. Virtual and Augmented Reality in Cardiovascular Care: State-of-the-Art and Future Perspectives. JACC Cardiovasc. Imaging 2022, 15, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Ng, J.C.; Awuah, W.A.; Huang, H.; Yarlagadda, R.; Mehta, A.; Nansubuga, E.P.; Jiffry, R.; Abdul-Rahman, T.; Ou Yong, B.M.; et al. NeuroVerse: Neurosurgery in the Era of Metaverse and Other Technological Breakthroughs. Postgrad. Med. J. 2023, 99, 240–243. [Google Scholar] [CrossRef]
- Wolff, G.; Bruno, R.R.; Reiter, M.; Kantzow, B.; Kelm, M.; Jung, C. Virtual Reality Device Training for Extracorporeal Membrane Oxygenation. Crit. Care 2020, 24, 390. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.; Belec, J.; Sheikh, A.; Chepelev, L.; Althobaity, W.; Chow, B.J.W.; Mitsouras, D.; Christensen, A.; Rybicki, F.J.; La Russa, D.J. Applying Modern Virtual and Augmented Reality Technologies to Medical Images and Models. J. Digit. Imaging 2019, 32, 38–53. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Rodriguez, R.A.; Gonzalez, M.; Bernardy, M.; Peña, R.; Beck, W.; Patterson, D.R.; Meyer, W.J. Immersive Virtual Reality as an Adjunctive Non-Opioid Analgesic for Pre-Dominantly Latin American Children with Large Severe Burn Wounds During Burn Wound Cleaning in the Intensive Care Unit: A Pilot Study. Front. Hum. Neurosci. 2019, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Puel, F.; Minville, V.; Vardon-Bounes, F. What Place for Virtual Reality in the Intensive Care Unit during Medical Procedures? J. Intensive Care 2021, 9, 30. [Google Scholar] [CrossRef]
- Silva, J.N.A.; Southworth, M.; Raptis, C.; Silva, J. Emerging Applications of Virtual Reality in Cardiovascular Medicine. JACC Basic Transl. Sci. 2018, 3, 420–430. [Google Scholar] [CrossRef]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N.; the TREND Group. Improving the Reporting Quality of Nonrandomized Evaluations of Behavioral and Public Health Interventions: The TREND Statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef]
- Marteau, T.M.; Bekker, H. The Development of a Six-Item Short-Form of the State Scale of the Spielberger State—Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 1992, 31, 301–306, Correction in Br. J. Clin. Psychol. 2020, 59, 276. [Google Scholar] [CrossRef]
- Cope, S.M.; Liu, X.-C.; Verber, M.D.; Cayo, C.; Rao, S.; Tassone, J.C. Upper Limb Function and Brain Reorganization after Constraint-Induced Movement Therapy in Children with Hemiplegia. Dev. Neurorehabilit. 2010, 13, 19–30. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385. [Google Scholar] [CrossRef]
- Washburn, R.A.; Montoye, H.J. The assessment of physical activity by questionnaire. Am. J. Epidemiol. 1986, 123, 563–576. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Namara, M.M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II 2011; American Psychological Association: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Kodraliu, G.; Mosconi, P.; Groth, N.; Carmosino, G.; Perilli, A.; Gianicolo, E.A.; Rossi, C.; Apolone, G. Subjective Health Status Assessment: Evaluation of the Italian Version of the SF-12 Health Survey. Results from the MiOS Project. J. Epidemiol. Biostat. 2001, 6, 305–316. [Google Scholar] [CrossRef]
- Finstad, K. The Usability Metric for User Experience. Interact. Comput. 2010, 22, 323–327. [Google Scholar] [CrossRef]
- Dilgul, M.; Martinez, J.; Laxhman, N.; Priebe, S.; Bird, V. Cognitive Behavioural Therapy in Virtual Reality Treatments across Mental Health Conditions: A Systematic Review. Consort. Psychiatr. 2020, 1, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Matos, R.; Castillo-García, M.; Magni, E.; Pabón-Carrasco, M. Effectiveness of Virtual Reality for Managing Pain, Fear, and Anxiety in Children and Adolescents Undergoing Needle-Related Procedures: Systematic Review with Meta-Analysis. Nurs. Rep. 2024, 14, 2456–2484. [Google Scholar] [CrossRef]
- Meurer, K.J.; Presciutti, A.M.; Bannon, S.M.; Kubota, R.; Baskaran, N.; Kim, J.; Zhang, Q.; Reichman, M.; Fishbein, N.S.; Lichstein, K.; et al. Characterizing Stressors and Coping Strategies Among Caregivers of Patients with Severe Acute Brain Injury by Level of Distress. Neurocrit. Care 2025. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, B.; Buntinx, F.; De Lepeleire, J. The Relation between Care Giving and the Mental Health of Caregivers of Demented Relatives: A Cross-Sectional Study. Eur. J. Gen. Pract. 2009, 15, 99–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Li, Q.; Cui, J.; Tu, S.; Deng, Z.; Yang, R.; Wang, Y. Effectiveness of Virtual Reality on the Caregiving Competence and Empathy of Caregivers for Elderly with Chronic Diseases: A Systematic Review and Meta-Analysis. J. Nurs. Manag. 2023, 2023, 5449955. [Google Scholar] [CrossRef]
- Li Pira, G.; Aquilini, B.; Davoli, A.; Grandi, S.; Ruini, C. The Use of Virtual Reality Interventions to Promote Positive Mental Health: Systematic Literature Review. JMIR Ment. Health 2023, 10, e44998. [Google Scholar] [CrossRef]
- Botha, F.; Dahmann, S.C. Locus of Control, Self-Control, and Health Outcomes. SSM-Popul. Health 2024, 25, 101566. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.P.; Storrow, A.; Liu, D.; Jenkins, C.A.; Miller, K.F.; Kampe, C.; Butler, J. Examining Whether a Self-Care Program Reduces Healthcare Use and Improves Health Among Patients with Acute Heart Failure—The Guided HF Study; PCORI Final Research Reports; Patient-Centered Outcomes Research Institute (PCORI): Washington, DC, USA, 2021. [Google Scholar]
- Ko, E.; Wongvibul, T.; Rose, K.M.; Jun, J. The Effects of Self-Guided Interventions on Stress, Burden, and Mental Health in Caregivers of People Living with Dementia: A Systematic Review. Int. J. Nurs. Stud. Adv. 2023, 5, 100141. [Google Scholar] [CrossRef]
- Habermann, B.; Hines, D.; Davis, L. Caring for Parents with Neurodegenerative Disease: A Qualitative Description. Clin. Nurse Spec. CNS 2013, 27, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Khalaila, R.; Cohen, M. Emotional Suppression, Caregiving Burden, Mastery, Coping Strategies and Mental Health in Spousal Caregivers. Aging Ment. Health 2016, 20, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Keng, S.-L.; Smoski, M.J.; Robins, C.J. Effects of Mindfulness on Psychological Health: A Review of Empirical Studies. Clin. Psychol. Rev. 2011, 31, 1041–1056. [Google Scholar] [CrossRef]
- Bruschetta, R.; Maggio, M.G.; Naro, A.; Ciancarelli, I.; Morone, G.; Arcuri, F.; Tonin, P.; Tartarisco, G.; Pioggia, G.; Cerasa, A.; et al. Gender Influences Virtual Reality-Based Recovery of Cognitive Functions in Patients with Traumatic Brain Injury: A Secondary Analysis of a Randomized Clinical Trial. Brain Sci. 2022, 12, 491. [Google Scholar] [CrossRef]
- Christian, L.M.; Wilson, S.J.; Madison, A.A.; Prakash, R.S.; Burd, C.E.; Rosko, A.E.; Kiecolt-Glaser, J.K. Understanding the Health Effects of Caregiving Stress: New Directions in Molecular Aging. Ageing Res. Rev. 2023, 92, 102096. [Google Scholar] [CrossRef]
- Nikou, S.; Agahari, W.; Keijzer-Broers, W.; De Reuver, M. Digital Healthcare Technology Adoption by Elderly People: A Capability Approach Model. Telemat. Inform. 2020, 53, 101315. [Google Scholar] [CrossRef]
- Bauge, K.; Babic, A. Gaming for Cognitive Assessment and Enhancement in Elders: A Secondary Analysis of Literature and Applications. Stud. Health Technol. Inform. 2025, 328, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef] [PubMed]


| Instrument | Description | Clinical Cut-off |
|---|---|---|
| State-Trait Anxiety Inventory (STAI) [30] | Measures both transient (state) and enduring (trait) anxiety symptoms | >40 indicates clinically relevant anxiety (varies by age/gender) |
| Coping Orientation to Problems Experienced (COPE) [31] | Assesses a wide range of coping strategies (e.g., social support, avoidance, problem-solving) | No specific cut-off; scores analyzed by subscale |
| Perceived Stress Scale (PSS) [32] | Evaluates perceived stress over the past month | ≥20 suggests high perceived stress |
| International Physical Activity Questionnaire (IPAQ-SF) [33] | Assesses weekly physical activity including walking, moderate, and vigorous exercise | <600 Metabolic Equivalent of Task minutes per week (MET-min/week) = low activity |
| Caregiver Burden Inventory (CBI) [34] | Measures caregiver burden across five dimensions (time-dependence, developmental, physical, social, emotional) | ≥24 indicates clinically significant burden |
| Beck Depression Inventory-II (BDI-II) [35] | Self-report inventory assessing depressive symptoms | 14–19 mild; 20–28 moderate; 29–63 severe |
| Short Form Health Status Survey-12 (SF-12) [36] | Self-report questionnaire assessing perceived physical and mental health status | >50 indicates better than average physical and health functioning |
| System Usability Scale (SUS) [37] | Assesses user satisfaction and usability of digital systems | >68 acceptable usability; >80 excellent usability |
| Section | Description | Duration |
|---|---|---|
| Warm-up (stretching and mobility exercises) |
| 5–10 min |
| Work-out (posture, mobility, strength, and balance) |
| 20–25 min |
| Cool-down (relaxation and decompression exercises) |
| 5 min |
| Characteristic | |
|---|---|
| Age | |
| mean ± SD | 58.9 ± 18.2 |
| min, max | 29, 77 |
| Gender | |
| Male (%) | 6 (60%) |
| Female (%) | 4 (40%) |
| Education Level | |
| mean ± SD | 11.4 ± 5.3 |
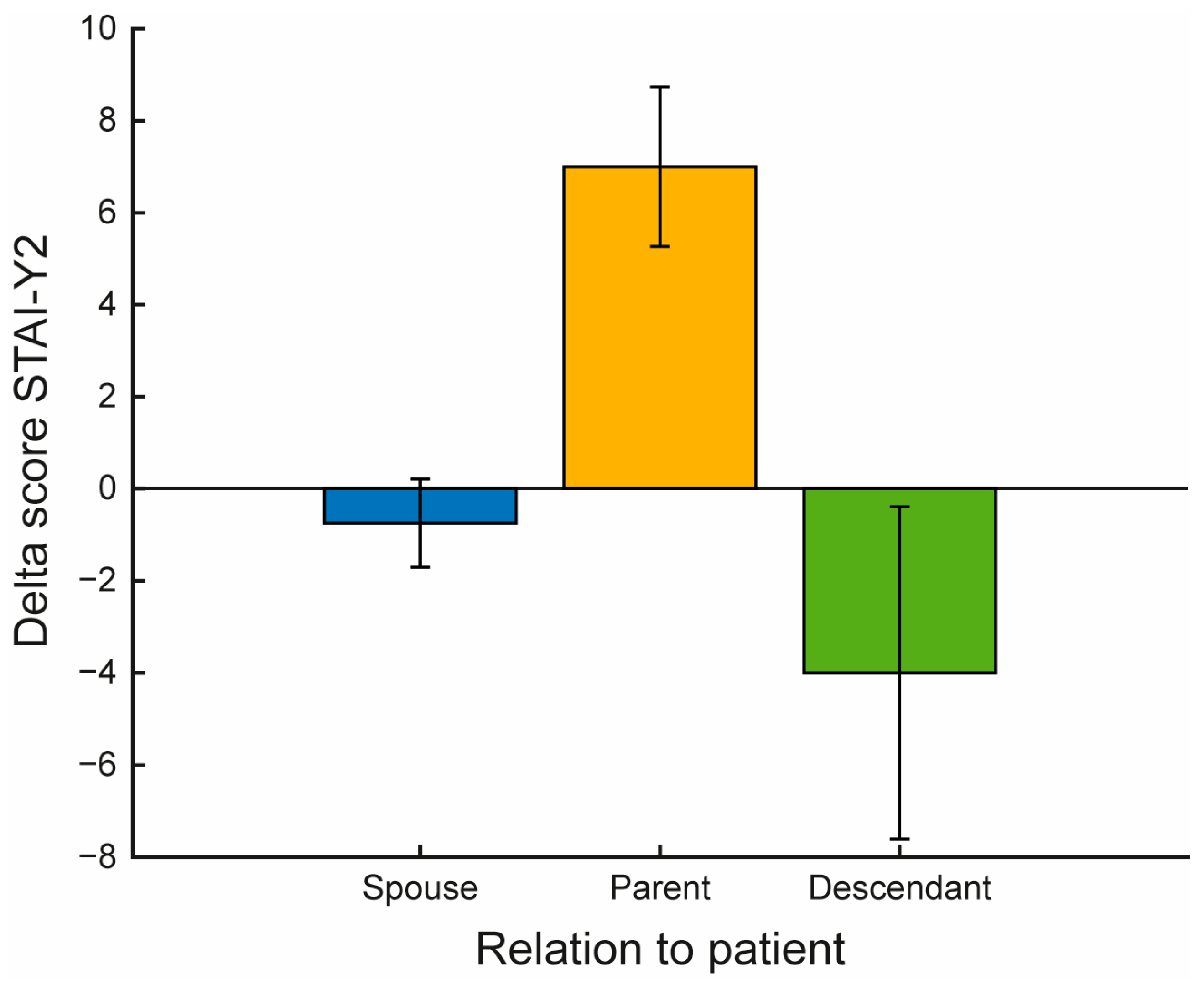
| Relationship with patient | |
| Spouse (%) | 4 (40%) |
| Parent (%) | 3 (30%) |
| Descendant (%) | 3 (30%) |
| Employment Status | |
| Retired (%) | 6 (60%) |
| Employee (%) | 4 (40%) |
| Clinical Scale | T0 | T1 | p-Value | Hodges-Lehmann Median Diff | 95% CI | |
|---|---|---|---|---|---|---|
| PSS | 16 (11–23) | 12 (10–18) | 0.426 | −1 | [−9, 2] | |
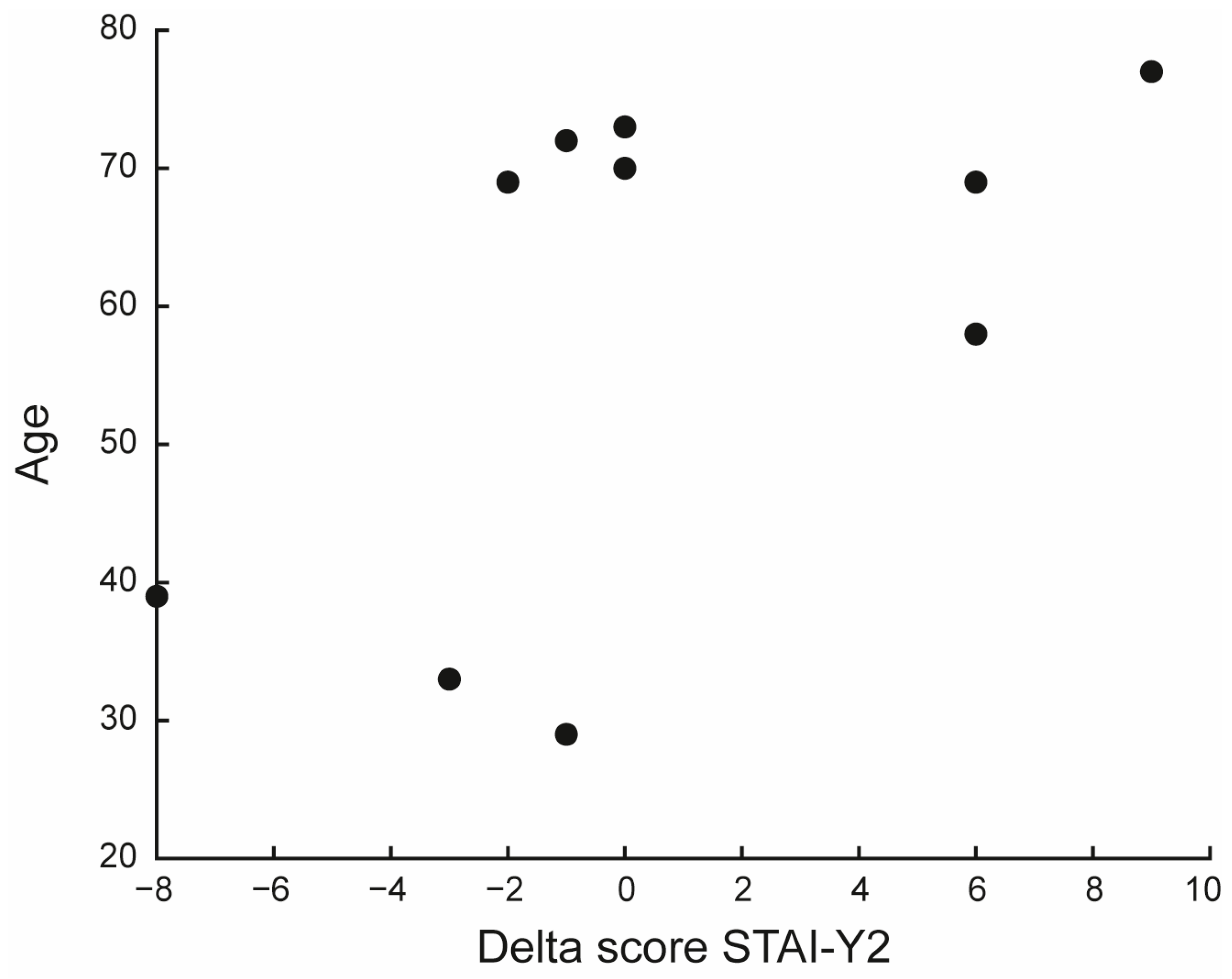
| STAI-Y | 1 | 36 (32–46) | 40.5 (26–48) | 0.590 | −2.5 | [−7, 5] |
| 2 | 35 (32–46) | 37 (31–45) | 0.930 | −0.5 | [−2.5, 6] | |
| CBI | 13.5 (9–23) | 15 (12–28) | 0.414 | 1 | [−2, 6] | |
| BDI-II | 7 (2–13) | 9 (1–12) | 0.906 | 0 | [−2.5, 1.5] | |
| SF-12 | 33.5 (30–34) | 33 (32–34) | 0.535 | −0.5 | [−2, 3] | |
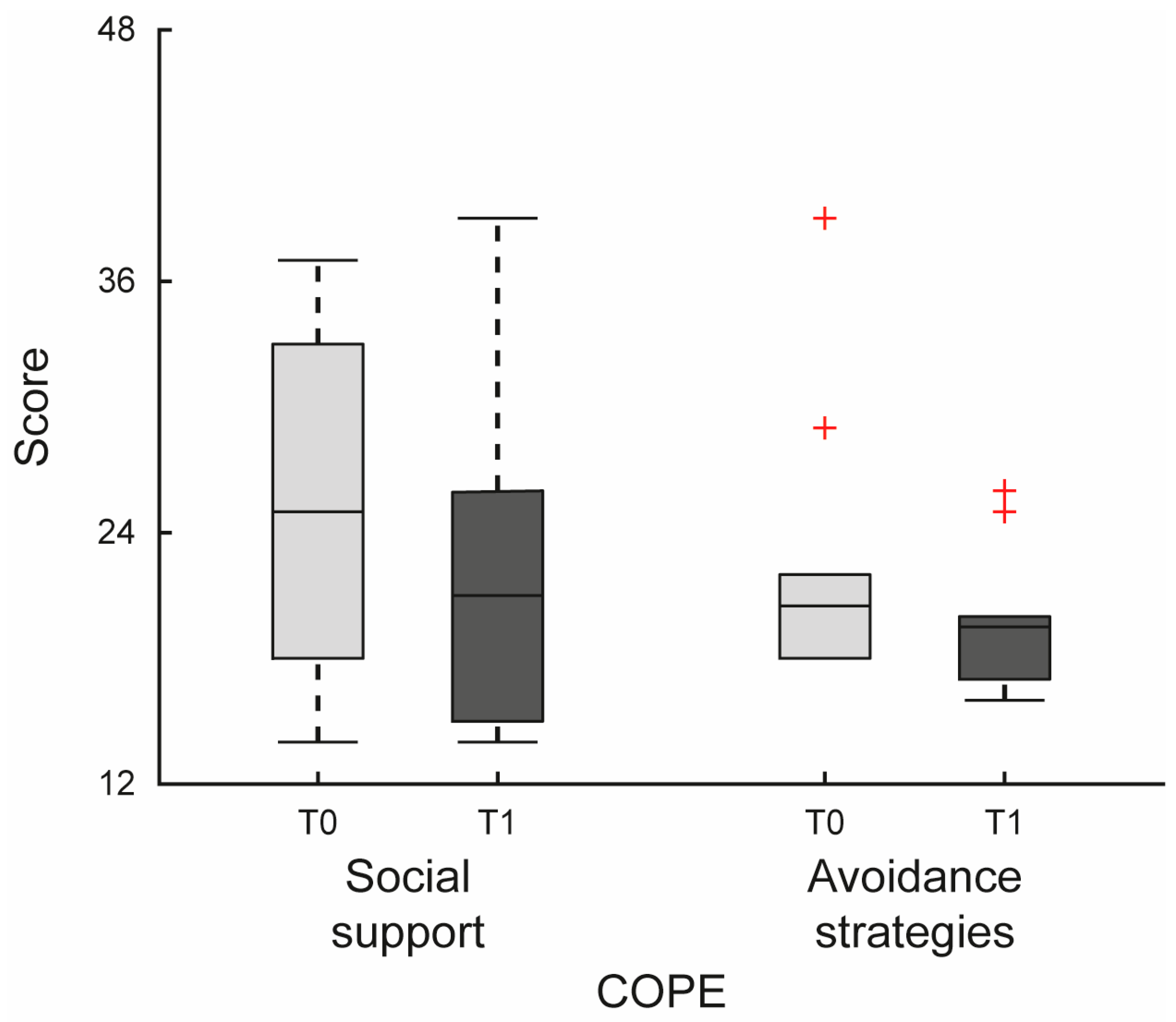
| COPE | Social Support | 25 (18–33) | 21 (15–26) | 0.023 | −3.5 | [−6, 0] |
| Avoidance Strategies | 20.5 (18–22) | 19.5 (17–20) | 0.033 | −3 | [−3.5, 0.5] | |
| Positive Attitude | 31.5 (28–34) | 30.5 (27–33) | 0.867 | 0 | [−4.5, 3] | |
| Orientation Problem | 34.5 (31–36) | 32.5 (29–38) | 0.945 | 0 | [−4, 4] | |
| Transcendent Orientation | 25 (22–27) | 22 (20–30) | 0.992 | −0.5 | [−2.5, 3] | |
| IPAQ | Vigorous IPAQ | 3660 (0–5760) | 1800 (960–4320) | 0.391 | 0 | [−2880, 1080] |
| Moderate-IPAQ | 1320 (0–3360) | 1320 (480–5040) | 0.723 | 480.0 | [−1560, 1440] | |
| Walking-IPAQ | 478.5 (0–1188) | 643.5 (396–1478.4) | 0.900 | 346.5 | [−1930.5, 2277] | |
| IPAQ | 6714 (2457–10,764) | 5310 (2259–9198) | 1.000 | 2395.2 | [−6129, 4158] | |
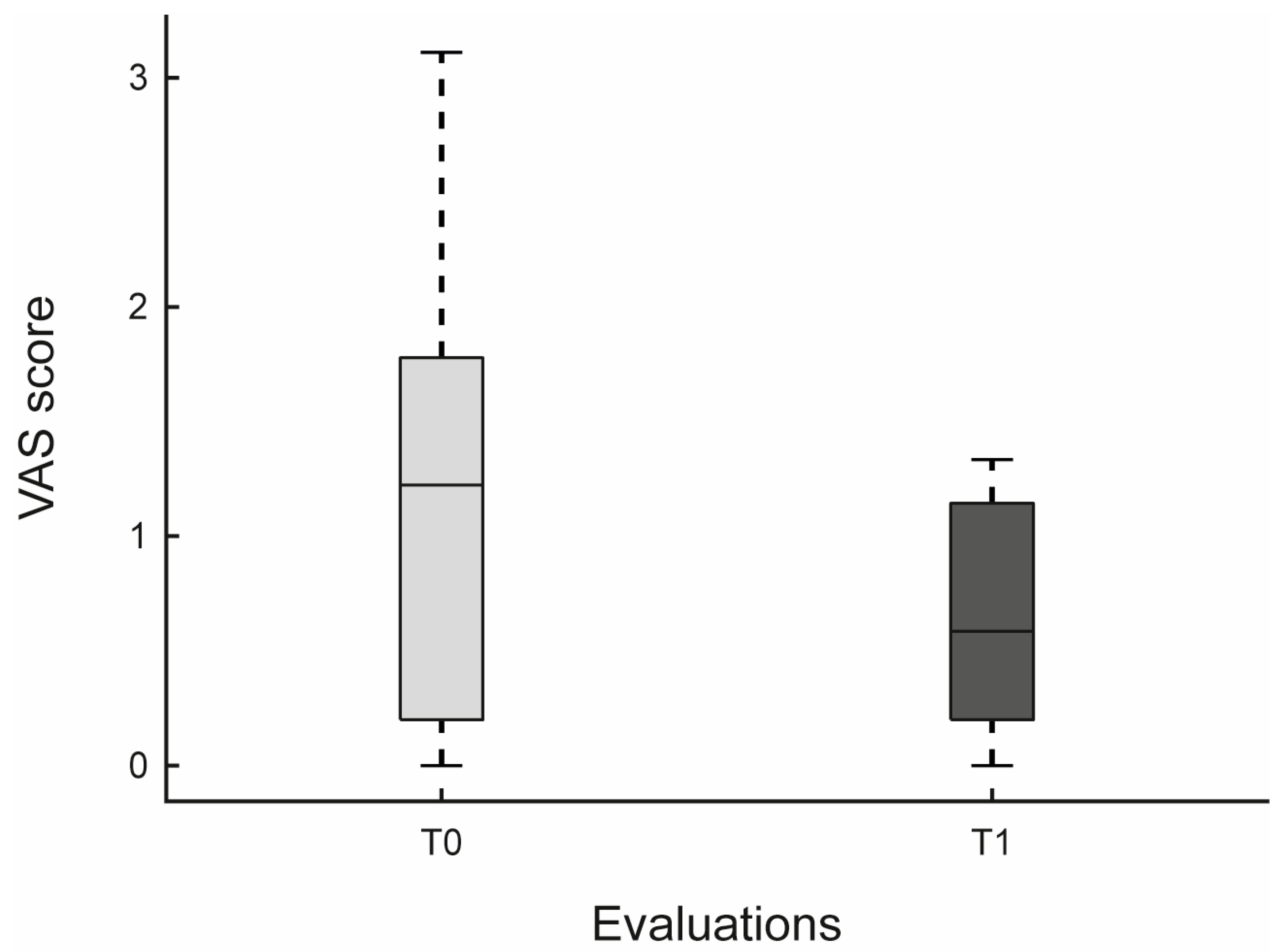
| VAS | 1.3 (1.27–1.27) | 0.6 (0.61–0.61) | 0.065 | −0.656 | [−1.333, −0.151] | |
| Execution difficulty | 0.6 (0.6–0.6) | 0.4 (0.4–0.4) | 0.813 | 0 | [−1, 0] | |
| Muscular difficulty | 0.4 (0.4–0.4) | 0.4 (0.4–0.4) | 0.703 | 0 | [−1, 1] | |
| Balance difficulty | 1.4 (1.4–1.4) | 0.9 (0.9–0.9) | 0.453 | −1 | [−2, 1] | |
| Instrumental data | ||||||
| Mobility left | 0.8 (0.78–0.78) | 0.8 (0.81–0.81) | 0.275 | 0.051 | [−0.017, 0.107] | |
| Mobility right | 0.8 (0.82–0.82) | 0.8 (0.85–0.85) | 0.160 | 0.049 | [−0.014, 0.080] | |
| Mobility full | 0.7 (0.7–0.7) | 0.7 (0.72–0.72) | 0.432 | 0.032 | [−0.047, 0.090] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, M.G.; Maione, R.; Migale, S.; Lombardo Facciale, A.; Pergolizzi, L.; Buonasera, P.; Fonti, B.; Bonanno, M.; Pistorino, G.; De Pasquale, P.; et al. Low-Intensity Virtual Reality Exercise for Caregivers of People with Mild Cognitive Impairment: A Pilot Study. J. Funct. Morphol. Kinesiol. 2025, 10, 353. https://doi.org/10.3390/jfmk10030353
Maggio MG, Maione R, Migale S, Lombardo Facciale A, Pergolizzi L, Buonasera P, Fonti B, Bonanno M, Pistorino G, De Pasquale P, et al. Low-Intensity Virtual Reality Exercise for Caregivers of People with Mild Cognitive Impairment: A Pilot Study. Journal of Functional Morphology and Kinesiology. 2025; 10(3):353. https://doi.org/10.3390/jfmk10030353
Chicago/Turabian StyleMaggio, Maria Grazia, Raffaela Maione, Silvia Migale, Antonino Lombardo Facciale, Luca Pergolizzi, Piero Buonasera, Bartolo Fonti, Mirjam Bonanno, Giulia Pistorino, Paolo De Pasquale, and et al. 2025. "Low-Intensity Virtual Reality Exercise for Caregivers of People with Mild Cognitive Impairment: A Pilot Study" Journal of Functional Morphology and Kinesiology 10, no. 3: 353. https://doi.org/10.3390/jfmk10030353
APA StyleMaggio, M. G., Maione, R., Migale, S., Lombardo Facciale, A., Pergolizzi, L., Buonasera, P., Fonti, B., Bonanno, M., Pistorino, G., De Pasquale, P., & Calabrò, R. S. (2025). Low-Intensity Virtual Reality Exercise for Caregivers of People with Mild Cognitive Impairment: A Pilot Study. Journal of Functional Morphology and Kinesiology, 10(3), 353. https://doi.org/10.3390/jfmk10030353








