Toward a Better Understanding of Hip Adductor Function: Internal Rotation Capability Revealed by Anatomical and MRI Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Functional Anatomy—Methods
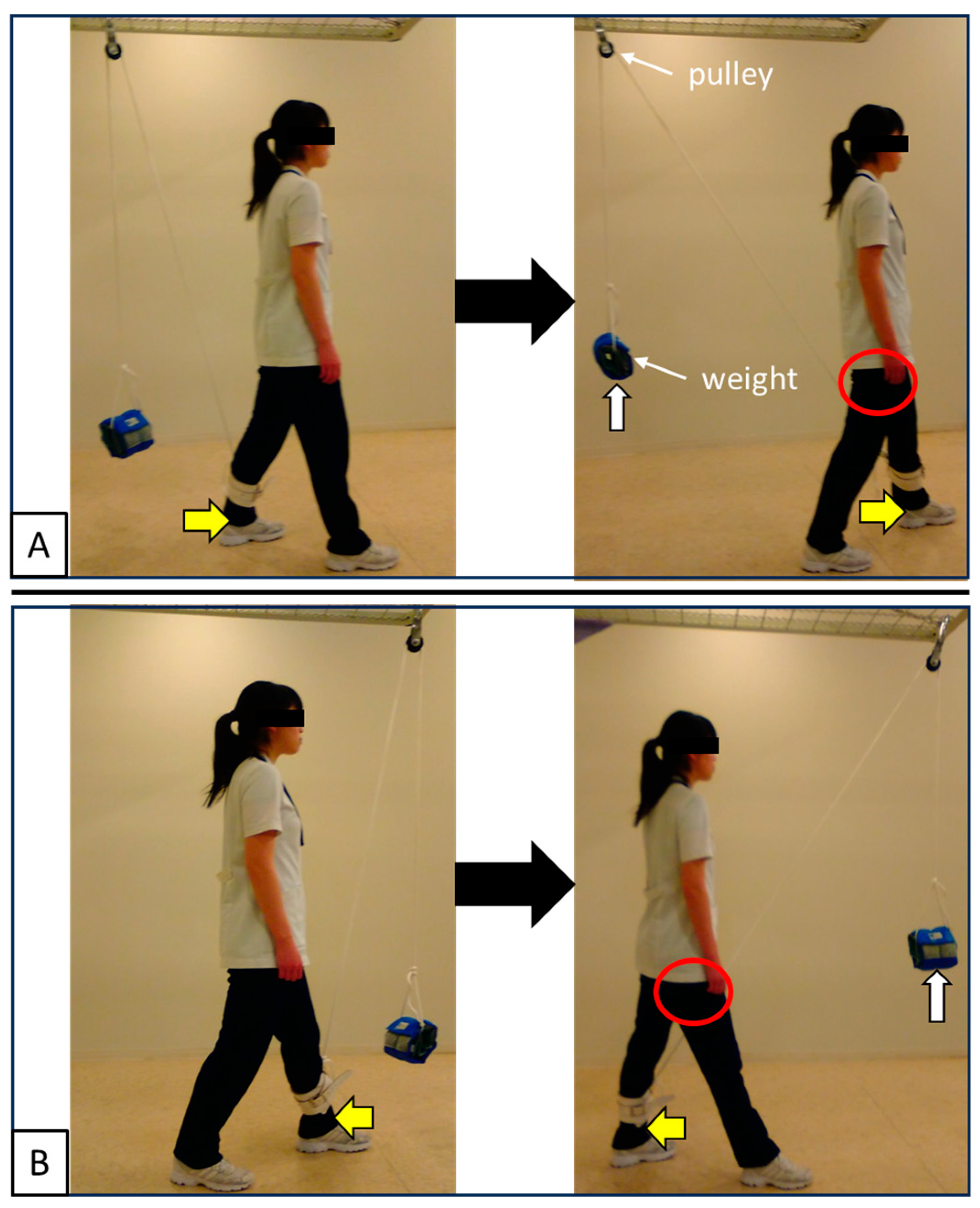
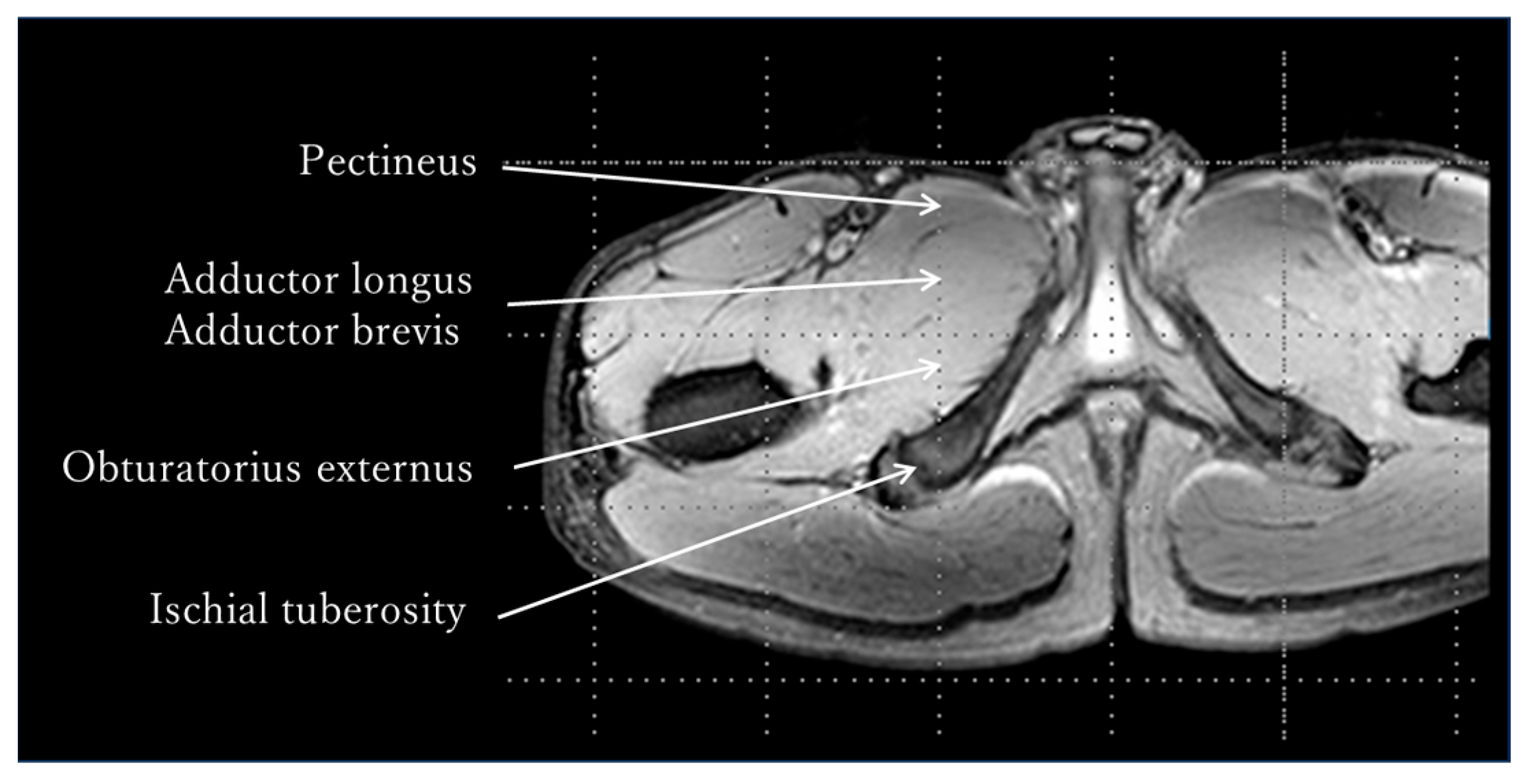
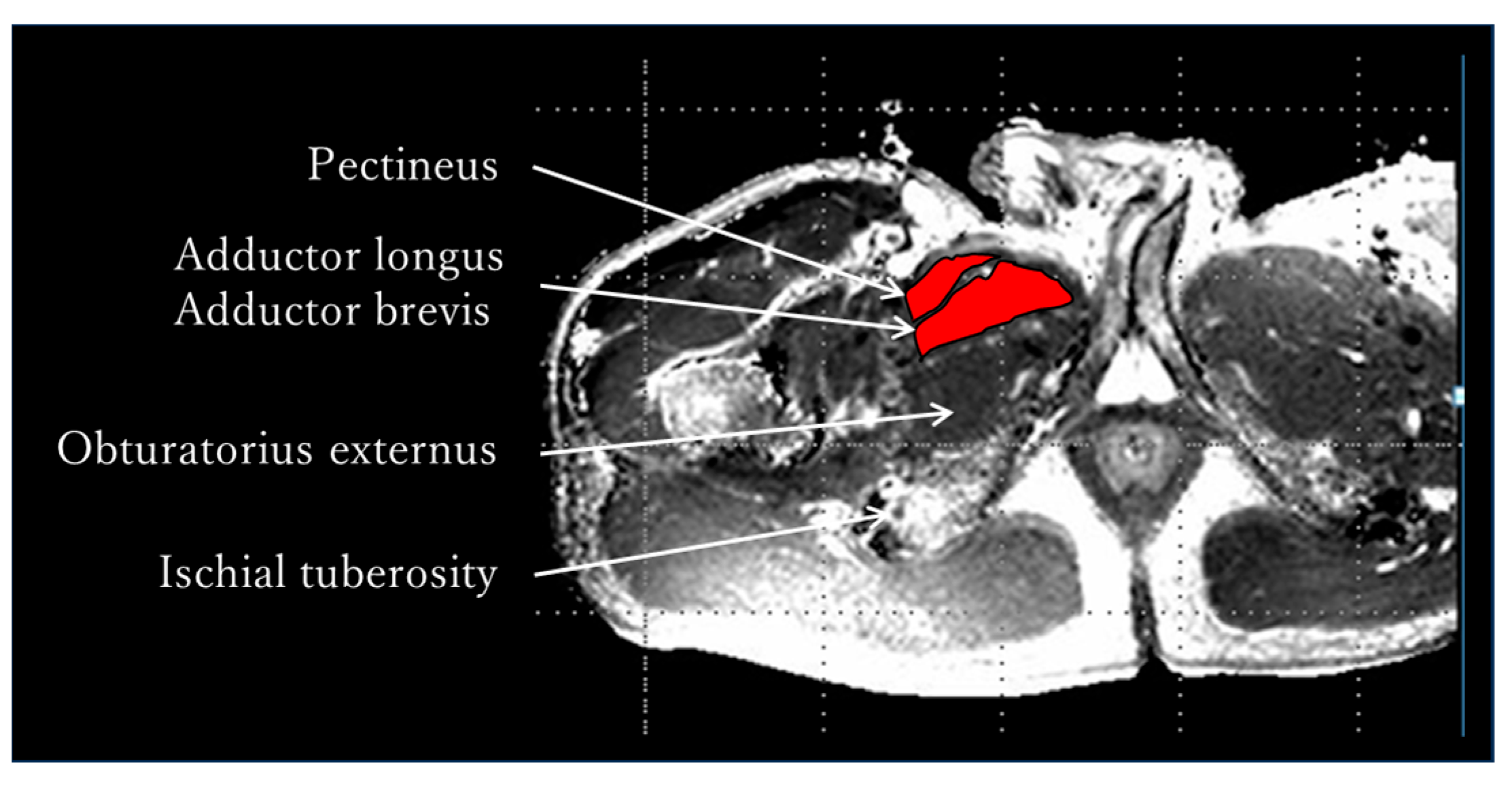
2.2. Evaluation of Skeletal Muscle Activity by MRI—Methods
3. Results
3.1. Functional Anatomy—Results
3.2. Evaluation of Skeletal Muscle Activity by MRI—Results
4. Discussion
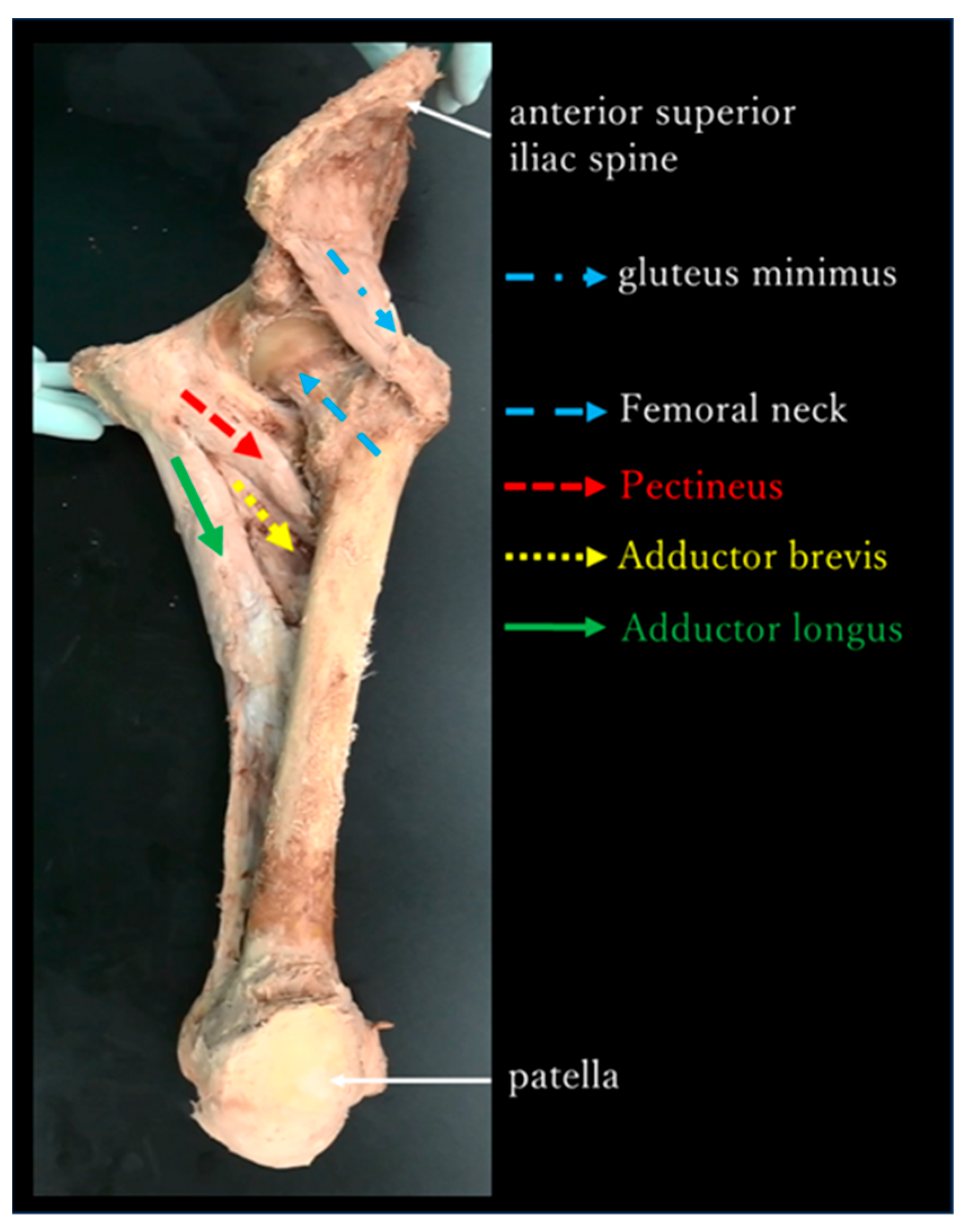
- The adductor muscle group in the neutral position of the hip joint was almost parallel to the femoral neck and gluteus minimus in the frontal plane (Figure 5).
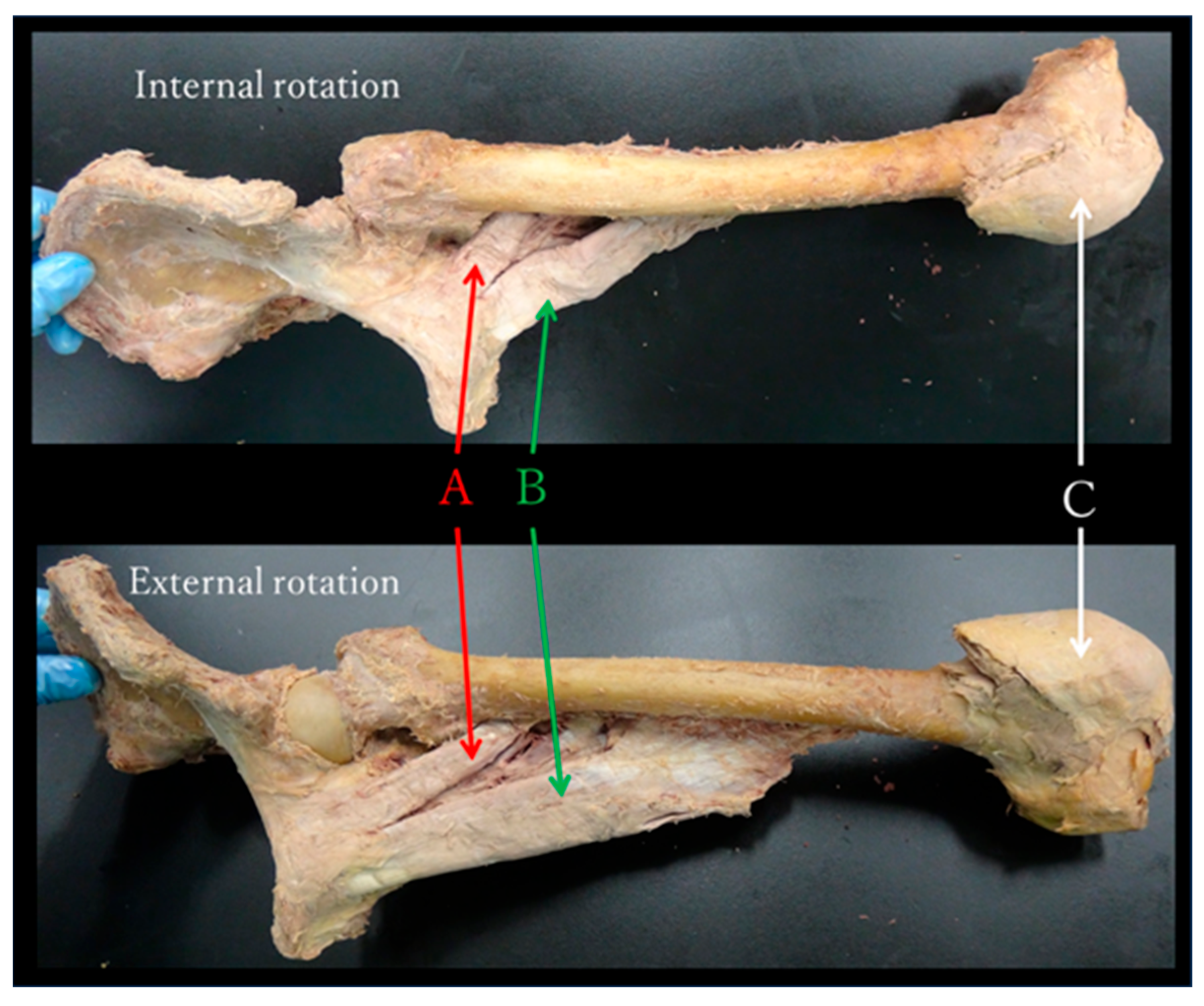
- During passive movement, the internal rotation of the hip joint shortened the adductor muscle group (Figure 6).
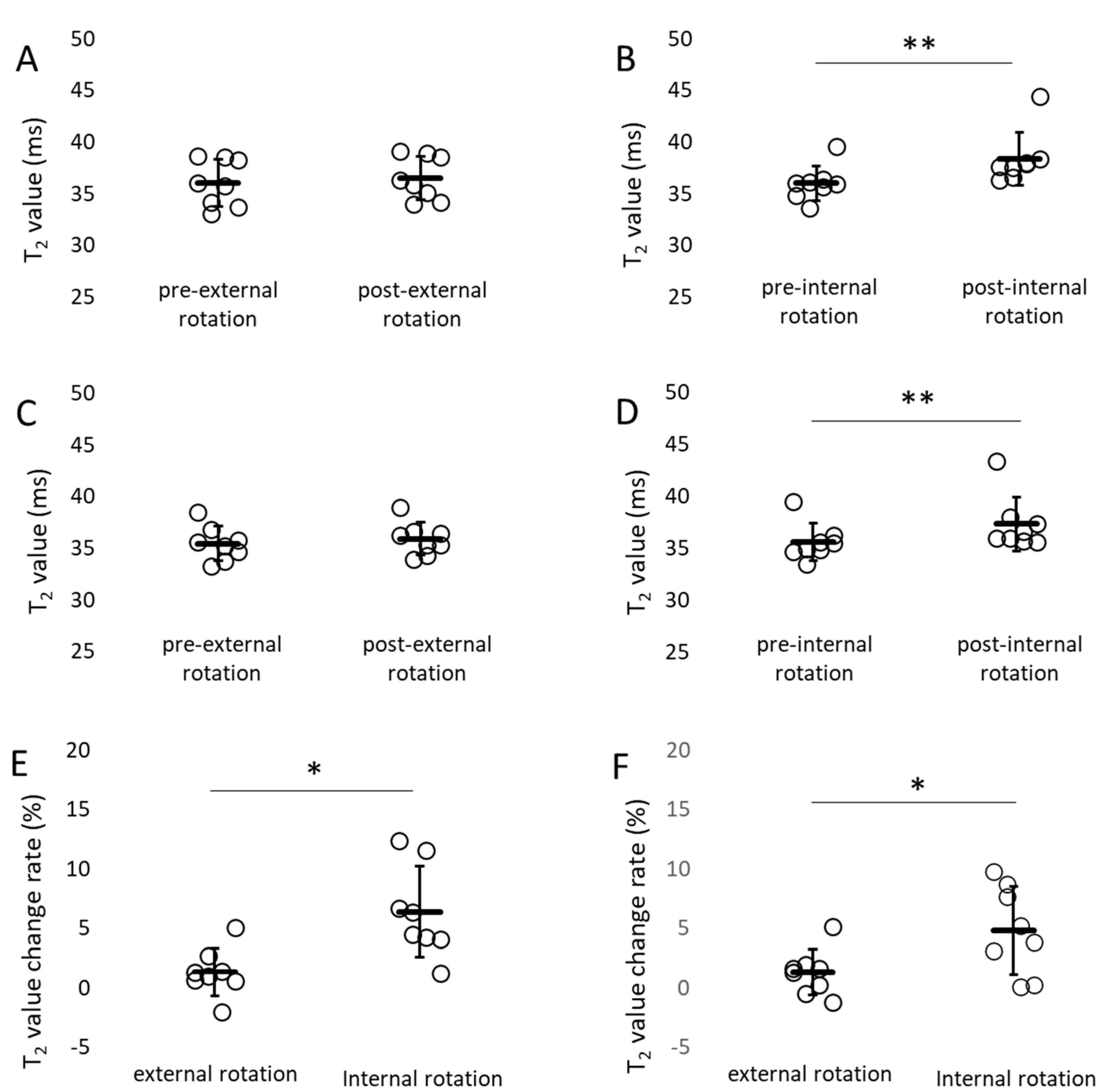
- The percent change in T2 values pre- and post-internal rotation exercise in the adductor muscle group was significantly higher than that pre- and post-external rotation exercise (Figure 7).
4.1. Functional Anatomy—Discussion
4.2. Evaluation of Skeletal Muscle Activity by MRI—Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| ROI | Region of Interest |
| ICC | Intraclass Correlation Coefficient |
| CKC | Closed Kinetic Chain |
| OKC | Open Kinetic Chain |
References
- Dostal, W.F.; Soderberg, G.L.; Andrews, J.G. Actions of Hip Muscles. Phys. Ther. 1986, 66, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.A. Kinesiology of the hip: A focus on muscular actions. J. Orthop. Sports Phys. Ther. 2010, 40, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Saladin, K. Human Anatomy, 3rd ed.; McGraw-Hill Science/Engineering/Math: New York, NY, USA, 2010; pp. 311–312. [Google Scholar]
- Standring, S. (Ed.) Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1374–1386. [Google Scholar]
- Tortora, G.J.; Grabowski, S.R. Principles of Anatomy and Physiology, 9th ed.; Wiley: Hoboken, NJ, USA, 2000; p. 359. [Google Scholar]
- Moore, K.L.; Agur, A.M.R.; Dalley, A.F., II. Essential Clinical Anatomy; Lippincott Williams and Wilkins: Baltimore, MD, USA, 1996; p. 234. [Google Scholar]
- Agur, A.M.R.; Dalley, A.F., II. Grant’s Atlas of Anatomy, 15th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2021; p. 500. [Google Scholar]
- Gilroy, A.M.; MacPherson, B.R.; Schuenke, M.; Schulte, E.; Schumacher, U. Atlas of Anatomy, 3rd ed.; Thieme Medical Publishers: New York, NY, USA, 2016; p. 422. [Google Scholar]
- Kapandji, I.A. The Physiology of the Joints, Vol 2, Lower Limb, 5th ed.; Churchill Livingstone: New York, NY, USA, 1988; p. 328. [Google Scholar]
- Snell, R.S. Anonymous Clinical Anatomy by Regions, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 578–584. [Google Scholar]
- Leighton, R.D. A functional model to describe the action of the adductor muscles at the hip in the transverse plane. Physiother. Theory Pract. 2006, 22, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Delp, S.L.; Hess, W.E.; Hungerford, D.S.; Jones, L.C. Variation of rotation moment arms with hip flexion. J. Biomech. 1999, 32, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Kourosh, S.; Leveau, B. The functional anatomy of tensor fasciae latae and gluteus medius and minimus. J. Anat. 1989, 166, 179–189. [Google Scholar] [PubMed]
- Fleckenstein, J.L.; Canby, R.C.; Parkey, R.W.; Peshock, R.M. Acute effects of exercise on MR imaging of skeletal muscle in normal volunteers. Am. J. Roentgenol. (1976) 1988, 151, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.J.; Meyer, R.A.; Adams, G.R.; Foley, J.M.; Potchen, E.J. Direct relationship between proton T2 and exercise intensity in skeletal muscle MR images. Investig. Radiol. 1990, 25, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.; Alexander, A.L.; Laidlaw, D.H.; Gmitro, A.F.; Unger, E.C.; Enoka, R.M. Sensitivity of muscle proton spin-spin relaxation time as an index of muscle activation. J. Appl. Physiol. (1985) 1994, 77, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T. 1H-nuclear magnetic resonance evidence for acto-myosin-dependent structural changes of the intracellular water of frog skeletal muscle fiber. Biochim. Biophys. Acta 1998, 1379, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.R.; Duvoisin, M.R.; Dudley, G.A. Magnetic resonance imaging and electromyography as indexes of muscle function. J. Appl. Physiol. (1985) 1992, 73, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.; Shiba, N.; Higuchi, F.; Nishimura, H.; Inoue, A. Functional evaluation of hip abductor muscles with use of magnetic resonance imaging. J. Orthop. Res. 1997, 15, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Kinoshita, K.; Thida, M.; Kawai, Y.; Kamikubo, T.; Abo, M. The function of the iliacus using Magnetic Resonance Imaging (MRI)—Based on anatomical findings by cadaver. Rigakuryouhougaku 2010, 37, 356–363. (In Japanese) [Google Scholar]
- Takizawa, M.; Suzuki, D.; Ito, H.; Fujimiya, M.; Uchiyama, E. Why adductor magnus muscle is large: The function based on muscle morphology in cadavers. Scand J. Med. Sci. Sports 2014, 24, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.L.; Pizzari, T.; Rath, L.; Tucker, K.; Semciw, A.I. Adductor magnus: An EMG investigation into proximal and distal portions and direction specific action. Clin. Anat. 2018, 31, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.P.; Drought, A.B.; Kory, R.C. Walking patterns of normal men. J. Bone Jt. Surg. Am. 1964, 46, 335–360. [Google Scholar] [CrossRef]

| Shapiro-Wilk Test | F-Test | |||
|---|---|---|---|---|
| Pre- vs. Post-External Rotation Exercise | Pre- vs. Post-Internal Rotation Exercise | Pre- vs. Post-External Rotation Exercise | Pre- vs. Post-Internal Rotation Exercise | |
| Pectineus | 0.45 | 0.41 | 0.86 | 1 |
| Adductor longus and brevis | 0.36 | 0.75 | 0.29 | 0.35 |
| Effect Size Analysis (Hedges’ g) | Power (1 − β) | |||
|---|---|---|---|---|
| Pre- vs. Post-External Rotation Exercise | Pre- vs. Post-Internal Rotation Exercise | Pre- vs. Post-External Rotation Exercise | Pre- vs. Post-Internal Rotation Exercise | |
| Pectineus | 0.59 | 1.40 | 0.39 | 0.97 |
| Adductor longus and brevis | 0.58 | 1.10 | 0.38 | 0.86 |
| 95% CI | t-Test Statistics (t-Value and Degrees of Freedom) | |||
|---|---|---|---|---|
| Pre- vs. Post-External Rotation Exercise | Pre- vs. Post-Internal Rotation Exercise | Pre- vs. Post-External Rotation Exercise | Pre- vs. Post-Internal Rotation Exercise | |
| Pectineus | −0.10~1.04 | 1.11~3.51 | 1.94 7 | 4.55 7 |
| Adductor longus and brevis | −0.11~1.01 | 0.59~2.88 | 1.91 7 | 3.58 7 |
| ICC (1,1) | Pre-External Rotation Exercise | Post-External Rotation Exercise | Pre-Internal Rotation Exercise | Post-Internal Rotation Exercise |
| Pectineus | 0.82 | 0.99 | 0.99 | 0.97 |
| Adductor longus and brevis | 0.95 | 0.92 | 0.91 | 0.93 |
| ICC (2,1) | Pre-External Rotation Exercise | Post-External Rotation Exercise | Pre-Internal Rotation Exercise | Post-Internal Rotation Exercise |
| Pectineus | 0.89 | 0.86 | 0.85 | 0.94 |
| Adductor longus and brevis | 0.75 | 0.94 | 0.75 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, K.; Kinoshita, K.; Senoo, A.; Watanabe, M. Toward a Better Understanding of Hip Adductor Function: Internal Rotation Capability Revealed by Anatomical and MRI Evaluation. J. Funct. Morphol. Kinesiol. 2025, 10, 354. https://doi.org/10.3390/jfmk10030354
Hirano K, Kinoshita K, Senoo A, Watanabe M. Toward a Better Understanding of Hip Adductor Function: Internal Rotation Capability Revealed by Anatomical and MRI Evaluation. Journal of Functional Morphology and Kinesiology. 2025; 10(3):354. https://doi.org/10.3390/jfmk10030354
Chicago/Turabian StyleHirano, Kazuhiro, Kazuo Kinoshita, Atsushi Senoo, and Masaru Watanabe. 2025. "Toward a Better Understanding of Hip Adductor Function: Internal Rotation Capability Revealed by Anatomical and MRI Evaluation" Journal of Functional Morphology and Kinesiology 10, no. 3: 354. https://doi.org/10.3390/jfmk10030354
APA StyleHirano, K., Kinoshita, K., Senoo, A., & Watanabe, M. (2025). Toward a Better Understanding of Hip Adductor Function: Internal Rotation Capability Revealed by Anatomical and MRI Evaluation. Journal of Functional Morphology and Kinesiology, 10(3), 354. https://doi.org/10.3390/jfmk10030354






