Mobile Health Interventions for Individuals with Type 2 Diabetes and Overweight or Obesity—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. The Criteria for Considering Studies for Inclusion in the Review
2.2. Protocol for Electronic Searching
2.3. Study Selection and Data Extraction
2.4. Risk of Bias in Individual Studies
2.5. Quality of Evidence
2.6. Statistical Analysis
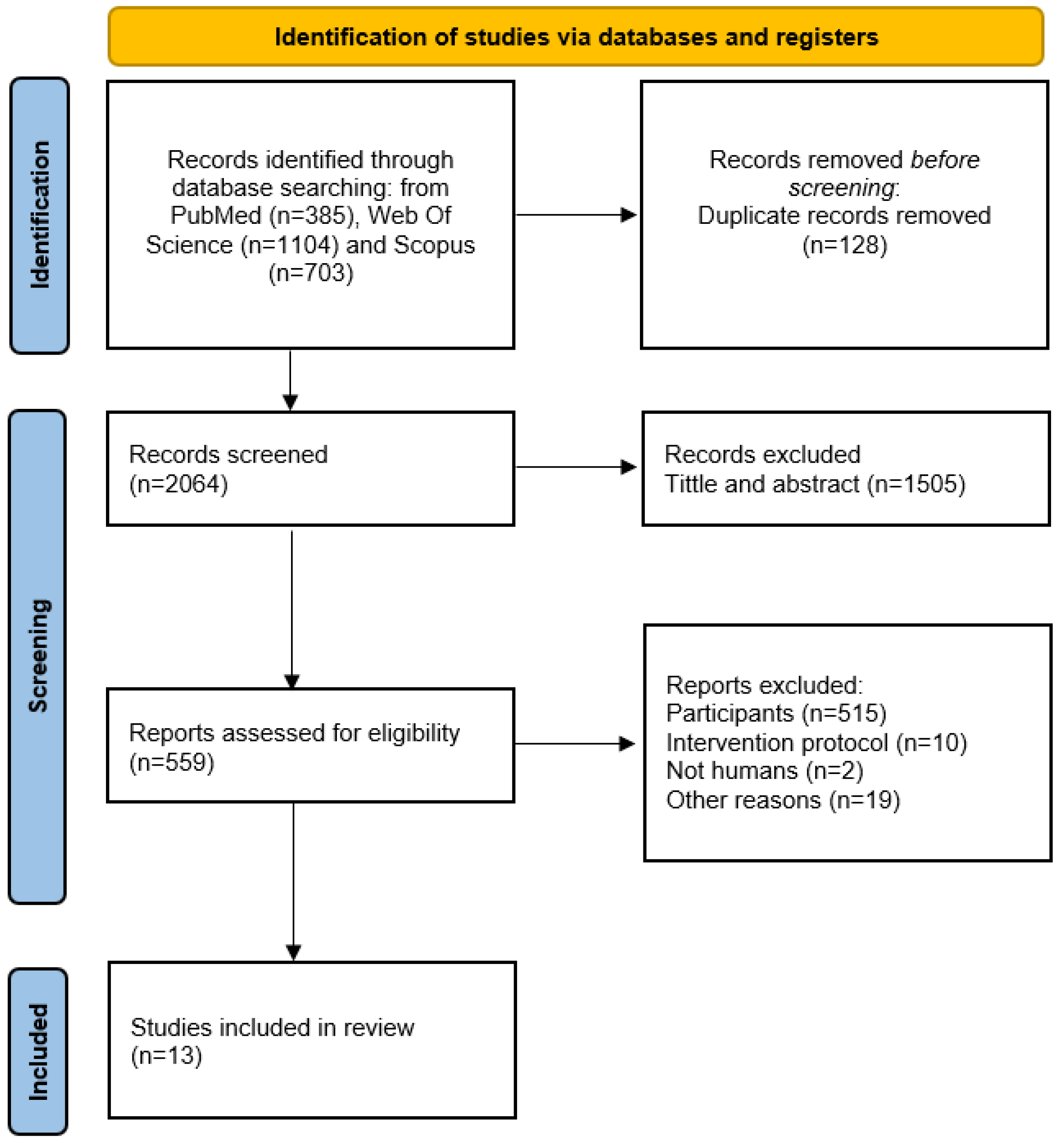
3. Results
3.1. Studies Selected
3.2. Description of Selected Studies
| Study (Year) | Setting | Total (EG/CG) | % Males | Age (Years), Mean (SD) | EG/CG Pre-Intervention | EG/CG Post Intervention | % Dropout | % HbA1c at Baseline or Range (mmol/mol) | BMI (kg/m2) at Baseline or Range | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bender et al. (2017) [54] | USA | n = 45 (EG, n = 22/CG, n = 23) | 38% | 57.6 (9.8) | n = 45 (22/23) | n = 45 (22/23) | 0% | 7.42 (0.8) | 30.1 (4.6) | Adherence to additional mHealth engagement measures | Weight, BMI, HbA1c, and daily step counts. |
| Bentley et al. (2016) [66] | UK | n = 27 (EG, n = 18/CG, n = 9) | 44% | 52.9 (8.6) | n = 27 (18/9) | n = 20 (13/7) | 25% | Range between 57.6 and 65.8 | Range between 25 and 40 | Adherence to using the device | Wright and HbA1c |
| Block et al. (2015) [62] | USA | n = 339 (EG, n = 163/CG, n = 176) | 69% | 55.0 (8.9) | n = 339 (163/176) | n = 292 (136/156) | 13% | 5.6 (0.3) | 31.2 (4.4) | HbA1c and fasting glucose. | Weight, BMI, waist circumference, TG to HDL ratio, and metabolic syndrome. |
| Christensen et al. (2022) [55] | Denmark | n = 340 (EG, n = 200/CG, n = 140) with T2D n = 168 (EG, n = 98/CG, n = 70) | With 24 months follow up: 38% Without 24 months follow up 35% | With 24 months follow-up: EG: 53.9 (9.2) CG: 53 (11.6) Without 24 months follow-up EG: 51 (10.9) CG: 51.7 (12.1) | n = 340 (200/140) with T2D n = 168 (98/70) | n = 132 (81/51) with T2D n = 65 (40/25) | 61% total participants | Range between 47.6 and 48.9 | 34.7 (3.9), 35.7 (3.8), 35.6 (3.7), 35.8 (5.0) | Weight | HbA1c level, waist/hip ratio (WHR), systolic and diastolic blood pressure, total TG, HDL, LDL, smoking status, and quality of life. |
| Christensen et al. (2022) [65] | Denmark | n = 170 (EG, n = 100/CG, n = 70) | EG 51%/CG 68% | EG 56.1 (7.3); CG 57.1 (9.9) | n = 170 (100/70) | n = 128 (75/53) | 24% | 7.4 (1.3) | 34.7 (3.29), 35.0 (4.40) | Weight | HbA1c |
| De Luca et al. (2023) [63] | Italy | n = 200 (EG, n = 100/CG, n = 100) | EG 83% CG 70% | EG 61.1 (9.4); CG 66.5 (9.0) | n = 200 (100/100) | n = 192 (92/100) | Not reported | 7 (0.9) | 29.6 (5.0) | Hb1Ac | Weight, blood pressure (systolic and diastolic), plasma cholesterol, plasma triglycerides, LDL, and HDL |
| Hesseldal at al (2022) [56] | Denmark | n = 338 (EG, n = 198/CG, n = 140) | 37% | 52.3 (11.0) | n = 338 (198/140) | n = 200 (127/73) | 40% | 6.6 (1.3) | 35.3 (3.8) | Weight | HbA1c |
| Kim et al. (2024) [67] | Korea | n = 200 (EG, n = 134/CG, n = 66) | 69% | EG 57.1 (7.2) CG 58.3 (5.8) | n = 200 (134/66) | n = 182 (119/63) | 17% | 7.1 (0.4) | 26.5 (2.6) | Step counts | HbA1c |
| Lim et al. (2022) [59] | Singapore | n = 148 (EG, n = 72/CG, n = 76) | 60% | 53.1 (9.3) | n = 148 (72/76) | n = 140 (67/73) | 5% | 5.9 (0.5) | 29.8 (4.1) | Weight (6 months) | HbA1c, FBG, blood pressure, serum lipids, creatinine, dietary intake and physical activity |
| Moravcová et al. (2024) [61] | Czech Republic | n = 100 (EG, n = 50/CG, n = 50) | 29% | 43.3 (9.5) | n = 84 (42/42) | n = 60 (32/28) | 40% | 5.7 (0.8) | 39.4 (6.8) | Weight | HbA1c, BMI, waist circumference, body fat, fasting glucose |
| Wang et al. (2018) [57] | USA | n = 26 (EG1, n = 11/EG2, n = 9/CG, n = 6) | 42% | 56.4 | n = 26 (11/9/6) | n = 24 (10/8/6) | 7% | 8.4% (2.3)–10.4% (2.4) | 38.1 kg/m2 | HbA1c | Weight |
| Whittemore at al (2020) [64] | Mexico | n = 47 (EG, n = 26/CG, n = 21) | 35% | EG 53.9 (9.2) CG 56.8 (8.3) | n = 47 (EG, n = 26/CG, n = 21) | n = 44 (EG, n = 24/CG, n = 20) | 6% | 9.2% (1.5) | EG: 31.0 (6.1) CG: 29.5 (5.0) | HbA1c | BMI, diastolic and systolic blood pressure, and PA |
| Yin et al. (2022) [58] | China | n = 120 (EG, n = 60/CG, n = 60) | 40% | 47.3 | n = 120 (60/60) | n = 99 (52/47) | 17% | 8.5% (0.8) | EC: 29.05 kg/m2 (3.31); CG: 29.2 kg/m2 (2.9) | HbA1c | Postprandial blood glucose, FBG, BMI, total cholesterol, TG, LDL and HDL, blood urea nitrogen, creatinine, and estimated glomerular filtration rate |
| Study (Year) | Type of Intervention | Type of Technology Used/mHealth Tools Needed in the Intervention | Groups Description | Hybrid Intervention * | Duration of Intervention | Follow-Up | Adherence (%) | Observations |
|---|---|---|---|---|---|---|---|---|
| Bender et al. (2017) [54] | Lifestyle intervention based on diabetes prevention program, modified to incorporate mobile technologies (Fitbit accelerometer plus app with diary) and private Facebook group for healthy behaviors tracking, real-time feedback, coaching, and virtual social support. | Mobile-based, virtual support and Fitbit accelerometer/wearable plus associated mobile app | EG: Phase 1: (3 months): Self-monitor real-time PA steps and daily food/calorie intake, and weekly weight. Virtual social support, coaching, weekly education topics, and individualized weight loss goals. Phase 2: Transitioned to a 3-month follow-up, removed from private Facebook group, and asked to continue using their Fitbit and app with diary to track health behaviors and maintain weight goals. CG: The control group was a waitlist group. Phase 1: Received only the Fitbit accelerometer and training about daily wear. Phase 2: At the 3-month office visit, they transitioned to receive the PilAm Go4health Intervention. | No | 6 months | EG: monthly for phase 1 Months 4 and 6 for phase 2 CG: months 1 and 3 for phase 1 Months 4, 5 and 6 for phase 2 | Attendance to all visits: EG, 95%; CG, 100% Wearing the Fitbit at least 5 days/week: EG, 97%; CG, 91% | N/A |
| Bentley et al. (2016) [66] | Training on appropriate behaviors to lose weight and control HbA1c that included automatic recording of PA and nutritional intake for eating healthily by using a wearable device called AiperMotion 500 plus qualitative interviews. | Mobile-based, wearable device, email support service/wearable plus associated mobile app | EG: Divided into groups 2 and 3: both groups received 90 min group training around appropriate behaviors to lose weight and control their HbA1c, specifically: diet, maximizing PA, and neuro-linguistic programming. Group 2 received additional 60 min training in the use of the AiperMotion 500. They were asked to enter individual characteristics and dietary information. They were asked to wear the device during walking hours. They received motivational feedback. Group 3 was asked to send weekly emails to the research team describing any positive or negative events that had impacted their conformance with the study or motivation to lose weight. CG: Received 90 min group training around appropriate behaviors to lose weight and control their HbA1c, specifically: diet, maximizing PA and Neuro-Linguistic Programming but no further training on how to use the device. Impossible to download the data from the device (no feedback) | Yes | 3 months | 4 months | % days worn from total Weeks 1–6 (G2: 62%, G3: 61%); weeks 7–12 (G2: 65%, G3: 69%); weeks 13–16 (G2: 75%, G3: 94%). % diet entries (at least 950 kcal) from total Weeks 1–6 (G2: 62%, G3:61%); weeks 7–12 (G2: 59%, G3: 70%); weeks 13–16 (G2: 49%, G3: 70%). % emails asked (G3): 31% | N/A |
| Block et al. (2015) [62] | Alive-PD (program design). Alive-PD provides a 1-year program of regular contact and goal setting, weekly in the first 6 months and biweekly thereafter, plus midweek automated email and mobile phone reminders. The program includes individually tailored weekly goal setting and other activities delivered via web and email, supplemented by automated IVR phone calls and a supportive mobile phone app. | Mobile-based, web-based, and email supplemented by automated IVR phone calls/mobile app | EG: Received the Alive-PD. CG: No mobile-based interventions, emails, or phone calls were provided to this group. Participants continued receiving their usual care through the health center. They received no further contact from the online Alive-PD system except reminders to complete a 3-month and 6-month online follow-up questionnaire. | No | 6 months | 3 and 6 months, optional additional clinic visits at 9 and 12 | EG, n = 163 set behavioral goals or otherwise interacted with the online Alive-PD in a median of 17 of the 24 weeks (70.8% of the weeks). In all, 87.1% (142/163) interacted with the program in 4 or more of the 24 weeks, and 70.6% (115/163) were still interacting with the program in the last month of the 6-month period. | N/A |
| Christensen et al. (2022) [55] | Telehealth lifestyle-coaching program (Liva 1.0) leads to long-term (24 months) weight loss compared to usual care. | Mobile-based and web-based telehealth lifestyle coaching program/mobile app | EG: Intervention using the Liva app telehealth lifestyle–coaching, starting with online face-to-face consultation to define SMART goals. After the first session, coaching was performed asynchronously. The first 6 months of structured educational material and motivational support were provided weekly from the lifestyle coaches, biweekly for the next 6 months, and after 12 months, participants only received structured educational material and lifestyle coaching every third month. CG: Participants randomized to the control group were offered to receive the standard municipal secondary or tertiary preventive care service with information about diet and physical activity, and how to develop healthy habits. A few of them included the promotion of well-being and social participation. The participants in the control group were not offered a specific ‘usual care’ program but participated in whatever the local municipality offered in accordance with the Danish Health Care Act of 2005. | No | 24 months | 6, 12, and 24 months | Not reported | Most of the dropouts were random or due to coronavirus disease 2019 restrictions |
| Christensen et al. (2022) [65] | eHealth app LIVA 2.0 (long-term lifestyle change intervention and eHealth application) combined with BCTs such as self-monitoring, reminders, tailored information, personal feedback, and face-to-face support. | Mobile-based and health coaching/mobile app | EG: They received the individualized digital lifestyle coaching LIVA 2.0. Each patient and their health coach discussed and agreed on goals for diet, physical exercise, sleep, and any other relevant lifestyle areas. Weekly coaching for the first three months, and biweekly for the next three months. The intervention included a high degree of BCTs. CG: Examinations at the same frequency as the intervention group. At the first examination, and after they were randomized in the control group, they were advised to contact their general practitioner (GP) who could provide guidance about their health problems and further refer them to diabetes programs in their municipalities that included education about diet, exercise, and different forms of behavioral change techniques (BCTs). The control group did not have access to the app, nor did they receive any digital interventions from LIVA 2.0. | Yes | 6 months | 6 months | Not reported | 25% of patients lost to follow-up at six months due to unknown reasons |
| De Luca et al. (2017) [63] | The ProEmpower solutions enabled the collection of clinical parameters by the patient, using a smartphone integrated with medical devices. The data collected by the integrated devices (glucometer, sphygmomanometer, scale, smart watch for heart rate monitoring, and step counter) were automatically sent to the shared care plan. | Mobile-based/mobile app (integrated with medical devices such as glucometer, sphygmomanometer, scale, smart watch for heart rate monitoring, and step counter) | EG: At baseline and after an average follow-up of 8 months, glycosylated hemoglobin, body weight, blood pressure, and blood lipids were measured in the experimental group using the ProEmpower solutions. CG: Participants randomized to this group did not receive the ProEmpower mobile-based intervention. They continued with their pre-study habits and served as a comparison group for the analysis of outcomes. | No | 8 months | 8 months | Not reported | The pandemic restrictions affected the completeness of the data (follow-up visits and scheduled measurement) |
| Hesseldal at al (2022) [56] | Digital coaching intervention: initial 1 h face-to-face motivational interview followed by digital coaching using behavioral change techniques enabled by individual live monitoring. | Mobile-based and virtual coaching/mobile app | EG: Usual care plus digital lifestyle coaching. After the initial interview from the health care professionals, they received the health coach weekly (asynchronous digital coaching for each participant) that included inspiring them, commending them on goal attainment, and seeking to help them stay motivated. The subsequent asynchronous eHealth coaching sessions were carried out once a week for the first 6 months and then once a month for the last 6 months, as maintenance. CG: They received only the usual care preferred by the patient and their doctor. | No | 12 months | 6 and 12 months | Not reported | Many of the dropouts occurred at random due to COVID-19 restrictions; this may explain the nonsignificant effect of the intervention on HbA1c |
| Kim et al. (2024) [67] | Physical activity encouragement intervention based on a smartphone personal health record (PHR) application on step count increases, glycemic control, and body weight. | Mobile-based/mobile app with step count | CG: Used a smartphone PHR app. EG: Used the app and received individualized motivational text messages (intervention group) to increase daily steps. | No | 3 months | 3 and 6 months | Not reported | N/A |
| Lim et al. (2021) [59] | Intervention through the nBuddy Diabetes mobile app and educated to self-monitor their weight, diet, physical activity, and blood glucose levels for 6 months. | Mobile-based/mobile app | EG: At baseline received standard face-to-face dietary advice from a dietitian, were provided with a digital weighing scale, and were encouraged to 150 min per week of moderate intensity PA. They used the Nutritionist Buddy Diabetes mobile app that includes goal–setting, stimulus control, problem solving, self-monitoring their diet, PA, weight and blood glucose levels, cognitive restructuring, and motivational interviewing. CG: At baseline, received standard face-to-face dietary advice from a dietitian, were provided with a digital weighing scale, and were encouraged to 150 min per week of moderate intensity PA. | No | 6 months | 3 and 6 months | Median overall app utilization in the intervention group was 97.8% during the first 3 months and 91.7% during 4 to 6 months of the intervention period. The average two-way dietitian-to-participant interactions via the app’s chat function were 3 days per week in the first 3 months, and 2 days per week in the subsequent 3 months. | N/A |
| Moravcová et al. (2024) [61] | Comparison between the effects of an intensive in-person weight loss intervention program and Vitadio digital therapy (e-health). | Mobile-based and virtual coaching/mobile app | EG: Intervention through Vitadio, which is a certified class I medical device designed to support diabetes patients in making healthy lifestyle choices and improving their self-management, consisting of a 3-month intensive phase followed by a sustaining phase. The application uses a series of personalized daily tasks and automated messages to help patients establish a new, healthy routine. CG: This group was offered access to five in-person lifestyle consultations with a physician, dietitian, and/or educational nurse with a nutrition background from the Department of Exercise Medicine and Cardiovascular Rehabilitation. | Yes | 6 months | 3 and 6 months | Not reported | Plans to extend the study to evaluate the durability of these effects were hindered by high attrition rates following the intervention period due to the COVID-19 pandemic, which created significant obstacles for RCTs requiring in-person clinical assessments in hospital settings |
| Wang et al. (2018) [57] | Behavioral lifestyle intervention using mobile or paper-based tools for self-monitoring. | Mobile health-based self-monitoring and online telehealth/smartphone and mobile app and devices (pedometers, weight scales, and food scales) | EG: Divided into 2 groups (mobile group and paper group) received a standard behavioral lifestyle intervention comprising 11 group sessions—weekly for month 1, biweekly for months 2 and 3, and monthly for months 4 to 6, and an individual session after month 3. Participants received training on how to self-monitor their diet and exercise habits, weight, and blood glucose in the first two sessions. Both groups were instructed to record their exercise activities (minutes and type), specify the foods they ate (amount, number of calories, fat grams, and carbohydrates), their weight, and their blood glucose using a paper diary or an electronic diary, depending on their group randomization. CG: Individual visits or interactive group classes. Patients were not asked to self-monitor diet, activity, and weight on a daily basis. | Yes | 6 months | 3 and 6 months | The median rate of session attendance at the 11 group sessions was 100% for the mobile group and 81.8% for the paper group. Mobile group: the median percentage of days with at least one self-monitoring entry for diet, PA, weight, and glucose was 96.6%, 37.3%, 49.7%, and 72.7%, respectively. The paper group was 8.1%, 1.2%, 2.5%, and 2.5%, respectively. | Rural area: none of the participants reported owning a smartphone |
| Whittemore et al. (2020) [64] | Intervention through the ¡Sí, yo puedo! program that incorporated relevant theoretical underpinnings, educational content, and interactive strategies of 4 evidence-based programs for Hispanic adults with T2D to meet the needs of adults with T2D with limited resources, expertise of providers, and the systems of care of the Seguro Popular clinics in Mexico City. | Mobile-based self-management, text/picture messages, and face-to-face visits | EG: Received standard T2D care at the Seguro Popular clinic as aforementioned. They also received the ¡Sí, Yo Puedo! program which was developed after formative research with adults with T2D in Mexico. The program included 7 interactive group-based educational sessions on diabetes self-management. The nutrition component was central in the delivery of the intervention. based on “the smart plate” (modified for T2D). Behavioral support was also provided in all sessions, weekly goals, phone calls every two weeks, and text/picture messages daily during the 6 months of intervention. CG: No mobile-based intervention was implemented with participants in the control group. They were placed on a waiting list and continued with their pre-study habits. | Yes | 6 months | 3 and 6 months | Attendance was high at 89% across all sessions, and attrition was low at 6.4% (n = 3) at 6 mo. A total of 96% of participants received the text at 3 months and 100% at 6 months, and for picture messages, 83% at 3 months and 88% at 6 months. Adherence to protocol implementation was high, with goals and objectives completely fulfilled in 91% of the sessions and mostly achieved in 7% of sessions. | N/A |
| Yin et al. (2022) [58] | Telemedicine | Mobile health-based self-monitoring and online telehealth/mobile app and device (glucometers) | EG: They were followed up four times a week in the first 3 months and twice a week in the next 3 months. Doctors used reminders for diet guidance and exercise advice, including energy intake and food exchange methods. They uploaded their daily dietary intake on the telemedicine app. Additionally, the app recorded the patients’ daily steps and automatically transferred them to the medical server. Further, exercise guidance was provided to each patient. Blood glucose levels were monitored using a glucometer and were automatically transferred to the hospital telemedicine app. CG: They were followed up through conventional outpatient clinic appointments every 2 weeks, and telephone follow-up was used during the isolation period for the glucose data management. Traditional health education, which included diet, exercise, and medication guidance, was provided during clinic visits. | No | 6 months | 21 days, 3 months, and 6 months | Not reported | In the framework of the COVID-19 disease, all patients underwent an initial physical examination and blood sample collection, followed by a mandatory home quarantine for 21 days |
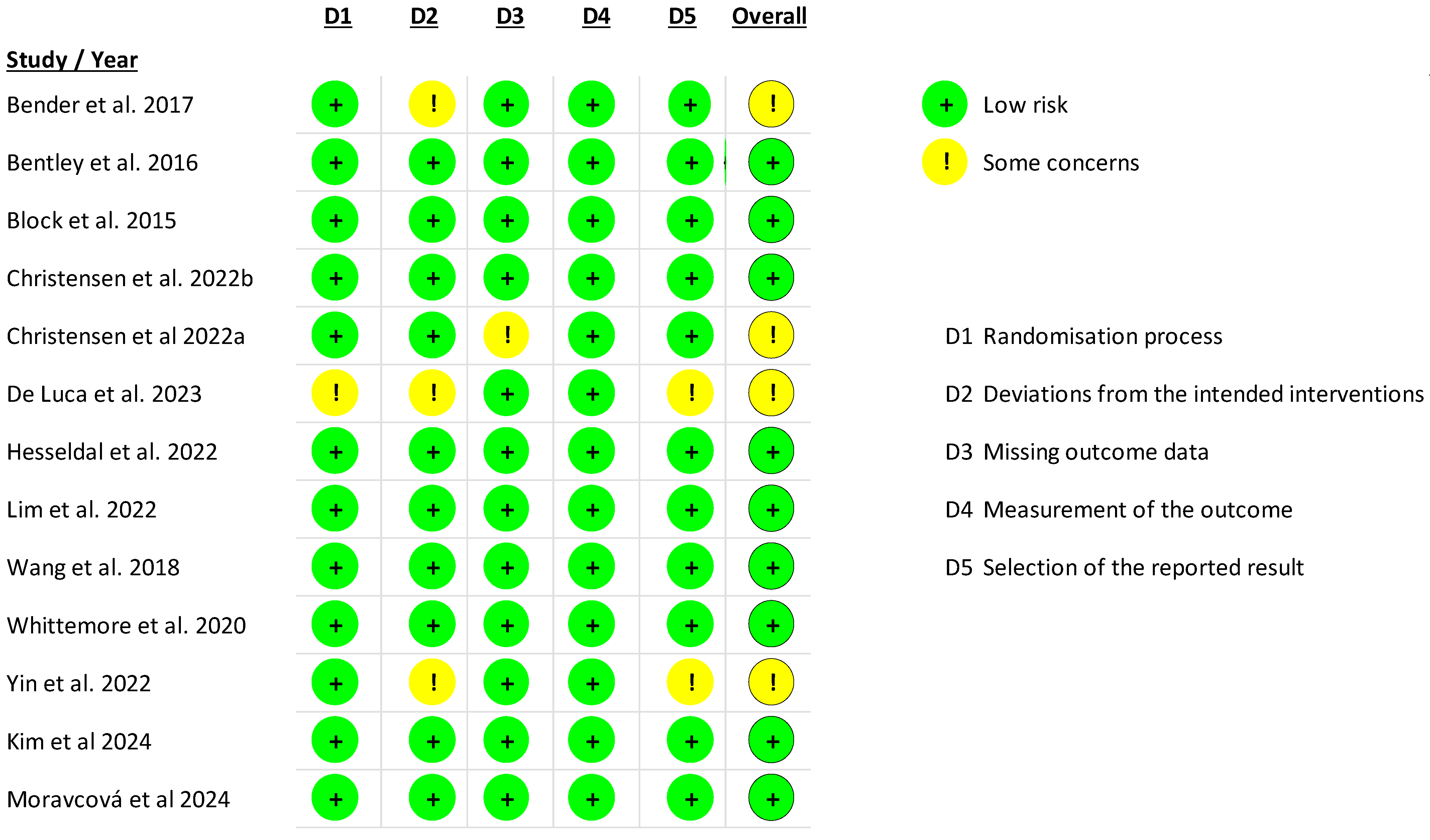
3.3. Risks of Bias in Included Studies
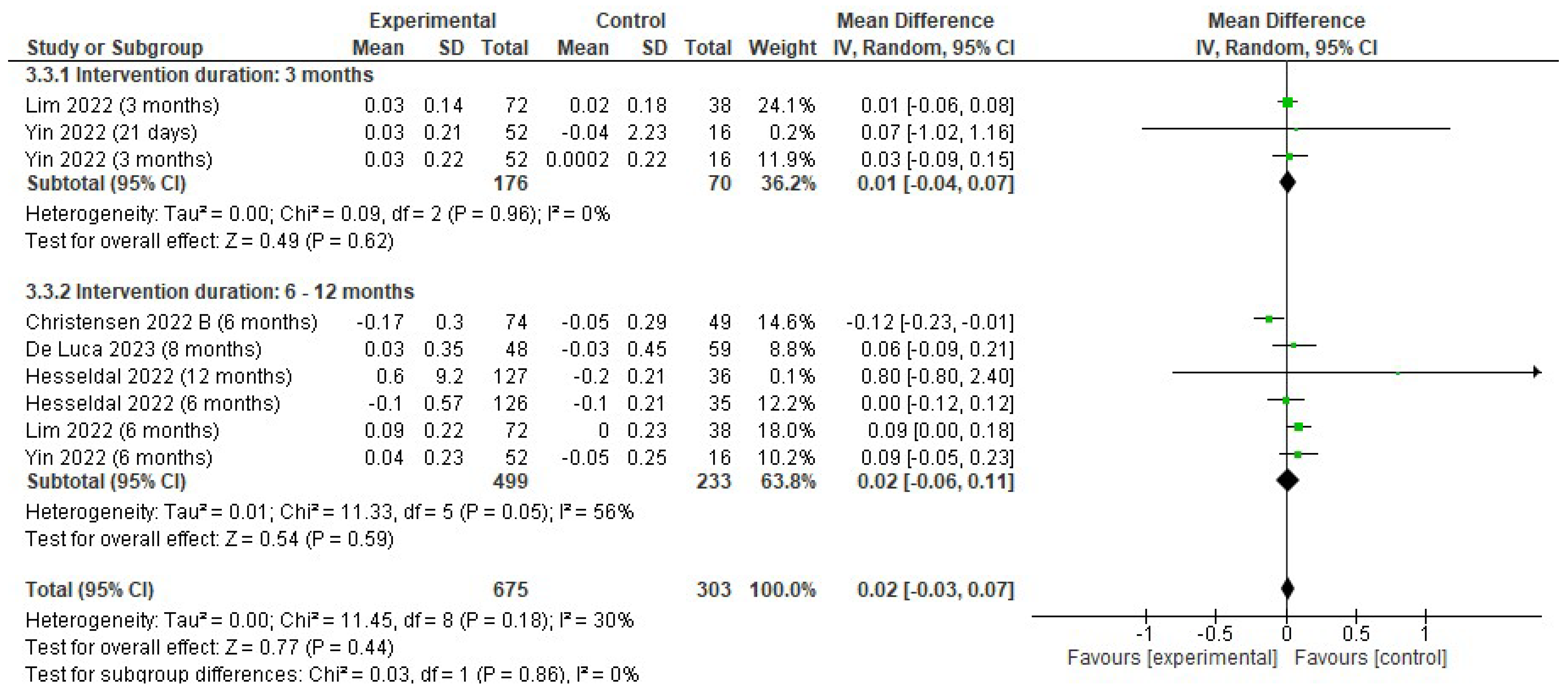
3.4. Effects of the Interventions
3.4.1. Changes in Hb1Ac (%)
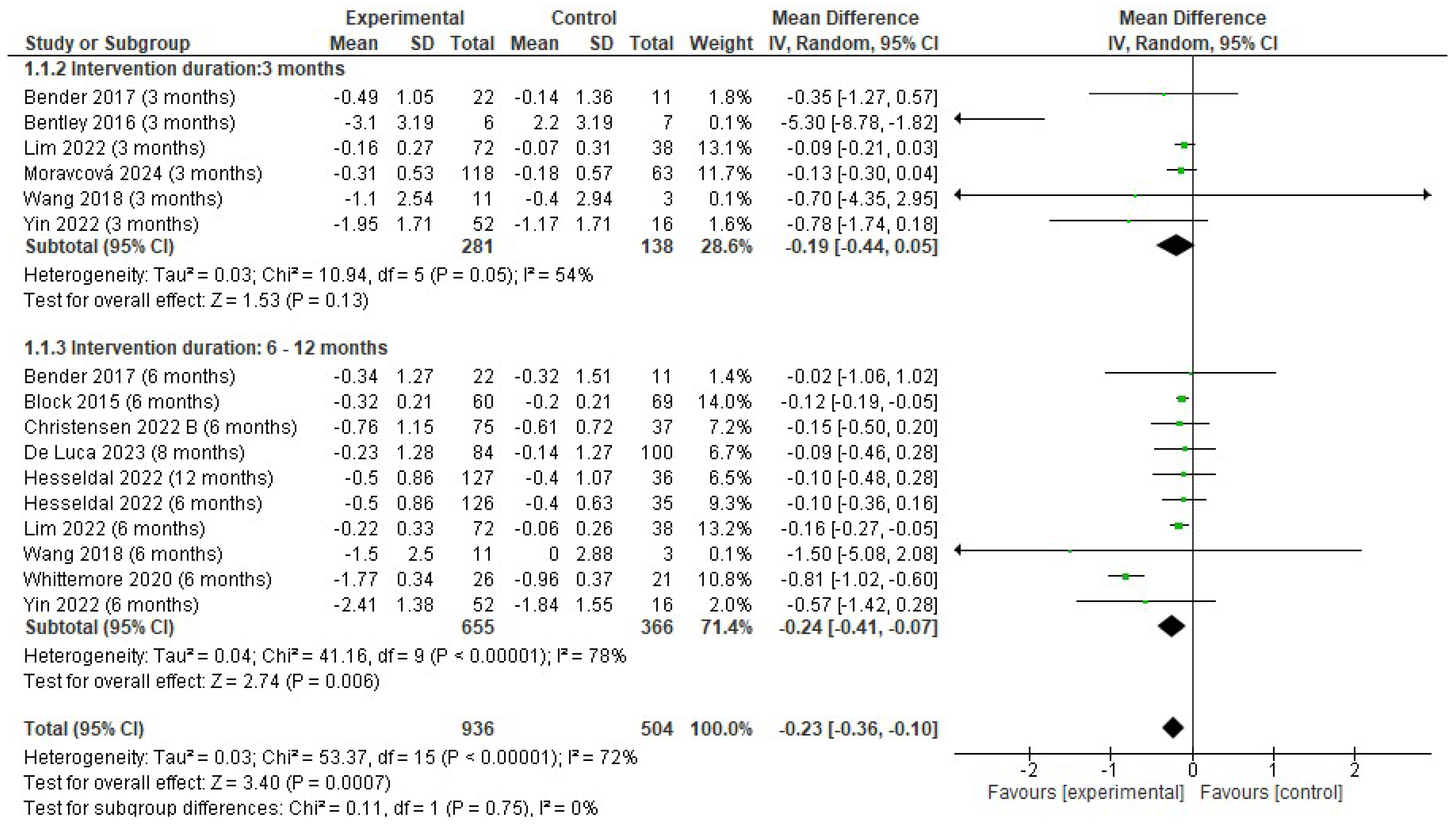
3.4.2. Changes in Body Weight
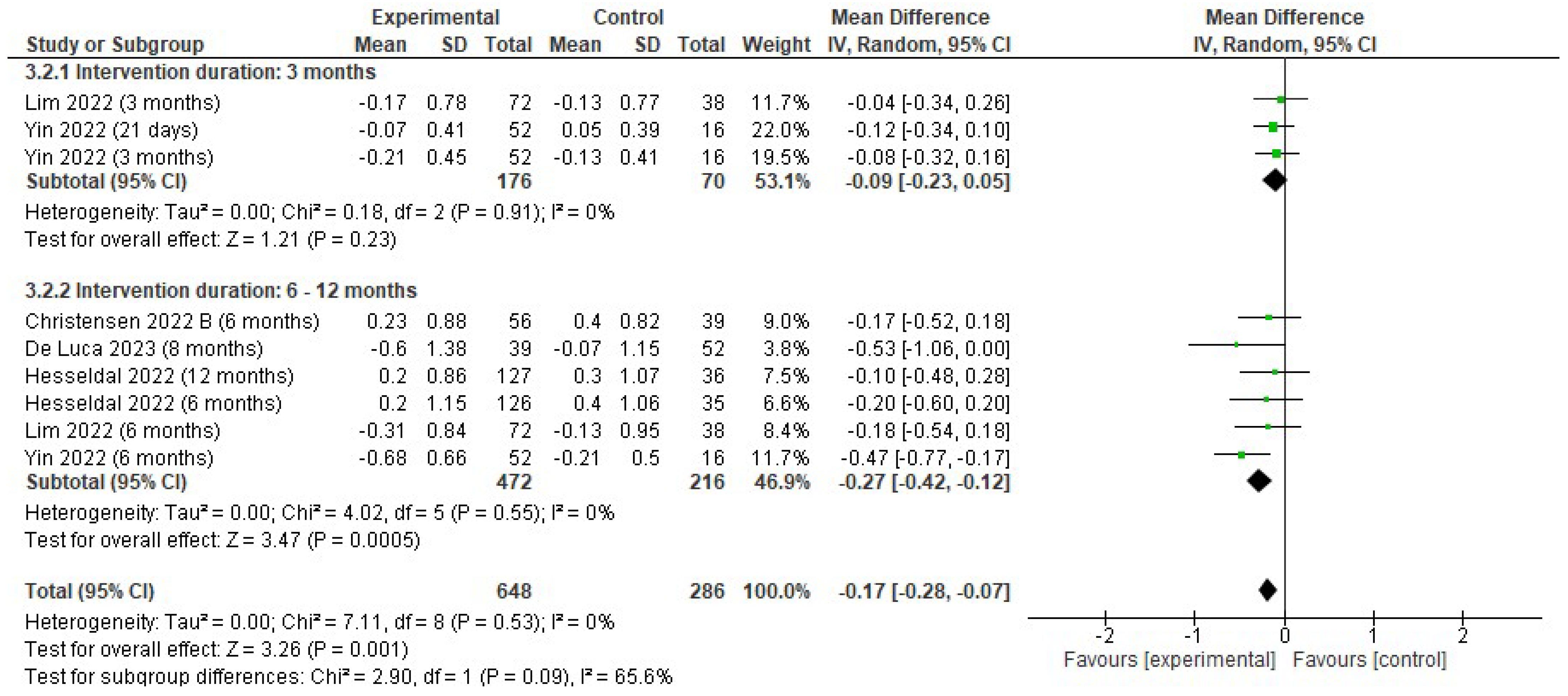
3.4.3. Changes in Triglycerides
3.4.4. Changes in Cholesterol, LDL, and HDL
4. Discussion
4.1. Effects on Hb1Ac
4.2. Effects on Body Weight
4.3. Effects on Lipid Profiles: Triglycerides, Cholesterol, LDL, and HDL
4.4. Adherence to mHealth Interventions
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| DM | Diabetes Mellitus |
| Hb1Ac | Glycosylated Hemoglobin |
| HDL | High-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| PA | Physical Activity |
| RCTs | Randomized Controlled Trials |
| TG | Triglycerides |
| T2D | Type 2 Diabetes |
References
- Gojka, R. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; p. 86. [Google Scholar]
- Chobot, A.; Górowska-Kowolik, K.; Sokołowska, M.; Jarosz-Chobot, P. Obesity and diabetes—Not only a simple link between two epidemics. Diabetes/Metab. Res. Rev. 2018, 34, e3042. [Google Scholar] [CrossRef]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; pp. 1–17. [Google Scholar] [CrossRef]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and Diabetes in the Developing World—A Growing Challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Farag, Y.M.K.; Gaballa, M.R. Diabesity: An overview of a rising epidemic. Nephrol. Dial. Transplant. 2011, 26, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Boye, K.S.; Lage, M.J.; Terrell, K. Healthcare outcomes for patients with type 2 diabetes with and without comorbid obesity. J. Diabetes Complicat. 2020, 34, 107730. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Rahim, N.E.; Flood, D.; Marcus, M.E.; Theilmann, M.; Aung, T.N.; Agoudavi, K.; Aryal, K.K.; Bahendeka, S.; Bicaba, B.; Bovet, P.; et al. Diabetes risk and provision of diabetes prevention activities in 44 low-income and middle-income countries: A cross-sectional analysis of nationally representative, individual-level survey data. Lancet Glob. Health 2023, 11, e1576–e1586. [Google Scholar] [CrossRef]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of Weight Loss With Lifestyle Intervention on Risk of Diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Rietz, M.; Lehr, A.; Mino, E.; Lang, A.; Szczerba, E.; Schiemann, T.; Herder, C.; Saatmann, N.; Geidl, W.; Barbaresko, J.; et al. Physical Activity and Risk of Major Diabetes-Related Complications in Individuals With Diabetes: A Systematic Review and Meta-Analysis of Observational Studies. Diabetes Care 2022, 45, 3101–3111. [Google Scholar] [CrossRef]
- Sigal, R.J.; Kenny, G.P.; Wasserman, D.H.; Castaneda-Sceppa, C.; White, R.D. Physical Activity/Exercise and Type 2 Diabetes. Diabetes Care 2006, 29, 1433–1438. [Google Scholar] [CrossRef]
- Narita, Z.; Inagawa, T.; Stickley, A.; Sugawara, N. Physical activity for diabetes-related depression: A systematic review and meta-analysis. J. Psychiatr. Res. 2019, 113, 100–107. [Google Scholar] [CrossRef]
- Absil, H.; Baudet, L.; Robert, A.; Lysy, P.A. Benefits of physical activity in children and adolescents with type 1 diabetes: A systematic review. Diabetes Res. Clin. Pract. 2019, 156, 107810. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Lipska, K.; Miller, M.E.; Rushing, J.; Cohen, R.A.; Verghese, J.; McDermott, M.M.; King, A.C.; Strotmeyer, E.S.; Blair, S.N.; et al. Effects of Physical Activity Intervention on Physical and Cognitive Function in Sedentary Adults With and Without Diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 861–866. [Google Scholar] [CrossRef]
- Kohl, H.W.; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S.; Lancet Physical Activity Series Working Group. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of Inactivity in Chronic Diseases: Evolutionary Insight and Pathophysiological Mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Booth, F.W. Health benefits of exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- Strain, T.; Flaxman, S.; Guthold, R.; Semenova, E.; Cowan, M.; Riley, L.M.; Bull, F.C.; Stevens, G.A.; Country Data Author Group. National, regional, and global trends in insufficient physical activity among adults from 2000 to 2022: A pooled analysis of 507 population-based surveys with 5·7 million participants. Lancet Glob. Health 2024, 12, e1232–e1243. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2214109X24001505 (accessed on 12 May 2024). [CrossRef] [PubMed]
- Ding, D.; Mutrie, N.; Bauman, A.; Pratt, M.; Hallal, P.R.C.; Powell, K.E. Physical activity guidelines 2020: Comprehensive and inclusive recommendations to activate populations. Lancet 2020, 396, 1780–1782. [Google Scholar] [CrossRef] [PubMed]
- Goode, A.D.; Reeves, M.M.; Eakin, E.G. Telephone-delivered interventions for physical activity and dietary behavior change: An updated systematic review. Am. J. Prev. Med. 2012, 42, 81–88. [Google Scholar] [CrossRef]
- Cradock, K.A.; Ólaighin, G.; Finucane, F.M.; Gainforth, H.L.; Quinlan, L.R.; Ginis, K.A.M. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 18. [Google Scholar] [CrossRef]
- Khokhar, B.; Jones, J.; Ronksley, P.E.; Armstrong, M.J.; Caird, J.; Rabi, D. Effectiveness of mobile electronic devices in weight loss among overweight and obese populations: A systematic review and meta-analysis. BMC Obes. 2014, 1, 22. [Google Scholar] [CrossRef]
- Foster, C.; Hillsdon, M.; Thorogood, M.; Kaur, A.; Wedatilake, T. Interventions for promoting physical activity. Cochrane Database Syst. Rev. 2005, 2005, CD003180. [Google Scholar] [CrossRef]
- O’hAlloran, P.D.; Blackstock, F.; Shields, N.; Holland, A.; Iles, R.; Kingsley, M.; Bernhardt, J.; Lannin, N.; Morris, M.E.; Taylor, N.F. Motivational interviewing to increase physical activity in people with chronic health conditions: A systematic review and meta-analysis. Clin. Rehabil. 2014, 28, 1159–1171. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Stewart, J.; Internationals, O. Use of Consumer Wearable Devices to Promote Physical Activity: A Review of Health Intervention Studies. J. Environ. Health Sci. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Singh, B.; Ahmed, M.; Staiano, A.E.; Gough, C.; Petersen, J.; Vandelanotte, C.; Kracht, C.; Huong, C.; Yin, Z.; Vasiloglou, M.F.; et al. A systematic umbrella review and meta-meta-analysis of eHealth and mHealth interventions for improving lifestyle behaviours. npj Digit. Med. 2024, 7, 179. [Google Scholar] [CrossRef]
- Vandelanotte, C.; Müller, A.M.; Short, C.E.; Hingle, M.; Nathan, N.; Williams, S.L.; Lopez, M.L.; Parekh, S.; Maher, C.A. Past, Present, and Future of eHealth and mHealth Research to Improve Physical Activity and Dietary Behaviors. J. Nutr. Educ. Behav. 2016, 48, 219–228.e1. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.N.; Wodajo, B.; Gochipathala, K.; Paul, D.P.; Coustasse, A. Can mHealth Revolutionize the Way We Manage Adult Obesity? Perspect. Health Inf. Manag. 2017, 14, 1a. [Google Scholar] [PubMed]
- Park, S.H.; Hwang, J.; Choi, Y.K. Effect of mobile health on obese adults: A systematic review and meta-analysis. Health Inform. Res. 2019, 25, 12–26. [Google Scholar] [CrossRef]
- Fortuin, J.; Salie, F.; Abdullahi, L.H.; Douglas, T.S. The impact of mHealth interventions on health systems: A systematic review protocol. Syst. Rev. 2016, 5, 200. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, H.; Huang, Y.; Huang, L.; Zhang, D. A systematic review of application and effectiveness of mHealth interventions for obesity and diabetes treatment and self-management. Adv. Nutr. 2017, 8, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, E.; O’nEal, A.L.; Garcia, L.C.; Mendoza, K.; Lee, R.E. Electronic Health Interventions for Type 2 Diabetes and Obesity in Hispanic or Latino Adults: A Systematic Review of English and Spanish Studies. Diabetes Spectr. 2024, 37, 65–85. [Google Scholar] [CrossRef]
- Enyioha, C.; Hall, M.; Voisin, C.; Jonas, D. Effectiveness of Mobile Phone and Web-Based Interventions for Diabetes and Obesity Among African American and Hispanic Adults in the United States: Systematic Review. JMIR Public Health Surveill. 2022, 8, e25890. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.; Spruijt-Metz, D.; Wen, C.K.F.; Hingle, M.D. Prevention and treatment of pediatric obesity using mobile and wireless technologies: A systematic review. Pediatr. Obes. 2015, 10, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Mateo, G.F.; Granado-Font, E.; Ferré-Grau, C.; Montaña-Carreras, X. Mobile phone apps to promote weight loss and increase physical activity: A systematic review and meta-analysis. J. Med. Internet Res. 2015, 17, e253. [Google Scholar] [CrossRef]
- Baron, J.; McBain, H.; Newman, S. The impact of mobile monitoring technologies on glycosylated hemoglobin in diabetes: A systematic review. J. Diabetes Sci. Technol. 2012, 6, 1185–1196. [Google Scholar] [CrossRef]
- Mallow, J.A.; Theeke, L.A.; Barnes, E.R.; Whetsel, T.; Mallow, B.K. Using mHealth Tools to Improve Rural Diabetes Care Guided by the Chronic Care Model. Online J. Rural. Nurs. Health Care 2014, 14, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Cotter, A.P.; Durant, N.; Agne, A.A.; Cherrington, A.L. Internet interventions to support lifestyle modification for diabetes management: A systematic review of the evidence. J. Diabetes Its Complicat. 2014, 28, 243–251. [Google Scholar] [CrossRef]
- Hood, M.; Wilson, R.; Corsica, J.; Bradley, L.; Chirinos, D.; Vivo, A. What do we know about mobile applications for diabetes self-management? A review of reviews. J. Behav. Med. 2016, 39, 981–994. [Google Scholar] [CrossRef]
- Dobson, R.; Whittaker, R.; Dale, L.P.; Maddison, R. The effectiveness of text message-based self-management interventions for poorly-controlled diabetes: A systematic review. Digit. Health 2017, 3, 205520761774031. [Google Scholar] [CrossRef]
- Kebede, M.M.; Liedtke, T.P.; Möllers, T.; Pischke, C.R. Characterizing active ingredients of ehealth interventions targeting persons with poorly controlled type 2 diabetes mellitus using the behavior change techniques taxonomy: Scoping review. J. Med. Internet Res. 2017, 19, e348. [Google Scholar] [CrossRef]
- De Ridder, M.; Kim, J.; Jing, Y.; Khadra, M.; Nanan, R. A systematic review on incentive-driven mobile health technology: As used in diabetes management. J. Telemed. Telecare 2017, 23, 26–35. [Google Scholar] [CrossRef]
- Cui, M.; Wu, X.; Mao, J.; Wang, X.; Nie, M.; Barengo, N.C. T2DM Self-Management via Smartphone Applications: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166718. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, X.; Vespasiani, G.; Nicolucci, A.; Dong, Y.; Kwong, J.; Li, L.; Sun, X.; Tian, H.; Li, S. Mobile app-based interventions to support diabetes self-management: A systematic review of randomized controlled trials to identify functions associated with glycemic efficacy. JMIR Mhealth Uhealth 2017, 5, e35. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Review Manager (RevMan), version 5.4; The Cochrane Collaboration: London, UK, 2020.
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: New York, NY, USA, 2008; 649p. [Google Scholar]
- Takkouche, B.; Cadarso-Suárez, C.; Spiegelman, D. Evaluation of Old and New Tests of Heterogeneity in Epidemiologic Meta-Analysis. Am. J. Epidemiol. 1999, 150, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Lipsey, M.W.; Wilson, D.B. Practical_Meta-Analysis; SAGE Publications: Thousand Oaks, CA, USA, 2001. [Google Scholar]
- Bender, M.S.; Cooper, B.A.; Park, L.G.; Padash, S.; Arai, S. A feasible and efficacious mobile-phone based lifestyle intervention for filipino americans with type 2 diabetes: Randomized controlled trial. JMIR Diabetes 2017, 2, e30. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.R.; Hesseldal, L.; Olesen, T.B.; Olsen, M.H.; Jakobsen, P.R.; Laursen, D.H.; Lauridsen, J.T.; Nielsen, J.B.; Søndergaard, J.; Brandt, C.J. Long-term weight loss in a 24-month primary care-anchored telehealth lifestyle coaching program: Randomized controlled trial. J. Telemed. Telecare 2022, 28, 764–770. [Google Scholar] [CrossRef]
- Hesseldal, L.; Christensen, J.R.; Olesen, T.B.; Olsen, M.H.; Jakobsen, P.R.; Laursen, D.H.; Lauridsen, J.T.; Nielsen, J.B.; Søndergaard, J.; Brandt, C.J. Long-term Weight Loss in a Primary Care–Anchored eHealth Lifestyle Coaching Program: Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e39741. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, C.; Padhye, N.; Orlander, P.; Zare, M. A Behavioral Lifestyle Intervention Enhanced With Multiple-Behavior Self-Monitoring Using Mobile and Connected Tools for Underserved Individuals With Type 2 Diabetes and Comorbid Overweight or Obesity: Pilot Comparative Effectiveness Trial. JMIR Mhealth Uhealth 2018, 6, e92. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Liu, Y.; Hu, H.; Sun, J.; Liu, Y.; Wang, Z. Telemedicine management of type 2 diabetes mellitus in obese and overweight young and middle-aged patients during COVID-19 outbreak: A single-center, prospective, randomized control study. PLoS ONE 2022, 17, e0275251. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Ong, K.W.; Johal, J.; Han, C.Y.; Yap, Q.V.; Chan, Y.H.; Zhang, Z.P.; Chandra, C.C.; Thiagarajah, A.G.; Khoo, C.M. A Smartphone App-Based Lifestyle Change Program for Prediabetes (D’LITE Study) in a Multiethnic Asian Population: A Randomized Controlled Trial. Front. Nutr. 2022, 8, 780567. [Google Scholar] [CrossRef]
- Rücker, G.; Cates, C.J.; Schwarzer, G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res. Synth. Methods 2017, 8, 392–403. [Google Scholar] [CrossRef]
- Moravcová, K.; Sovová, M.; Ožana, J.; Karbanová, M.; Klásek, J.; Kolasińska, A.B.; Sovová, E. Comparing the Efficacy of Digital and In-Person Weight Loss Interventions for Patients with Obesity and Glycemic Disorders: Evidence from a Randomized Non-Inferiority Trial. Nutrients 2024, 16, 1510. [Google Scholar] [CrossRef]
- Block, G.; Azar, K.M.; Romanelli, R.J.; Block, T.J.; Hopkins, D.; Carpenter, H.A.; Dolginsky, M.S.; Hudes, M.L.; Palaniappan, L.P.; Block, C.H. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: A randomized controlled trial among persons with prediabetes. J. Med. Internet Res. 2015, 17, e240. [Google Scholar] [CrossRef]
- De Luca, V.; Bozzetto, L.; Giglio, C.; Tramontano, G.; De Simone, G.; Luciano, A.; Lucibelli, L.; Maffettone, A.; Riccio, M.; Romano, G.; et al. Clinical outcomes of a digitally supported approach for self-management of type 2 diabetes mellitus. Front. Public Health 2023, 11, 1219661. [Google Scholar] [CrossRef]
- Whittemore, R.; Vilar-Compte, M.; De La Cerda, S.; Delvy, R.; Jeon, S.; Burrola-Méndez, S.; Pardo-Carrillo, M.; Lozano-Marrufo, A.; Pérez-Escamilla, R. ¡Sí, Yo Puedo Vivir Sano con Diabetes! A Self-Management Randomized Controlled Pilot Trial for Low-Income Adults with Type 2 Diabetes in Mexico City. Curr. Dev. Nutr. 2020, 4, nzaa074. [Google Scholar] [CrossRef]
- Christensen, J.R.; Laursen, D.H.; Lauridsen, J.T.; Hesseldal, L.; Jakobsen, P.R.; Nielsen, J.B.; Søndergaard, J.; Brandt, C.J. Reversing Type 2 Diabetes in a Primary Care-Anchored eHealth Lifestyle Coaching Programme in Denmark: A Randomised Controlled Trial. Nutrients 2022, 14, 3424. [Google Scholar] [CrossRef] [PubMed]
- Bentley, C.L.; Otesile, O.; Bacigalupo, R.; Elliott, J.; Noble, H.; Hawley, M.S.; Williams, E.A.; Cudd, P. Feasibility study of portable technology for weight loss and HbA1c control in type 2 diabetes. BMC Med. Inform. Decis. Mak. 2016, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, S.; Lee, Y.-B.; Jin, S.-M.; Hur, K.Y.; Kim, J.H. A randomized controlled trial of an app-based intervention on physical activity and glycemic control in people with type 2 diabetes. BMC Med. 2024, 22, 185. [Google Scholar] [CrossRef]
- Eberle, C.; Löhnert, M.; Stichling, S. Effectiveness of disease-specific mHealth apps in patients with diabetes mellitus: Scoping review. JMIR Mhealth Uhealth 2021, 9, e23477. [Google Scholar] [CrossRef]
- Hou, C.; Carter, B.; Hewitt, J.; Francisa, T.; Mayor, S. Do mobile phone applications improve glycemic control (HbA<>1c<>) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care 2016, 39, 2089–2095. [Google Scholar]
- Timpel, P.; Oswald, S.; Schwarz, P.E.H.; Harst, L. Mapping the Evidence on the Effectiveness of Telemedicine Interventions in Diabetes, Dyslipidemia, and Hypertension: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Med. Internet Res. 2020, 22, e16791. Available online: http://www.jmir.org/2020/3/e16791/ (accessed on 4 November 2024). [CrossRef]
- Mokaya, M.; Kyallo, F.; Vangoitsenhoven, R.; Matthys, C. Clinical and patient-centered implementation outcomes of mHealth interventions for type 2 diabetes in low-and-middle income countries: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 1. [Google Scholar] [CrossRef]
- Verma, D.; Bahurupi, Y.; Kant, R.; Singh, M.; Aggarwal, P.; Saxena, V. Effect of mHealth interventions on glycemic control and hba1c improvement among type II diabetes patients in asian population: A systematic review and meta-analysis. Indian J. Endocrinol. Metab. 2021, 25, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; O’cOnnell, S.S.; Thomas, C.; Chimmanamada, R. Telehealth Interventions to Improve Diabetes Management Among Black and Hispanic Patients: A Systematic Review and Meta-Analysis. J. Racial Ethn. Health Disparities 2022, 9, 2375–2386. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Miyazaki, Y.; Pettiti, M.; Matsuda, M.; Mahankali, S.; Santini, E.; DeFronzo, R.A.; Ferrannini, E. Metabolic effects of visceral fat accumulation in type 2 diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 5098–5103. [Google Scholar] [CrossRef]
- Ros Pérez, M.; Medina-Gómez, G. Obesidad, adipogénesis y resistencia a la insulina. Endocrinol. Y Nutricion. 2011, 58, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- Zhao, X.; He, Q.; Zeng, Y.; Cheng, L. Effectiveness of combined exercise in people with type 2 diabetes and concurrent overweight/obesity: A systematic review and meta-analysis. BMJ Open 2021, 11, e046252. [Google Scholar] [CrossRef] [PubMed]
- Gar, C.; Rottenkolber, M.; Haenelt, M.; Potzel, A.L.; Kern-Matschilles, S.; Then, C.; Seissler, J.; Bidlingmaier, M.; Lechner, A. Altered metabolic and hormonal responses to moderate exercise in overweight/obesity. Metabolism 2020, 107, 154219. [Google Scholar] [CrossRef]
- Antoun, J.; Itani, H.; Alarab, N.; Elsehmawy, A. The Effectiveness of Combining Nonmobile Interventions With the Use of Smartphone Apps With Various Features for Weight Loss: Systematic Review and Meta-analysis. JMIR Mhealth Uhealth 2022, 10, e35479. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.S.J.; Koh, W.L.; Ng, J.S.H.Y.; Tan, K.K. Sustainability of Weight Loss Through Smartphone Apps: Systematic Review and Meta-analysis on Anthropometric, Metabolic, and Dietary Outcomes. J. Med. Internet Res. 2022, 24, e40141. [Google Scholar] [CrossRef]
- Apovian, C.M.; Okemah, J.; O’nEil, P.M. Body Weight Considerations in the Management of Type 2 Diabetes. Adv. Ther. 2019, 36, 44–58. [Google Scholar] [CrossRef]
- Maggio, C.A.; Pi-Sunyer, F.X. Obesity and type 2 diabetes. Endocrinol. Metab. Clin. N. Am. 2003, 32, 805–822. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Corrêa, R.; Tabak, B.M. The Influence of Behavioral Sciences on Adherence to Physical Activity and Weight Loss in Overweight and Obese Patients: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2024, 21, 630. [Google Scholar] [CrossRef]
- Grave, R.D.; Calugi, S.; Centis, E.; El Ghoch, M.; Marchesini, G. Cognitive-Behavioral Strategies to Increase the Adherence to Exercise in the Management of Obesity. J. Obes. 2011, 2011, 348293. [Google Scholar] [CrossRef]
- Baker, R.C.; Kirschenbaum, D.S. Self-monitoring may be necessary for successful weight control. Behav. Ther. 1993, 24, 377–394. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Vazou, S.; Bixby, W.R.; Georgiadis, E. The mysterious case of the public health guideline that is (almost) entirely ignored: Call for a research agenda on the causes of the extreme avoidance of physical activity in obesity. Obes. Rev. 2016, 17, 313–329. [Google Scholar] [CrossRef]
- Kitazawa, M.; Takeda, Y.; Hatta, M.; Horikawa, C.; Sato, T.; Osawa, T.; Ishizawa, M.; Suzuki, H.; Matsubayashi, Y.; Fujihara, K.; et al. Lifestyle Intervention With Smartphone App and isCGM for People at High Risk of Type 2 Diabetes: Randomized Trial. J. Clin. Endocrinol. Metab. 2024, 109, 1060–1070. [Google Scholar] [CrossRef]
- McDiarmid, S.; Harvie, M.; Aglan, A.; Winterbottom, H.; Mubita, W.; Hulme, A.; Davies, J.; Yates, J.; Krizak, S.; Perry, D.; et al. Manchester Intermittent and Daily diet Type 1 Diabetes App Study (MIDDAS-Type 1): Protocol for a randomised feasibility trial of an intermittent and continuous low-energy diet in patients with type 1 diabetes and overweight and obesity. BMJ Open 2023, 13, e071395. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- Akbari, M.; Lankarani, K.B.; Tahami, A.N.; Tabrizi, R.; Honarvar, B.; Kolahdooz, F.; Borhaninejad, V.; Asemi, Z. The effects of mobile health interventions on lipid profiles among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1949–1955. [Google Scholar] [CrossRef]
- Alfarwan, N.; Hodkinson, A.; Panagioti, M.; Hassan, L.; Kontopantelis, E. Clinical and cost-effectiveness of telemedicine among patients with type 2 diabetes in primary care: A systematic review and meta-analysis. Diabet. Med. 2024, 41, e15343. [Google Scholar] [CrossRef]
- Chew, H.S.J.; Rajasegaran, N.N.; Chin, Y.H.; Chew, W.S.N.; Kim, K.M. Effectiveness of Combined Health Coaching and Self-Monitoring Apps on Weight-Related Outcomes in People With Overweight and Obesity: Systematic Review and Meta-analysis. J. Med. Internet Res. 2023, 25, e42432. [Google Scholar] [CrossRef]
- Hamine, S.; Gerth-Guyette, E.; Faulx, D.; Green, B.B.; Ginsburg, A.S. Impact of mHealth Chronic Disease Management on Treatment Adherence and Patient Outcomes: A Systematic Review. J. Med. Internet Res. 2015, 17, e52. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Lam, C.N.; Burner, E.; Menchine, M. Implementation and Evaluation of an Automated Text Message–Based Diabetes Prevention Program for Adults With Pre-diabetes. J. Diabetes Sci. Technol. 2024, 18, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Boye, K.S.; Shinde, S.; Kennedy-Martin, T.; Robinson, S.; Thieu, V.T. Weight Change and the Association with Adherence and Persistence to Diabetes Therapy: A Narrative Review. Patient Prefer. Adherence 2022, 16, 23–39. [Google Scholar] [CrossRef] [PubMed]



| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | mHealth | [Usual Care] | Relative (95% CI) | Absolute (95% CI) | ||
| Glycated Hemoglobin (HbA1c) | ||||||||||||
| 11 | randomized trials | not serious a | very serious b | serious c | not serious d | very strong association | 936/1440 (65.0%) | 504/1440 (35.0%) | not estimable | 2 more per 1000 (from 1 more to 4 more) | ⨁⨁⨁◯ Moderate a,b,c,d | CRITICAL |
| Weight | ||||||||||||
| 9 | randomized trials | not serious a | very serious e | serious c | not serious f | very strong association | 861/1301 (66.2%) | 440/1301 (33.8%) | not estimable | 25 more per 1000 (from 16 more to 34 more) | ⨁⨁⨁◯ Moderate a,c,e,f | CRITICAL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Garcia, C.; Maher, C.A.; Sañudo, B.; Jurado-Castro, J.M. Mobile Health Interventions for Individuals with Type 2 Diabetes and Overweight or Obesity—A Systematic Review and Meta-Analysis. J. Funct. Morphol. Kinesiol. 2025, 10, 292. https://doi.org/10.3390/jfmk10030292
Gomez-Garcia C, Maher CA, Sañudo B, Jurado-Castro JM. Mobile Health Interventions for Individuals with Type 2 Diabetes and Overweight or Obesity—A Systematic Review and Meta-Analysis. Journal of Functional Morphology and Kinesiology. 2025; 10(3):292. https://doi.org/10.3390/jfmk10030292
Chicago/Turabian StyleGomez-Garcia, Carlos, Carol A. Maher, Borja Sañudo, and Jose Manuel Jurado-Castro. 2025. "Mobile Health Interventions for Individuals with Type 2 Diabetes and Overweight or Obesity—A Systematic Review and Meta-Analysis" Journal of Functional Morphology and Kinesiology 10, no. 3: 292. https://doi.org/10.3390/jfmk10030292
APA StyleGomez-Garcia, C., Maher, C. A., Sañudo, B., & Jurado-Castro, J. M. (2025). Mobile Health Interventions for Individuals with Type 2 Diabetes and Overweight or Obesity—A Systematic Review and Meta-Analysis. Journal of Functional Morphology and Kinesiology, 10(3), 292. https://doi.org/10.3390/jfmk10030292








