High-Intensity Interval Training Improves Cardiovascular Fitness and Induces Left-Ventricular Hypertrophy During Off-Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Organization of the Study
2.3. Physiological and Performance Measurements
2.4. Training Intervention
2.5. Echocardiographic Examination
2.6. Statistics
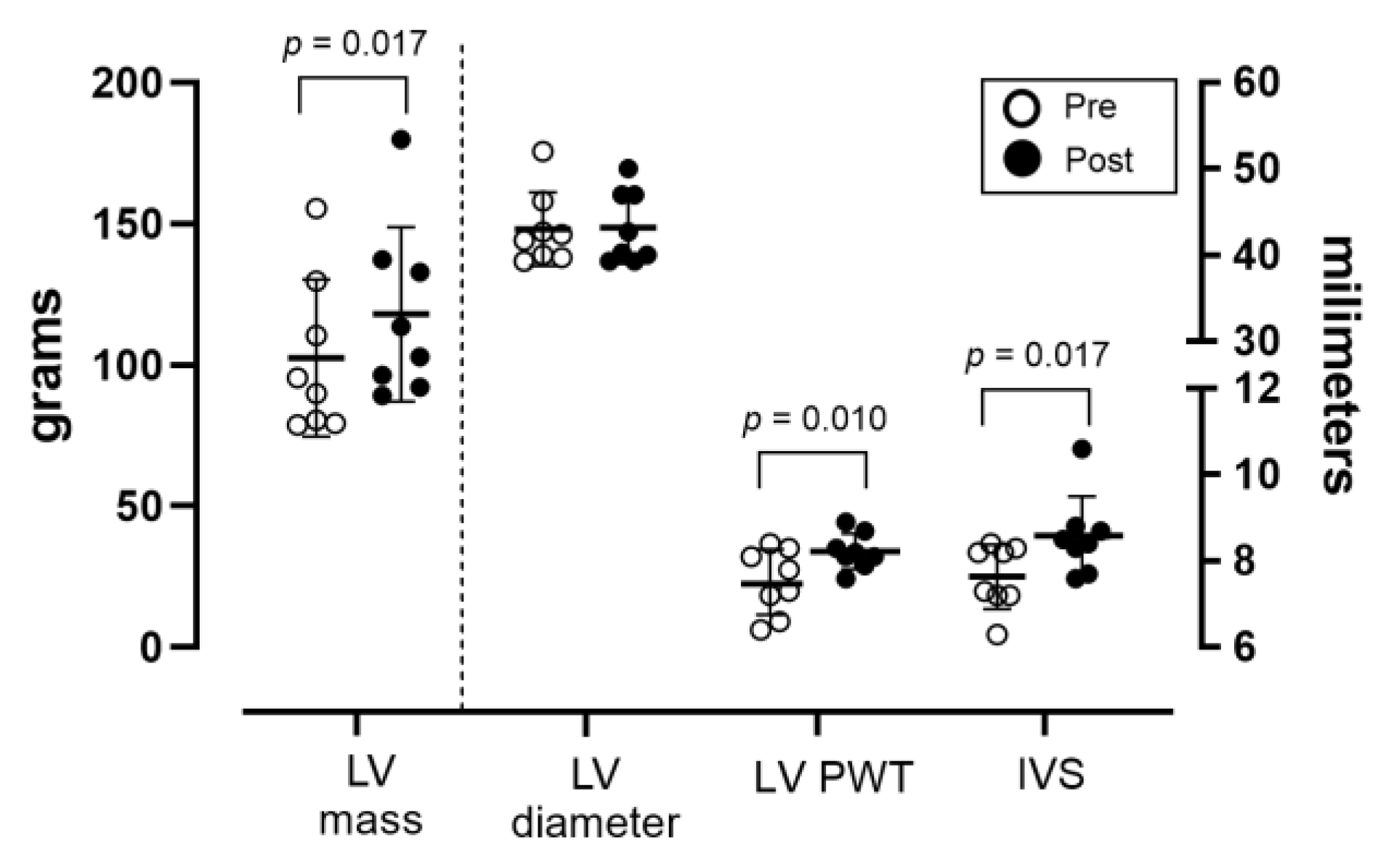
3. Results
4. Discussion
4.1. Factors of Cardiac Remodeling in Response to Exercise Training
4.2. Plausible Mechanisms of HIIT-Induced Cardiac Adaptation
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spirito, P.; Pelliccia, A.; Proschan, M.A.; Granata, M.; Spataro, A.; Bellone, P.; Caselli, G.; Biffi, A.; Vecchio, C.; Maron, B.J. Morphology of the ‘athlete’s heart’ assessed by echocardiography in 947 elite athletes representing 27 sports. Am. J. Cardiol. 1994, 74, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Spence, A.L.; Naylor, L.H.; Carter, H.H.; Buck, C.L.; Dembo, L.; Murray, C.P.; Watson, P.; Oxborough, D.; George, K.P.; Green, D.J. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J. Physiol. 2011, 589 Pt 22, 5443–5452. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.B.; DeLuca, J.R.; Wang, F.; Lin, J.; Wasfy, M.M.; Berkstresser, B.; Stöhr, E.; Shave, R.; Lewis, G.D.; Hutter, A.M.; et al. Exercise-Induced Left Ventricular Remodeling Among Competitive Athletes: A Phasic Phenomenon. Circ. Cardiovasc. Imaging 2015, 8, e003651. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Bittencourt, C.R.; de Oliveira Izar, M.C.; Schwerz, V.L.; Póvoa, R.M.D.S.; Fonseca, H.A.R.; Fonseca, M.I.H.; Bianco, H.T.; França, C.N.; Ferreira, C.E.D.S.; Fonseca, F.A.H. Effects of High-Intensity Training of Professional Runners on Myocardial Hypertrophy and Subclinical Atherosclerosis. PLoS ONE 2016, 11, e0166009. [Google Scholar] [CrossRef]
- Banks, L.; Bentley, R.F.; Currie, K.D.; Vecchiarelli, E.; Aslam, A.; Connelly, K.A.; Yan, A.T.; Konieczny, K.M.; Dorian, P.; Mak, S.; et al. Cardiac Remodeling in Middle-Aged Endurance Athletes and Recreationally Active Individuals: Challenges in Defining the ‘Athlete’s Heart’. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020, 33, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Csajági, E.; Szauder, I.; Major, Z.; Pavlik, G. Left Ventricular Morphology in Different Periods of the Training Season in Elite Young Swimmers. Pediatr. Exerc. Sci. 2015, 27, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wasfy, M.M.; Weiner, R.B.; Wang, F.; Berkstresser, B.; Deluca, J.; Hutter, A.M.; Picard, M.H.; Baggish, A.L. Myocardial Adaptations to Competitive Swim Training. Med. Sci. Sports Exerc. 2019, 51, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Stanton, K.M.; Wylie, L.; Kotchetkova, I.; Coy, A.; Carroll, G.; LA Gerche, A.; Celermajer, D.S. Soldiers’ Heart: A Prospective Study of Cardiac Remodeling in Soldiers Undergoing Progressive Intensity Exercise Training. Med. Sci. Sports Exerc. 2022, 54, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.F.; Perlingeiro, P.; Hachul, D.T.; Gomes-Santos, I.L.; Tsutsui, J.M.; Negrao, C.E.; De Matos, L.D.N.J. Predominance of Intrinsic Mechanism of Resting Heart Rate Control and Preserved Baroreflex Sensitivity in Professional Cyclists after Competitive Training. PLoS ONE 2016, 11, e0148036. [Google Scholar] [CrossRef] [PubMed]
- Venckūnas, T.; Raugaliene, R.; Jankauskiene, E. Structure and function of distance runners’ heart. Medicina 2005, 41, 685–692. [Google Scholar] [PubMed]
- Venckūnas, T.; Stasiulis, A.; Raugaliene, R. Relationship between echocardiographic and aerobic capacity parameters in distance runners. Int. J. Cardiol. 2005, 102, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Venckunas, T.; Stasiulis, A.; Raugaliene, R. Concentric myocardial hypertrophy after one year of increased training volume in experienced distance runners. Br. J. Sports Med. 2006, 40, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Abergel, E.; Chatellier, G.; Hagege, A.A.; Oblak, A.; Linhart, A.; Ducardonnet, A.; Menard, J. Serial left ventricular adaptations in world-class professional cyclists: Implications for disease screening and follow-up. J. Am. Coll. Cardiol. 2004, 44, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskas, D.; Venckūnas, T.; Marcinkeviciene, J.; Bartkeviciene, A. Development of structural cardiac adaptation in basketball players. Eur. J. Cardiovasc. Prev. Rehabil. Off. J. Eur. Soc. Cardiol. Work. Groups Epidemiol. Prev. Card. Rehabil. Exerc. Physiol. 2006, 13, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Venckunas, T.; Raugaliene, R.; Mazutaitiene, B.; Ramoskeviciute, S. Endurance rather than sprint running training increases left ventricular wall thickness in female athletes. Eur. J. Appl. Physiol. 2008, 102, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Skalenius, M.; Mattsson, C.M.; Dahlberg, P.; Bergfeldt, L.; Ravn-Fischer, A. Performance and cardiac evaluation before and after a 3-week training camp for 400-meter sprinters—An observational, non-randomized study. PLoS ONE 2019, 14, e0217856. [Google Scholar] [CrossRef] [PubMed]
- Zacher, J.; Blome, I.; Schenk, A.; Gorr, E. Cardiac adaptations in elite female football- and volleyball-athletes do not impact left ventricular global strain values: A speckle tracking echocardiography study. Int. J. Cardiovasc. Imaging 2020, 36, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, G.; Ferrera, A.; Vespasiano, F.; Maestrini, V.; Monosilio, S.; Lemme, E.; Serdoz, A.; Mango, F.; Casciani, E.; Pelliccia, A.; et al. Insight on Exercise-Induced Heart Remodeling in Different Track and Field Disciplines. J. Clin. Med. 2024, 13, 6027. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.M.J.; Hedman, K.; Åström-Aneq, M.; Nylander, E.; Bouma, K.; Mandić, M.; Gustafsson, T.; Rullman, E. Evidence of Left Ventricular Cardiac Remodeling After 6 Weeks of Sprint Interval Training. Scand. J. Med. Sci. Sports 2024, 34, e70007. [Google Scholar] [CrossRef] [PubMed]
- Hottenrott, K.; Ludyga, S.; Schulze, S. Effects of high intensity training and continuous endurance training on aerobic capacity and body composition in recreationally active runners. J. Sports Sci. Med. 2012, 11, 483–488. [Google Scholar] [PubMed]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle: Part I: Cardiopulmonary emphasis. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Esfandiari, S.; Elmayergi, N.; Sasson, Z.; Goodman, J.M. Left atrial functional changes following short-term exercise training. Eur. J. Appl. Physiol. 2014, 114, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, H.; Le Blanc, O.; Paquette, M.; Imhoff, S.; Labrecque, L.; Drapeau, A.; Poirier, P.; Bédard, É.; Pibarot, P.; Brassard, P. Cardiac remodeling after six weeks of high-intensity interval training to exhaustion in endurance-trained men. Am. J. Physiology. Heart Circ. Physiol. 2019, 317, H685–H694. [Google Scholar] [CrossRef] [PubMed]
- Ryffel, C.P.; Eser, P.; Trachsel, L.D.; Brugger, N.; Wilhelm, M. Age at start of endurance training is associated with patterns of left ventricular hypertrophy in middle-aged runners. Int. J. Cardiol. 2018, 267, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, A.W.; Landgraff, H.E.; Stokke, T.M.; Murbræch, K.; Leirstein, S.; Aaeng, A.; Brun, H.; Haugaa, K.H.; Hallén, J.; Edvardsen, T.; et al. The developing athlete’s heart: A cohort study in young athletes transitioning through adolescence. Eur. J. Prev. Cardiol. 2019, 26, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Puccetti, L.; Pastorelli, M.; Pasqui, A.L.; Auteri, A.; Bruni, F. Transmitral and pulmonary venous flow study in elite male runners and young adults. Int. J. Cardiol. 2002, 84, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Howley, E.T.; Bassett, D.R.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.; Jøranson, K.; Olesen, B.V.; Hetlelid, K.J. Adaptations to aerobic interval training: Interactive effects of exercise intensity and total work duration. Scand. J. Med. Sci. Sports 2013, 23, 74–83. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Hicks, A.L.; MacDonald, J.R.; McKelvie, R.S.; Green, H.J.; Smith, K.M. Muscle performance and enzymatic adaptations to sprint interval training. J. Appl. Physiol. 1998, 84, 2138–2142. [Google Scholar] [CrossRef] [PubMed]
- Helgerud, J.; Høydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Venckunas, T.; Snieckus, A.; Trinkunas, E.; Baranauskiene, N.; Solianik, R.; Juodsnukis, A.; Streckis, V.; Kamandulis, S. Interval Running Training Improves Cognitive Flexibility and Aerobic Power of Young Healthy Adults. J. Strength Cond. Res. 2016, 30, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. Nutrition 1989, 5, 303–313, 303–311; discussion 312–313. [Google Scholar] [PubMed]
- Luijkx, T.; Cramer, M.J.; Prakken, N.H.J.; Buckens, C.F.; Mosterd, A.; Rienks, R.; Backx, F.J.G.; Mali, W.P.T.M.; Velthuis, B.K. Sport category is an important determinant of cardiac adaptation: An MRI study. Br. J. Sports Med. 2012, 46, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Csecs, I.; Czimbalmos, C.; Toth, A.; Dohy, Z.; Suhai, I.F.; Szabo, L.; Kovacs, A.; Lakatos, B.; Sydo, N.; Kheirkhahan, M.; et al. The impact of sex, age and training on biventricular cardiac adaptation in healthy adult and adolescent athletes: Cardiac magnetic resonance imaging study. Eur. J. Prev. Cardiol. 2020, 27, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Kemi, O.J.; Haram, P.M.; Loennechen, J.P.; Osnes, J.-B.; Skomedal, T.; Wisløff, U.; Ellingsen, Ø. Moderate vs. high exercise intensity: Differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc. Res. 2005, 67, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Rubal, B.J.; Al-Muhailani, A.R.; Rosentswieg, J. Effects of physical conditioning on the heart size and wall thickness of college women. Med. Sci. Sports Exerc. 1987, 19, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R. Athlete’s heart. Heart 2003, 89, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Dawes, T.J.W.; Corden, B.; Cotter, S.; de Marvao, A.; Walsh, R.; Ware, J.S.; Cook, S.A.; O’Regan, D.P. Moderate Physical Activity in Healthy Adults Is Associated with Cardiac Remodeling. Circ. Cardiovasc. Imaging 2016, 9, e004712. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, L.A.; Laprade, A.; Burggraf, G.W.; Norman, R. Cardiac responses of young women to conditioning for a 10 kilometer race. Int. J. Sports Med. 1992, 13, 384–389. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, A.; Bhuva, A.N.; van Zalen, J.; Bastiaenen, R.; Abdel-Gadir, A.; Jones, S.; Nadarajan, N.; Menacho Medina, K.D.; Ye, Y.; Augusto, J.; et al. Cardiovascular Remodeling Experienced by Real-World, Unsupervised, Young Novice Marathon Runners. Front. Physiol. 2020, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Arbab-Zadeh, A.; Perhonen, M.; Howden, E.; Peshock, R.M.; Zhang, R.; Adams-Huet, B.; Haykowsky, M.J.; Levine, B.D. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation 2014, 130, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.L.; Bennett, J.B.; Dudley, G.A. Exercise training-induced alterations of cardiac morphology. J. Appl. Physiol. 1986, 61, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, N.; Cox, D.; Jamnik, R. Endurance athletes’ stroke volume does not plateau: Major advantage is diastolic function. Med. Sci. Sports Exerc. 1994, 26, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsuda, T.; Tobina, T.; Yamada, Y.; Yamagishi, T.; Sakai, H.; Obara, S.; Higaki, Y.; Kiyonaga, A.; Brubaker, P.H. Product of heart rate and first heart sound amplitude as an index of myocardial metabolic stress during graded exercise. Circ. J. Off. J. Jpn. Circ. Soc. 2013, 77, 2736–2741. [Google Scholar] [CrossRef] [PubMed]
- Karaliute, R.; Raugaliene, R.; Venckunas, T. Relationship between left ventricular structure and post-exercise blood pressure in endurance athletes. Acta Cardiol. 2011, 66, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Conlee, R.K.; Jensen, R.; Fellingham, G.W.; George, J.D.; Fisher, A.G. Stroke volume does not plateau during graded exercise in elite male distance runners. Med. Sci. Sports Exerc. 2001, 33, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Lepretre, P.-M.; Koralsztein, J.-P.; Billat, V.L. Effect of exercise intensity on relationship between VO2max and cardiac output. Med. Sci. Sports Exerc. 2004, 36, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Solli, G.S.; Nyberg, S.K.; Hoff, J.; Helgerud, J. Stroke volume does not plateau in female endurance athletes. Int. J. Sports Med. 2012, 33, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Fontana, P.; Betschon, K.; Boutellier, U.; Toigo, M. Cardiac output but not stroke volume is similar in a Wingate and VO2peak test in young men. Eur. J. Appl. Physiol. 2011, 111, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Buchheit, M. Moderate Recovery Unnecessary to Sustain High Stroke Volume during Interval Training. A Brief Report. J. Sports Sci. Med. 2014, 13, 393–396. [Google Scholar] [PubMed]
- Zinner, C.; Wahl, P.; Achtzehn, S.; Reed, J.L.; Mester, J. Acute hormonal responses before and after 2 weeks of HIT in well trained junior triathletes. Int. J. Sports Med. 2014, 35, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Venckunas, T.; Krusnauskas, R.; Snieckus, A.; Eimantas, N.; Baranauskiene, N.; Skurvydas, A.; Brazaitis, M.; Kamandulis, S. Acute effects of very low-volume high-intensity interval training on muscular fatigue and serum testosterone level vary according to age and training status. Eur. J. Appl. Physiol. 2019, 119, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Nottin, S.; Nguyen, L.-D.; Terbah, M.; Obert, P. Cardiovascular effects of androgenic anabolic steroids in male bodybuilders determined by tissue Doppler imaging. Am. J. Cardiol. 2006, 97, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C.; Hooper, D.R. Reductions in testosterone are not indicative of exercise performance decrement in male endurance athletes. Aging Male Off. J. Int. Soc. Study Aging Male 2020, 23, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Heinicke, K.; Wolfarth, B.; Winchenbach, P.; Biermann, B.; Schmid, A.; Huber, G.; Friedmann, B.; Schmidt, W. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int. J. Sports Med. 2001, 22, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Menz, V.; Strobl, J.; Faulhaber, M.; Gatterer, H.; Burtscher, M. Effect of 3-week high-intensity interval training on VO2max, total haemoglobin mass, plasma and blood volume in well-trained athletes. Eur. J. Appl. Physiol. 2015, 115, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Letnes, J.M.; Nes, B.; Vaardal-Lunde, K.; Slette, M.B.; Mølmen-Hansen, H.E.; Aspenes, S.T.; Støylen, A.; Wisløff, U.; Dalen, H. Left Atrial Volume, Cardiorespiratory Fitness, and Diastolic Function in Healthy Individuals: The HUNT Study, Norway. J. Am. Heart Assoc. 2020, 9, e014682. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Mos, L.; Munari, L.; Mormino, P.; Del Torre, M.; Valle, F.; Penzo, M.; Pessina, A.C.; Dal Palù, C. Beats modulate blood pressure during running. Am. J. Hypertens. 1989, 2 Pt 1, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Kaşikçioğlu, E.; Oflaz, H.; Akhan, H.; Kayserilioğlu, A.; Umman, S. Peak pulse pressure during exercise and left ventricular hypertrophy in athletes. Anadolu Kardiyol. Derg. AKD=Anatol. J. Cardiol. 2005, 5, 64–65. [Google Scholar]
- Esfandiari, S.; Sasson, Z.; Goodman, J.M. Short-term high-intensity interval and continuous moderate-intensity training improve maximal aerobic power and diastolic filling during exercise. Eur. J. Appl. Physiol. 2014, 114, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Kooreman, Z.; Giraldeau, G.; Finocchiaro, G.; Kobayashi, Y.; Wheeler, M.; Perez, M.; Moneghetti, K.; Oxborough, D.; George, K.P.; Myers, J.; et al. Athletic Remodeling in Female College Athletes: The ‘Morganroth Hypothesis’ Revisited. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2019, 29, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.D.; Baggish, A.L.; Kovacs, R.J.; Link, M.S.; Maron, M.S.; Mitchell, J.H.; American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology; Council on Cardiovascular Disease in Young; Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology; American College of Cardiology. Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities: Task Force 1: Classification of Sports: Dynamic, Static, and Impact: A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation 2015, 132, e262–e266. [Google Scholar] [CrossRef] [PubMed]
- Scharf, M.; Schmid, A.; Kemmler, W.; von Stengel, S.; May, M.S.; Wuest, W.; Achenbach, S.; Uder, M.; Lell, M.M. Myocardial adaptation to high-intensity (interval) training in previously untrained men with a longitudinal cardiovascular magnetic resonance imaging study (Running Study and Heart Trial). Circ. Cardiovasc. Imaging 2015, 8, e002566. [Google Scholar] [CrossRef] [PubMed]
- Shave, R.E.; Lieberman, D.E.; Drane, A.L.; Brown, M.G.; Batterham, A.M.; Worthington, S.; Atencia, R.; Feltrer, Y.; Neary, J.; Weiner, R.B.; et al. Selection of endurance capabilities and the trade-off between pressure and volume in the evolution of the human heart. Proc. Natl. Acad. Sci. USA 2019, 116, 19905–19910. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, J.; Musha, H.; Takada, H.; Murayama, M. New upper limit of physiologic cardiac hypertrophy in Japanese participants in the 100-km ultramarathon. J. Am. Coll. Cardiol. 2003, 42, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
| Week No. | Session No. | Intervals (m) | No. of Intervals per Session | Rest Between Intervals (min) | Total Session Time (min) * | Total Distance per Session (m) | Total Distance per Week (m) | HIE Time per Session (min) | HIE Time per Week (min) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2000 + 200 (both all-out) | 2 | 10–15 | 11 | 2200 | 9 | ||
| 1 | 2 | 400 | 6 | 3 | 37 | 2400 | 9400 | 9 | 37 |
| 3 | 200 | 10 | 2 | 36 | 2000 | 6 | |||
| 4 | 1000 | 5 | 4 | 52 | 5000 | 22 | |||
| 2 | 5 | 5 × (200 + 1000) # | 10 | 2; 4 & | 68 | 6000 | 17,800 | 28 | 75 |
| 6 | 8 × (200 + 400) # | 16 | 2; 3 & | 67 | 4800 | 17 | |||
| 7 | 5 × (400 + 1000) # | 10 | 3; 4 & | 75 | 7000 | 30 | |||
| 3 | 8 | 200 | 14 | 2 | 46 | 2800 | 13,800 | 8 | 55 |
| 9 | 400 | 10 | 3 | 55 | 4000 | 15 | |||
| 10 | 1000 | 7 | 4 | 70 | 7000 | 32 | |||
| 4 | 11 | 10 × (400 + 200) # | 20 | 2; 3 & | 82 | 6000 | 21,600 | 22 | 88 |
| 12 | 6 × (1000 + 200) # | 12 | 2; 4 & | 76 | 7200 | 30 | |||
| 13 | 6 × (1000 + 400) # | 12 | 3; 4 & | 88 | 8400 | 36 | |||
| 5 | 14 | 400 | 12 | 3 | 64 | 4800 | 19,200 | 18 | 80 |
| 15 | 10 × 600 + 2 × 200 | 12 | 3 | 73 | 6400 | 26 | |||
| 16 | 1000 | 8 | 4 | 78 | 8000 | 36 | |||
| 6 | 17 | 10 × (400 + 200) # | 20 | 3 | 90 | 6000 | 23,200 | 20 | 89 |
| 18 | 8 × (1000 + 400) # | 16 | 3 | 105 | 11,200 | 22 | |||
| 20 | 400 | 15 | 3 | 77 | 6000 | 47 | |||
| 7 | 21 | 8 × (1000 + 200) # | 16 | 3; 4 & | 106 | 9600 | 19,600 | 40 | 84 |
| 22 | 1000 | 10 | 3 | 84 | 10,000 | 44 | |||
| 8 | 23 | 2000 + 200 (both all-out) | 2 | 10–15 | 10 | 2200 | 8 | ||
| Total | 1440 | 126,800 | 525 | ||||||
| Pre-Training | Post-Training | p Value | |
|---|---|---|---|
| Body weight, kg | 62.9 (11.3) | 63.1 (11.3) | 0.45 |
| Heart rate, bpm | 68.4 (9.5) | 64.3 (8.9) | 0.05 |
| LV mass index, g·m−2 | 49.2 (8.0) | 58.7 (6.6) | 0.02 |
| Relative wall thickness | 0.354 (0.033) | 0.387 (0.024) | 0.18 |
| Left atrium diameter, mm | 32.0 (2.5) | 33.5 (2.3) | 0.01 |
| RV diameter, mm | 29.6 (2.8) | 29.2 (2.1) | 0.65 |
| Right atrium diameter, mm | 38.7 (2.1) | 37.3 (3.7) | 0.68 |
| E/A | 1.91 (0.25) | 1.86 (0.34) | 0.87 |
| E’, cm·s−2 | 17.4 (3.6) | 17.0 (1.3) | 0.69 |
| E/E’ | 5.35 (0.92) | 5.35 (0.94) | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venckunas, T.; Gumauskiene, B.; Muanjai, P.; Cadefau, J.A.; Kamandulis, S. High-Intensity Interval Training Improves Cardiovascular Fitness and Induces Left-Ventricular Hypertrophy During Off-Season. J. Funct. Morphol. Kinesiol. 2025, 10, 271. https://doi.org/10.3390/jfmk10030271
Venckunas T, Gumauskiene B, Muanjai P, Cadefau JA, Kamandulis S. High-Intensity Interval Training Improves Cardiovascular Fitness and Induces Left-Ventricular Hypertrophy During Off-Season. Journal of Functional Morphology and Kinesiology. 2025; 10(3):271. https://doi.org/10.3390/jfmk10030271
Chicago/Turabian StyleVenckunas, Tomas, Birute Gumauskiene, Pornpimol Muanjai, Joan Aureli Cadefau, and Sigitas Kamandulis. 2025. "High-Intensity Interval Training Improves Cardiovascular Fitness and Induces Left-Ventricular Hypertrophy During Off-Season" Journal of Functional Morphology and Kinesiology 10, no. 3: 271. https://doi.org/10.3390/jfmk10030271
APA StyleVenckunas, T., Gumauskiene, B., Muanjai, P., Cadefau, J. A., & Kamandulis, S. (2025). High-Intensity Interval Training Improves Cardiovascular Fitness and Induces Left-Ventricular Hypertrophy During Off-Season. Journal of Functional Morphology and Kinesiology, 10(3), 271. https://doi.org/10.3390/jfmk10030271






