Contribution of Tibialis Anterior in Sit-to-Stand Motion: Implications for Its Role in Shifting the Center of Pressure Backward
Abstract
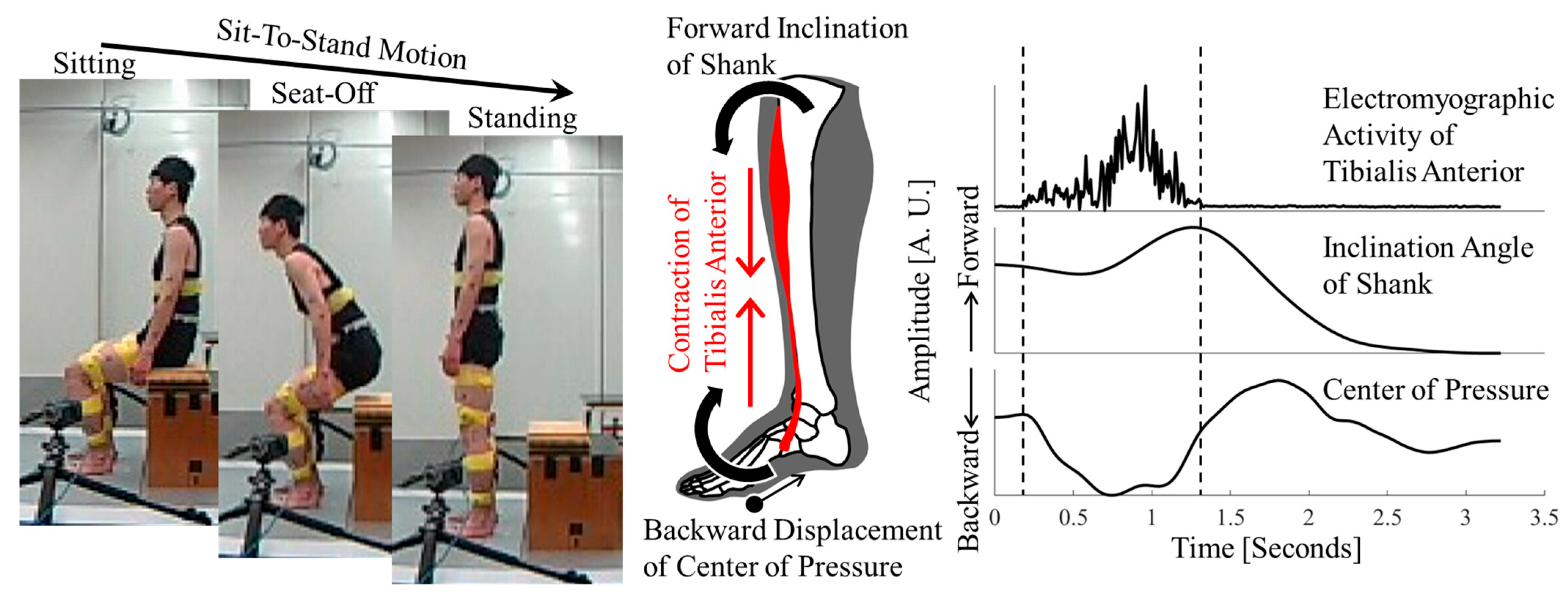
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Data Collection
2.4. Data Processing
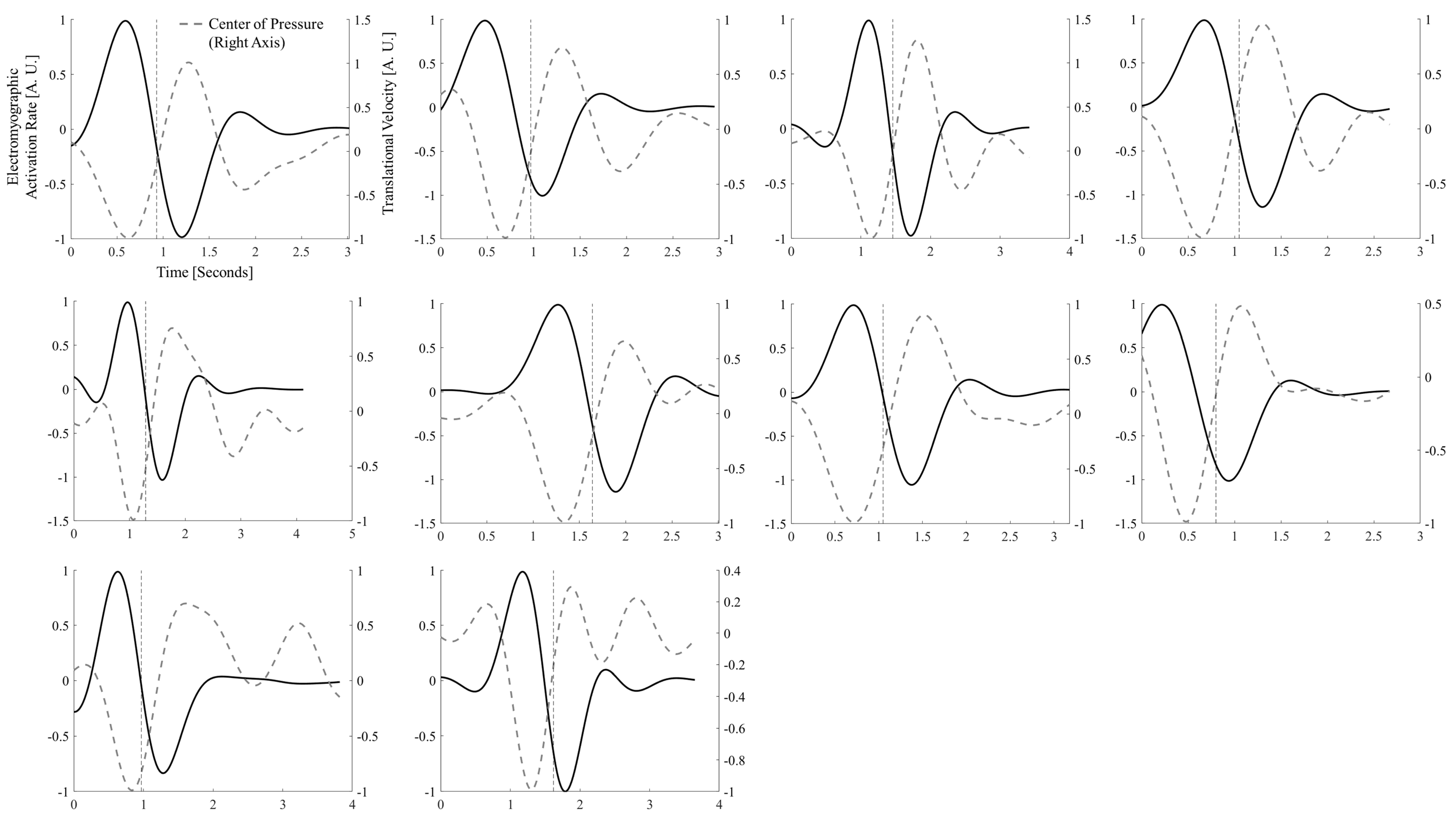
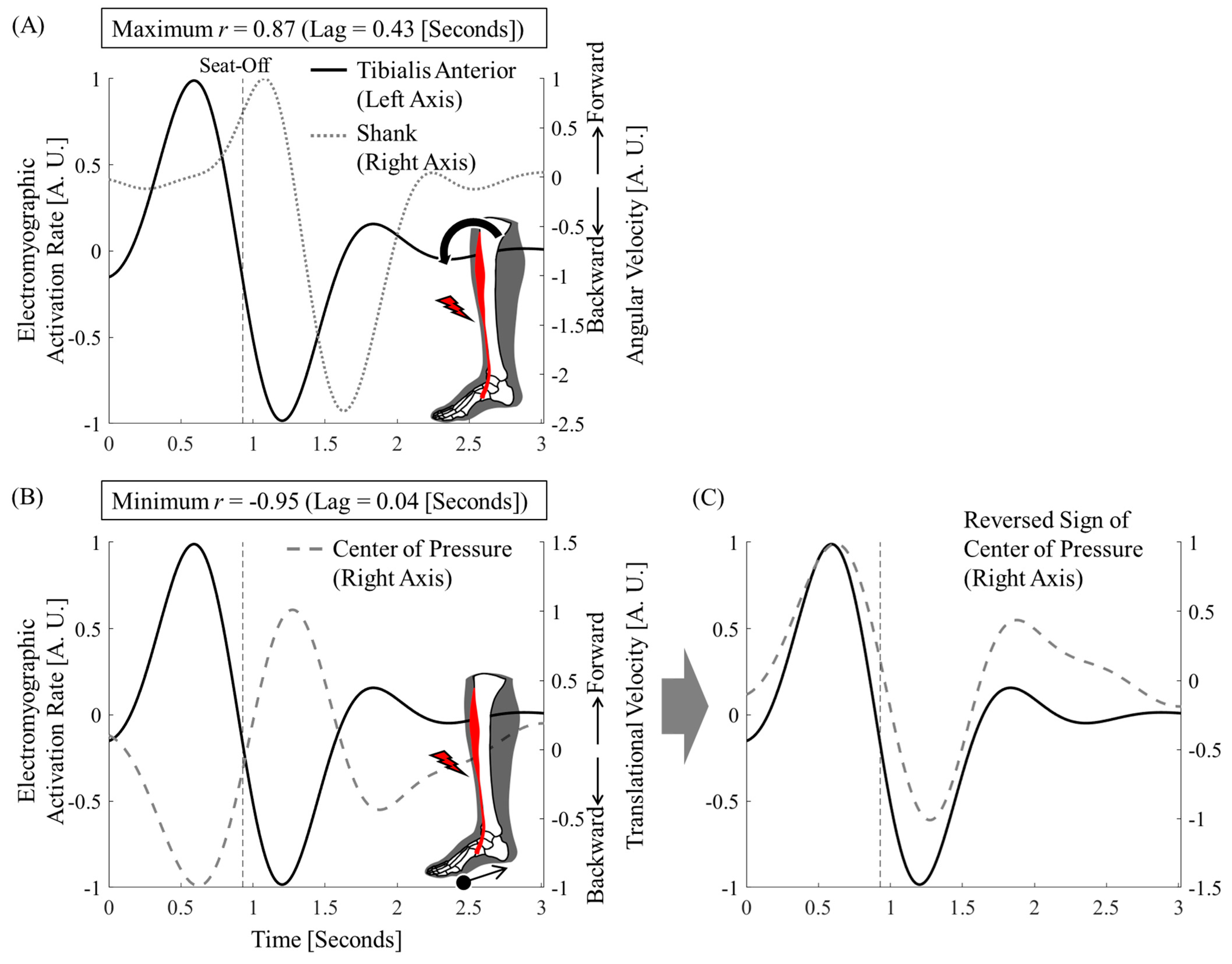
2.5. Correlation and Synchrony Between Tibialis Anterior, Shank Tilt, and Center of Pressure
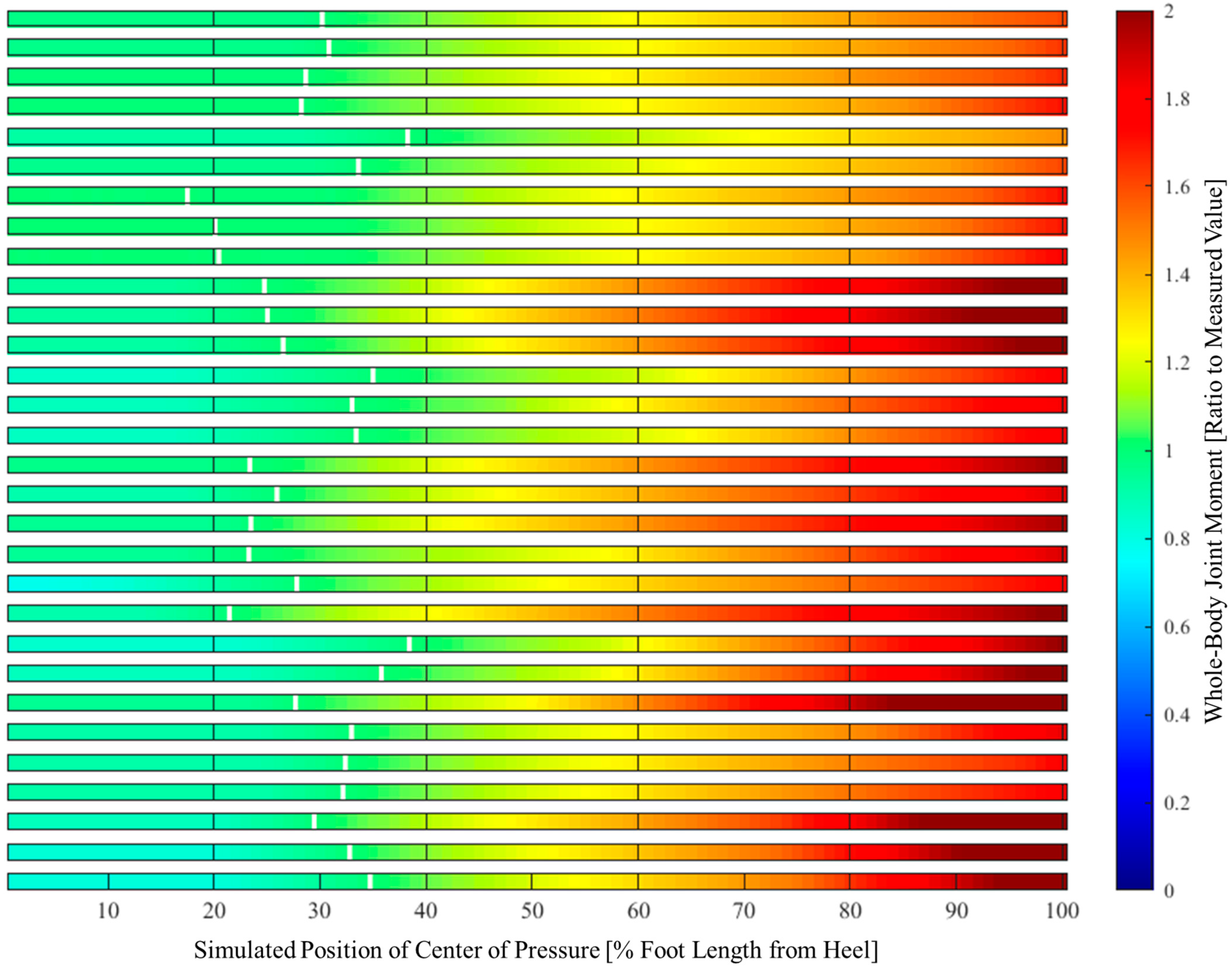
2.6. Center of Pressure and Its Relationship to Whole-Body Joint Moments
2.7. Relative Position of Center of Pressure and Mass and Angle of Ground Reaction Force
3. Results
4. Discussion
4.1. Tibialis Anterior Activity and Center-of-Pressure Shift
4.2. Effect of Center-of-Pressure Shift on Minimizing Whole-Body Joint Moments
4.3. Center of Pressure and Sit-to-Stand Balance
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMG | electromyography |
Appendix A

References
- Bramble, D.M.; Lieberman, D.E. Endurance running and the evolution of homo. Nature 2004, 432, 345–352. [Google Scholar] [CrossRef]
- Loram, I.D.; Maganaris, C.N.; Lakie, M. Paradoxical muscle movement in human standing. J. Physiol. 2004, 556, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Loram, I.D.; Lakie, M. Human balancing of an inverted pendulum: Position control by small, ballistic-like, throw and catch movements. J. Physiol. 2002, 540, 1111–1124. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Khemlani, M.M.; Carr, J.H.; Crosbie, W.J. Muscle synergies and joint linkages in sit-to-stand under two initial foot positions. Clin. Biomech. 1999, 14, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Vander Linden, D.W.; Brunt, D.; McCulloch, M.U. Variant and invariant characteristics of the sit-to-stand task in healthy elderly adults. Arch. Phys. Med. Rehabil. 1994, 75, 653–660. [Google Scholar] [CrossRef]
- Roebroeck, M.E.; Doorenbosch, C.A.; Harlaar, J.; Jacobs, R.; Lankhorst, G.J. Biomechanics and muscular activity during sit-to-stand transfer. Clin. Biomech. 1994, 9, 235–244. [Google Scholar] [CrossRef] [PubMed]
- De Leva, P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J. Biomech. 1996, 29, 1223–1230. [Google Scholar] [CrossRef]
- Kodama, J.; Watanabe, T. Examination of inertial sensor-based estimation methods of lower limb joint moments and ground reaction force: Results for squat and sit-to-stand movements in the sagittal plane. Sensors 2016, 16, 1209. [Google Scholar] [CrossRef]
- Demura, S.; Yamada, T. Height of chair seat and movement characteristics in sit-to-stand by young and elderly adults. Percept. Mot. Skills 2007, 104, 21–31. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 0470398183. [Google Scholar] [CrossRef]
- Hanawa, H.; Hirata, K.; Miyazawa, T.; Kubota, K.; Yokoyama, M.; Fujino, T.; Kanemura, N. Compensatory relationship of mechanical energy in paretic limb during sit-to-stand motion of stroke survivors. Hum. Mov. Sci. 2023, 88, 103052. [Google Scholar] [CrossRef]
- Pai, Y.-C.; Naughton, B.; Chang, R.; Rogers, M. Control of body centre of mass momentum during sit-to-stand among young and elderly adults. Gait Posture 1994, 2, 109–116. [Google Scholar] [CrossRef]
- Riley, P.O.; Krebs, D.E.; Popat, R.A. Biomechanical analysis of failed sit-to-Stand. IEEE Trans. Rehabil. Eng. 1997, 5, 353–359. [Google Scholar] [CrossRef]
- Sasagawa, S.; Shinya, M.; Nakazawa, K. Intersegmental coordination during quiet standing in adults and children: A cross-correlation analysis. Gait Posture 2009, 29, 59–63. [Google Scholar] [CrossRef]
- Yang, J.F.; Winter, D.A. Electromyographic amplitude normalization methods: Improving their sensitivity as diagnostic tools in gait analysis. Arch. Phys. Med. Rehabil. 1984, 65, 517–521. [Google Scholar] [PubMed]
- Robertson, D.G.E.; Caldwell, G.E.; Hamill, J.; Kamen, G.; Whittlesey, S.N. Research Methods in Biomechanics; Human Kinetics Publishers: Champaign, IL, USA, 2014. [Google Scholar] [CrossRef]
- Mathiyakom, W.; McNitt-Gray, J.L.; Requejo, P.; Costa, K. Modifying center of mass trajectory during sit-to-stand tasks redistributes the mechanical demand across the lower extremity joints. Clin. Biomech. 2005, 20, 105–111. [Google Scholar] [CrossRef]
- Horak, F.B. Clinical assessment of balance disorders. Gait Posture 1997, 6, 76–84. [Google Scholar] [CrossRef]
- Riley, P.O.; Mann, R.W.; Hodge, W.A. Modelling of the biomechanics of posture and balance. J. Biomech. 1990, 23, 503–506. [Google Scholar] [CrossRef]
- Smith, T.M.; Hester, G.M.; Ha, P.L.; Olmos, A.A.; Stratton, M.T.; VanDusseldorp, T.A.; Feito, Y.; Dalton, B.E. Sit-to-stand kinetics and correlates of performance in young and older males. Arch. Gerontol. Geriatr. 2020, 91, 104215. [Google Scholar] [CrossRef]
- Chorin, F.; Cornu, C.; Beaune, B.; Frère, J.; Rahmani, A. Sit to stand in elderly fallers vs non-fallers: New insights from force platform and electromyography data. Aging Clin. Exp. Res. 2016, 28, 871–879. [Google Scholar] [CrossRef]
- Cavanagh, P.R.; Komi, P.V. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur. J. Appl. Physiol. Occup. Physiol. 1979, 42, 159–163. [Google Scholar] [CrossRef]
- Nordez, A.; Gallot, T.; Catheline, S.; Guével, A.; Cornu, C.; Hug, F. Electromechanical delay revisited using very high frame rate ultrasound. J. Appl. Physiol. 2009, 106, 1970–1975. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.U.; Şendemir-Ürkmez, A.; Türker, K.S. Effect of gender, age, fatigue and contraction level on electromechanical delay. Clin. Neurophysiol. 2010, 121, 1700–1706. [Google Scholar] [CrossRef]
- Blackburn, J.T.; Bell, D.R.; Norcross, M.F.; Hudson, J.D.; Engstrom, L.A. Comparison of hamstring neuromechanical properties between healthy males and females and the influence of musculotendinous stiffness. J. Electromyogr. Kinesiol. 2009, 19, e362–e369. [Google Scholar] [CrossRef] [PubMed]
- Úbeda, A.; Del Vecchio, A.; Sartori, M.; Puente, S.T.; Torres, F.; Azorín, J.M.; Farina, D. Electromechanical delay in the tibialis anterior muscle during time-varying ankle dorsiflexion. IEEE Int. Conf. Rehabil. Robot. 2017, 2017, 68–71. [Google Scholar] [CrossRef]
- Ates, F.; Davies, B.L.; Chopra, S.; Coleman-Wood, K.; Litchy, W.J.; Kaufman, K.R. Intramuscular pressure of tibialis anterior reflects ankle torque but does not follow joint angle-torque relationship. Front. Physiol. 2018, 9, 312222. [Google Scholar] [CrossRef]
- Doorenbosch, C.A.M.; Harlaar, J.; Roebroeck, M.E.; Lankhorst, G.J. Two strategies of transferring from sit-to-stand; the activation of monoarticular and biarticular muscles. J. Biomech. 1994, 27, 1299–1307. [Google Scholar] [CrossRef]
- Goulart, F.R.; Valls-Solé, J. Patterned electromyographic activity in the sit-to-stand movement. Clin. Neurophysiol. 1999, 110, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Schultz, A.B.; Alexander, N.B.; Ashton-Miller, J.A. Biomechanical analyses of rising from a chair. J. Biomech. 1992, 25, 1383–1391. [Google Scholar] [CrossRef]
- Yoshioka, S.; Nagano, A.; Himeno, R.; Fukashiro, S. Computation of the kinematics and the minimum peak joint moments of sit-to-stand movements. Biomed. Eng. OnLine 2007, 6, 26. [Google Scholar] [CrossRef]
- Hicks, J.H. The mechanics of the foot: II. The plantar aponeurosis and the arch. J. Anat. 1954, 88, 25–30. [Google Scholar] [PubMed]

| ID | Age (Years) | Height (m) | Weight (kg) |
|---|---|---|---|
| 1 | 30 | 1.75 | 60.0 |
| 2 | 22 | 1.77 | 68.0 |
| 3 | 22 | 1.83 | 65.0 |
| 4 | 27 | 1.71 | 54.5 |
| 5 | 21 | 1.73 | 60.0 |
| 6 | 30 | 1.56 | 52.0 |
| 7 | 21 | 1.69 | 57.8 |
| 8 | 22 | 1.50 | 58.8 |
| 9 | 71 | 1.75 | 57.3 |
| 10 | 67 | 1.74 | 73.0 |
| CCFTA_Shank | CCFTA_CoP | |||
|---|---|---|---|---|
| r (Maximum) | Lag (s) | r (Minimum) | Lag (s) | |
| Mean | 0.85 | 0.37 | −0.90 | 0.13 |
| SD | 0.10 | 0.12 | 0.05 | 0.07 |
| Range | 0.49–0.97 | 0.05–0.53 | −0.98–−0.80 | 0.01–0.26 |
| Joint Moment (Ratio to Measured Value) | ||
|---|---|---|
| Minimum | Maximum | |
| Mean | 0.92 | 1.88 |
| SD | 0.06 | 0.23 |
| Range | 0.78–1.00 | 1.47–2.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanawa, H.; Miyazawa, T.; Hirata, K.; Kubota, K.; Fujino, T. Contribution of Tibialis Anterior in Sit-to-Stand Motion: Implications for Its Role in Shifting the Center of Pressure Backward. J. Funct. Morphol. Kinesiol. 2025, 10, 156. https://doi.org/10.3390/jfmk10020156
Hanawa H, Miyazawa T, Hirata K, Kubota K, Fujino T. Contribution of Tibialis Anterior in Sit-to-Stand Motion: Implications for Its Role in Shifting the Center of Pressure Backward. Journal of Functional Morphology and Kinesiology. 2025; 10(2):156. https://doi.org/10.3390/jfmk10020156
Chicago/Turabian StyleHanawa, Hiroki, Taku Miyazawa, Keisuke Hirata, Keisuke Kubota, and Tsutomu Fujino. 2025. "Contribution of Tibialis Anterior in Sit-to-Stand Motion: Implications for Its Role in Shifting the Center of Pressure Backward" Journal of Functional Morphology and Kinesiology 10, no. 2: 156. https://doi.org/10.3390/jfmk10020156
APA StyleHanawa, H., Miyazawa, T., Hirata, K., Kubota, K., & Fujino, T. (2025). Contribution of Tibialis Anterior in Sit-to-Stand Motion: Implications for Its Role in Shifting the Center of Pressure Backward. Journal of Functional Morphology and Kinesiology, 10(2), 156. https://doi.org/10.3390/jfmk10020156







