Platinum Atom-Functionalized Carbon Nanotubes as Efficient Sensors for CO and CO2: A Theoretical Investigation
Abstract
1. Introduction
2. Methodology
3. Results
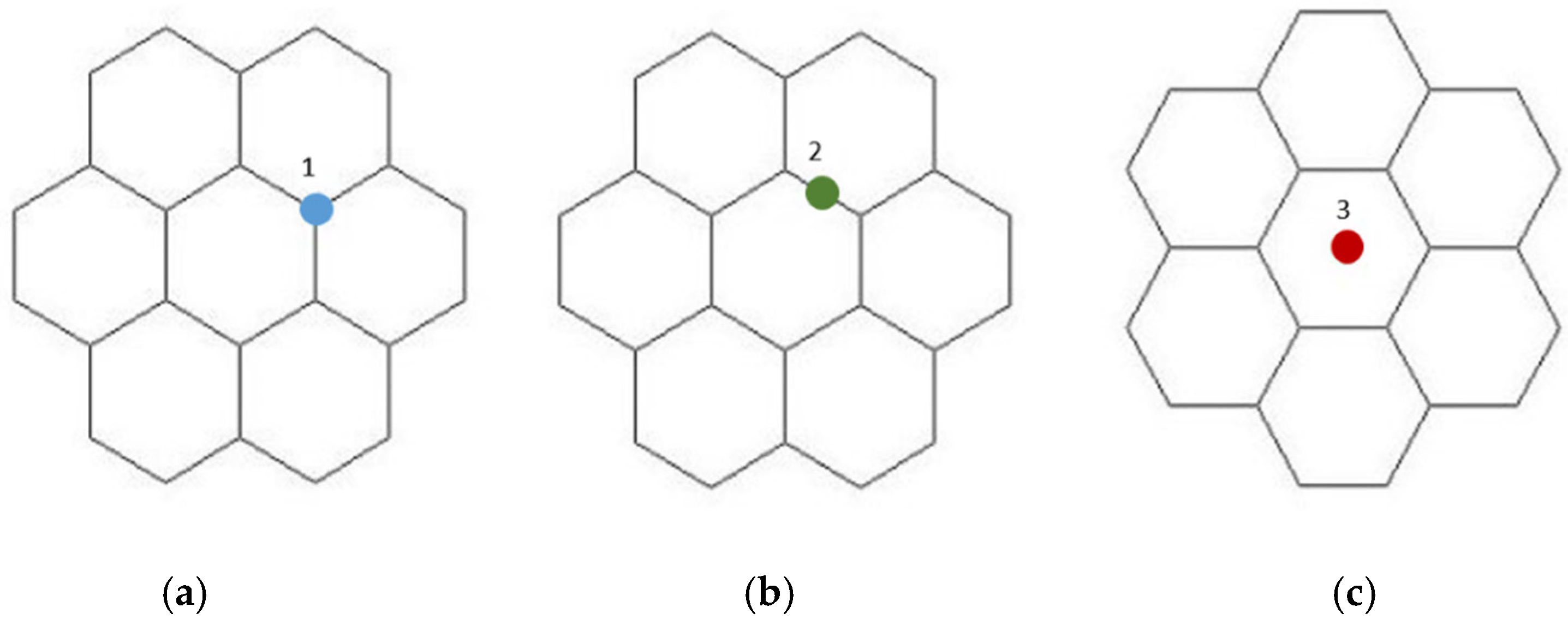
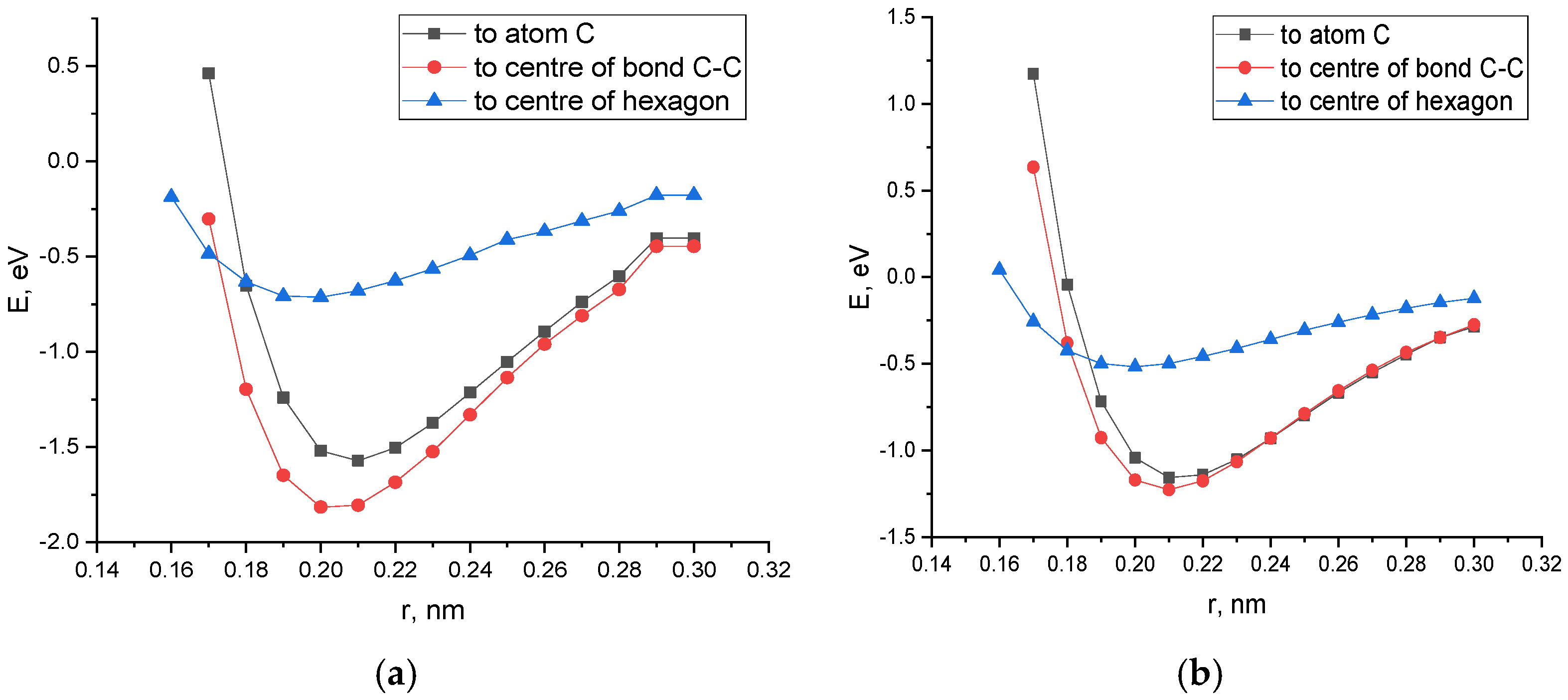
3.1. The Theoretical Study of the Possibile Mechanism of Interaction of SWCNTs of Types (6.0) and (6.6) with the Platinum Atom (Pt)
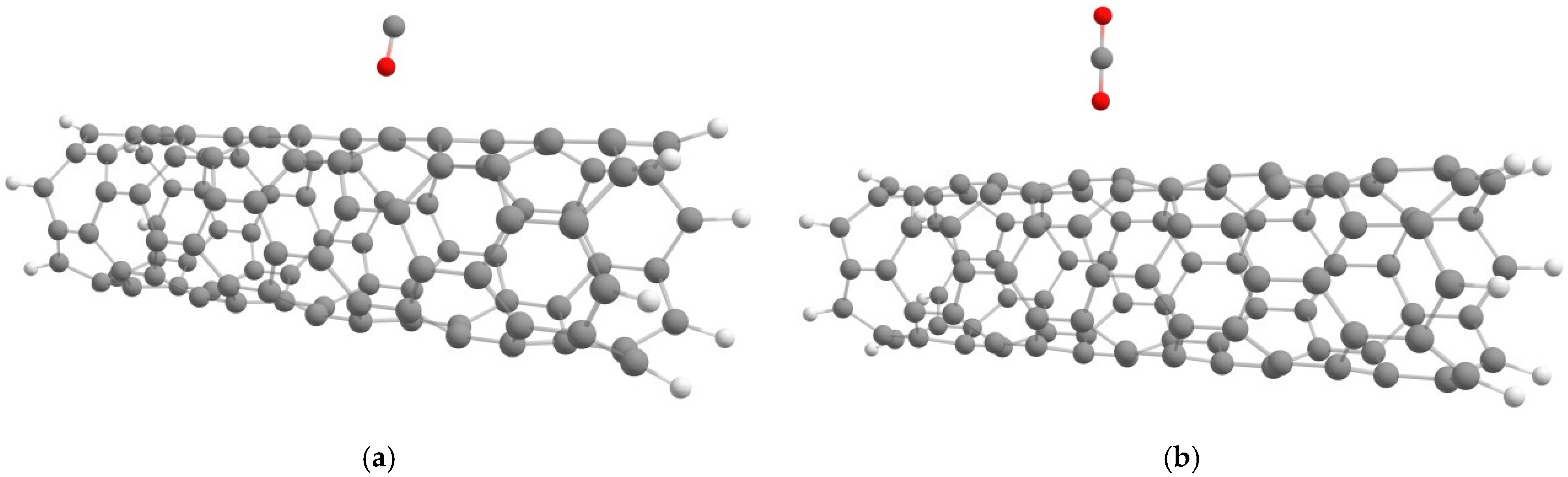
3.2. The Investigation of the SWCNT Interaction Modified by a Platinum Atom with a Carbon Monoxide Molecule
3.3. The Investigation of the SWCNT Interaction Modified by a Platinum Atom with a Molecule of Carbon Dioxide
4. Conclusions
- (1)
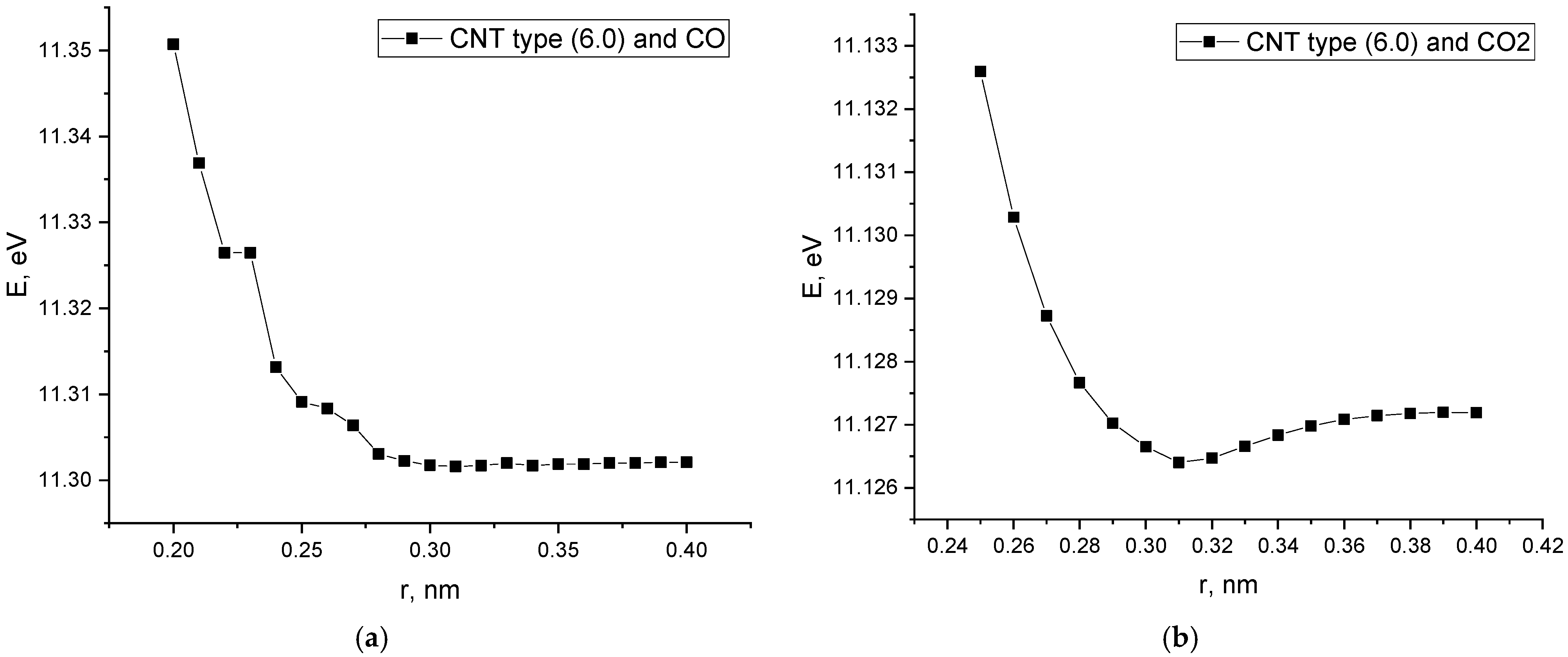
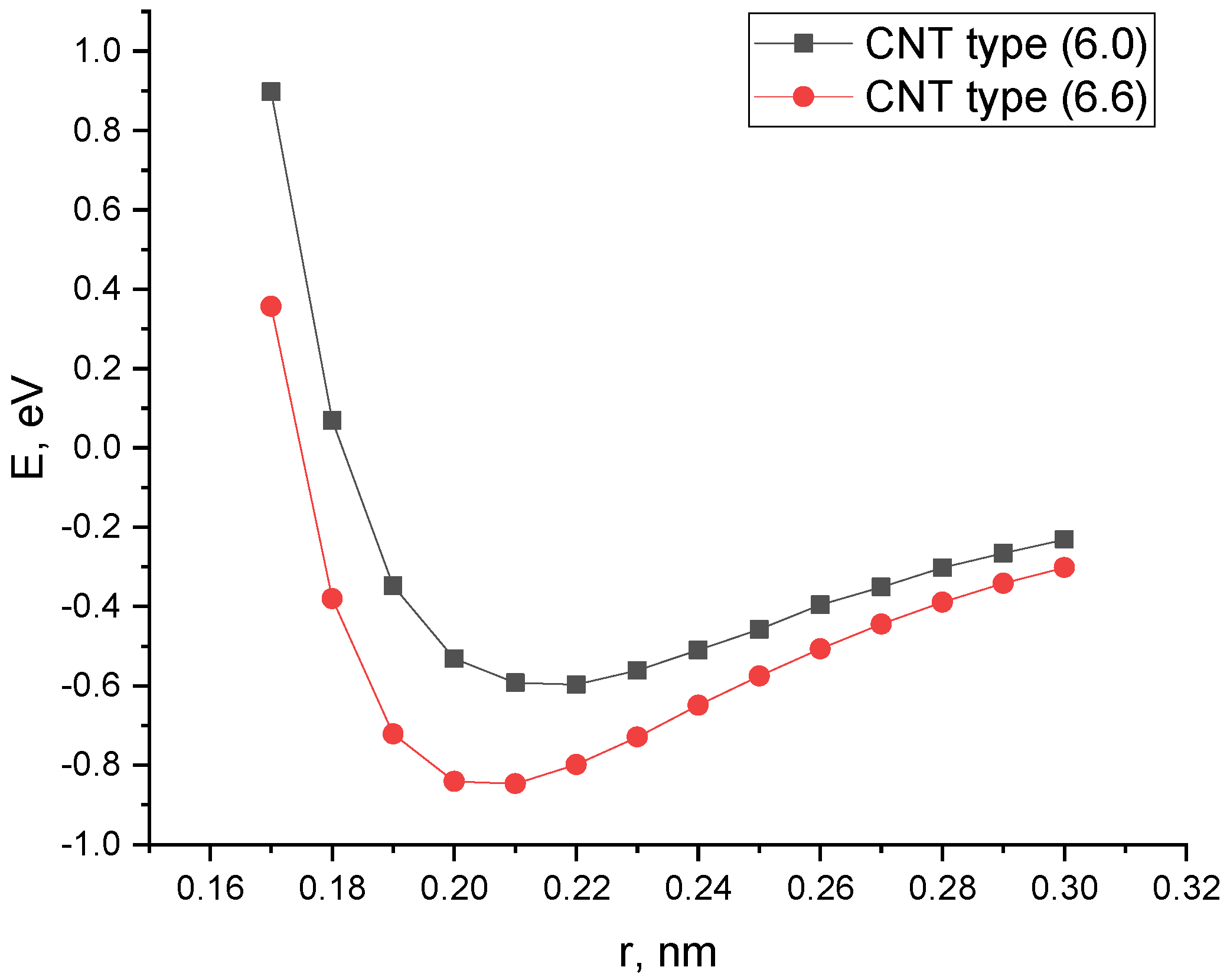
- An analysis of the energy curves of the interaction of a “pure” carbon nanotube with CO and CO2 showed the presence of a minimum interaction energy. However, the energy values are in the positive range; this indicates the instability of the obtained complexes and determines the prospects of work to improve the properties of SWCNTs for the adsorption of selected gases.
- (2)
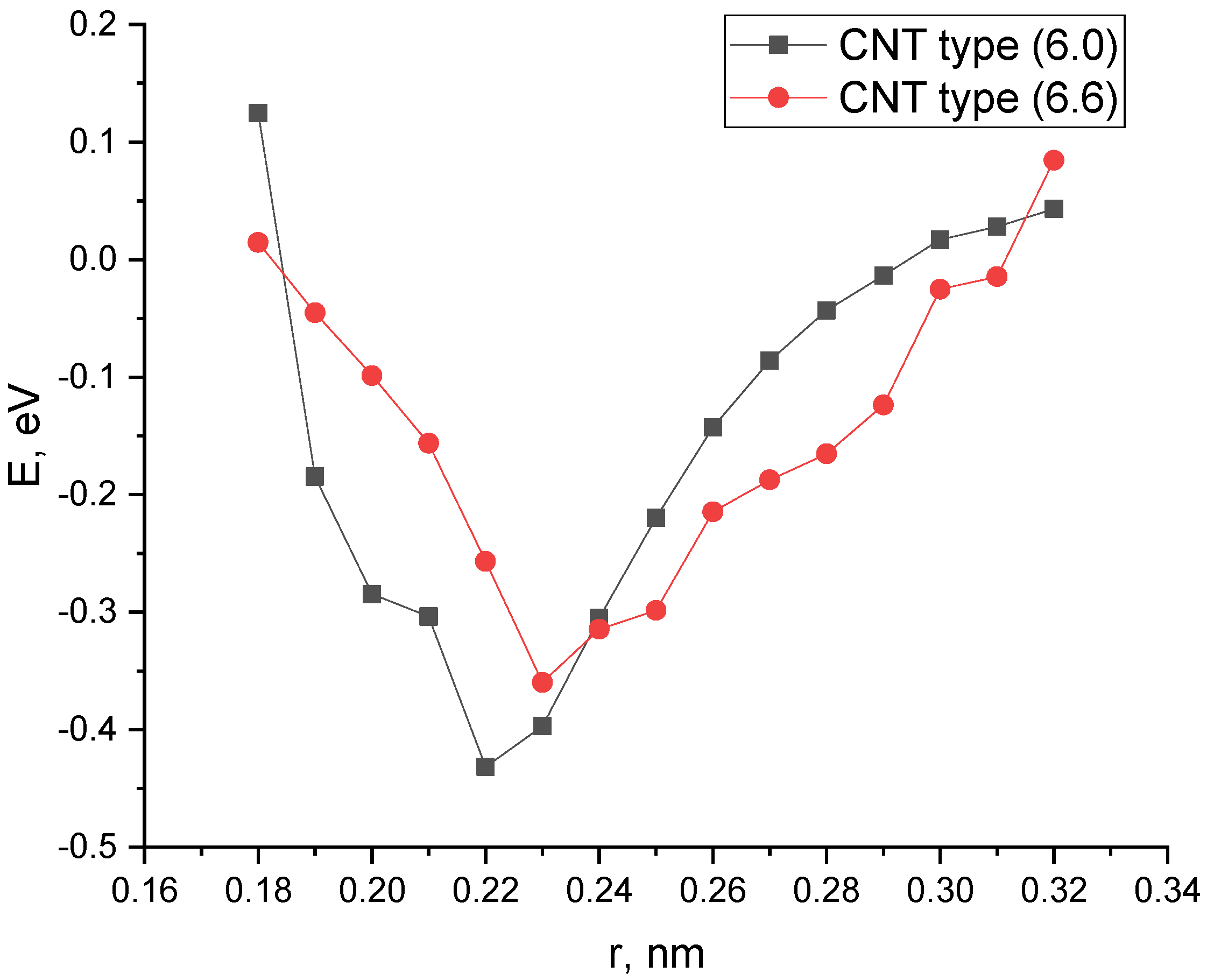
- The study of the interaction of a carbon nanotube with a platinum atom has shown that the most optimal position of the metal atom relative to the surface of the nanotubulene from an energy point of view and the stability of the configuration is its location in the center of the C-C bond.
- (3)
- Studies of the interactions of the SWCNT + Pt nanocomplex with carbon monoxide and carbon dioxide molecules have shown the presence of sorption interactions and the formation of stable complexes. The energy values correspond to the weak Van der Waals interaction. This type of interaction, unlike the chemical one, allows you to reuse the sensor without polluting it.
- (4)
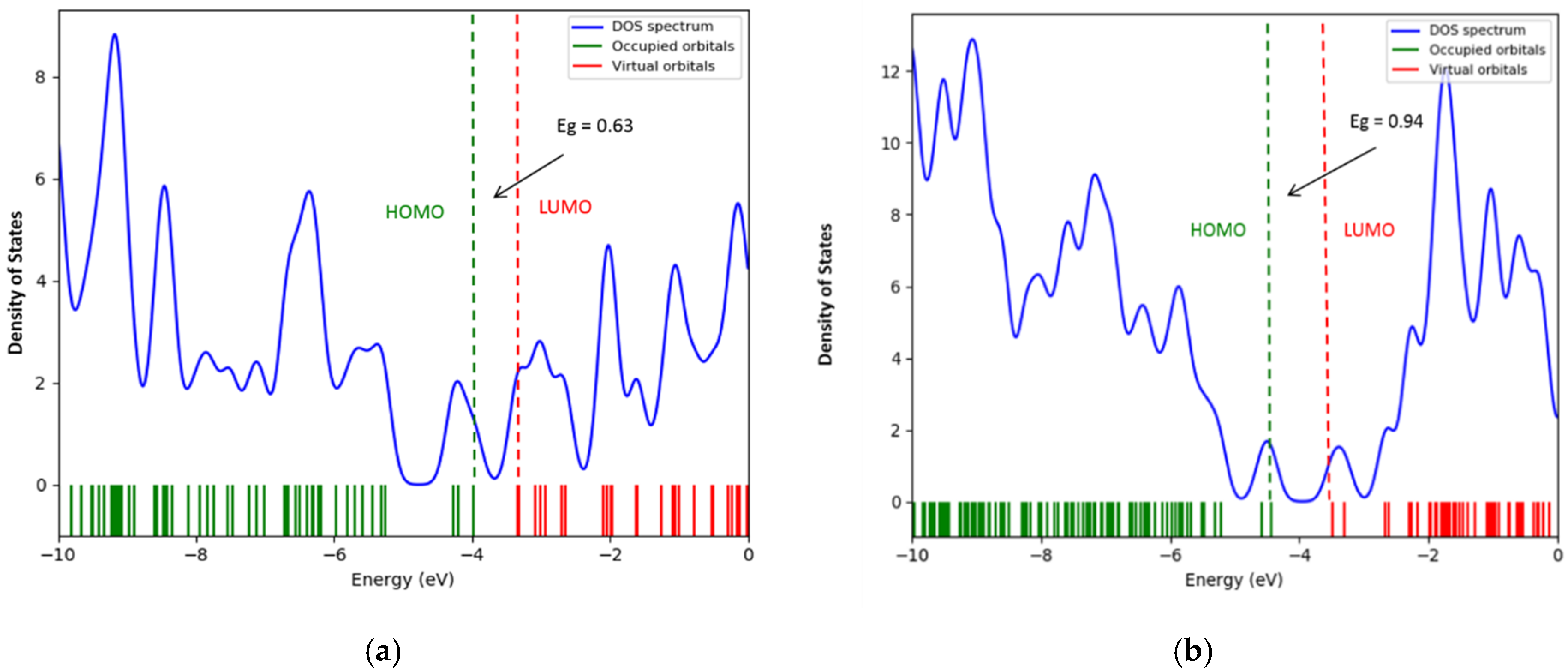
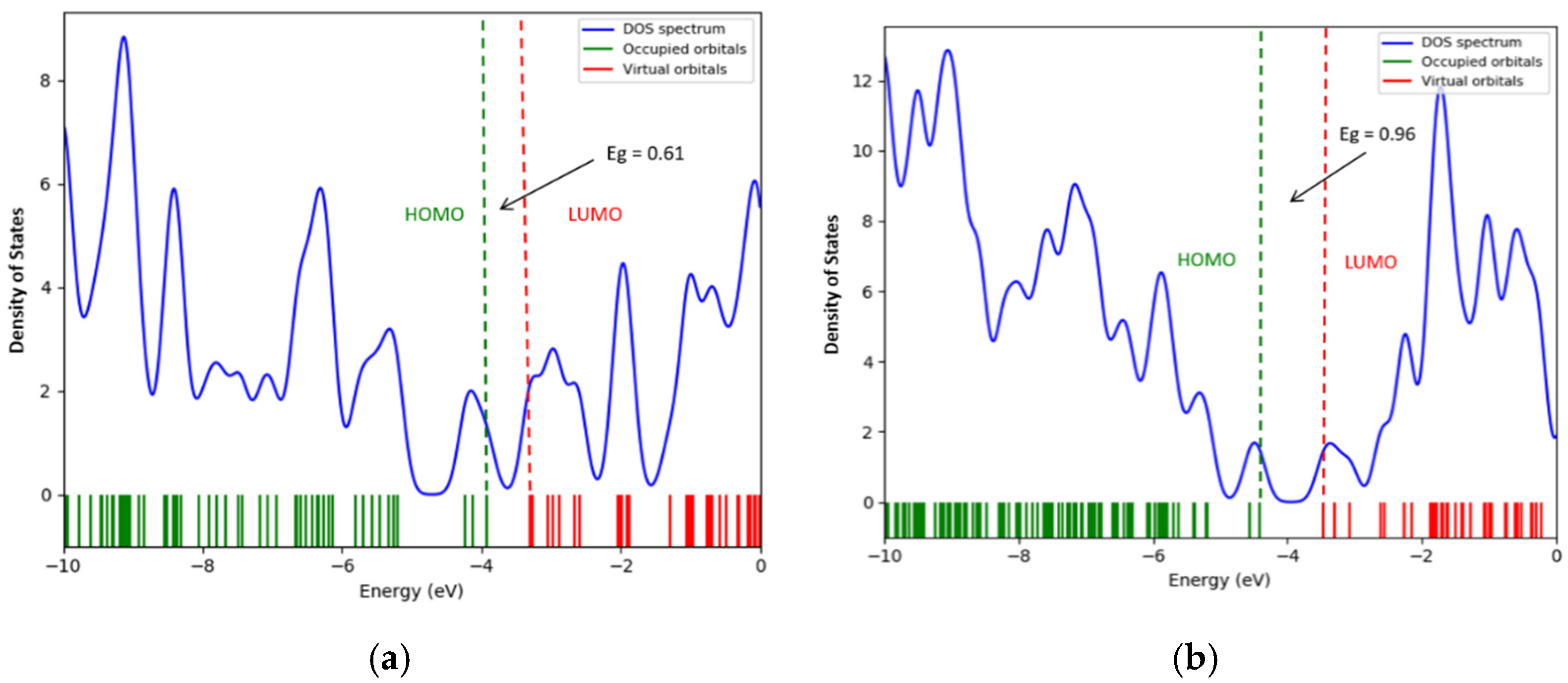
- The interaction of the SWCNT + Pt nanocomplex with carbon monoxide and carbon dioxide molecules is accompanied by a change in the band gap. Thus, sensor devices manufactured using such modified nanotube materials as sensors, work on the basis of fixing changes in the conductive characteristics of the system when additional charge carriers occur, caused by the redistribution of electron density. In the considered cases, the values of the band gap differ for the cases of the interaction of the systems with each selected gas.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, K.; Peng, H.; Zhang, B.; Chen, L.; Zhang, P.; Peng, Z.; Fu, X. Advances in carbon nanotube-based gas sensors: Exploring the path to the future. Coord. Chem. Rev. 2024, 518, 216049. [Google Scholar] [CrossRef]
- Onyancha, R.B.; Ukhurebor, K.E.; Aigbe, U.O.; Osibote, O.A.; Kusuma, H.S.; Darmokoesoemo, H.; Balogun, V.A. A systematic review on the detection and monitoring of toxic gases using carbon nanotube-based biosensors. Sens. Bio-Sens. Res. 2021, 34, 100463. [Google Scholar] [CrossRef]
- Romero-Guzmán, L.; Reyes-Gutiérrez, L.R.; Romero-Guzmán, E.T.; Savedra-Labastida, E. Carbon nanotube filters for removal of air pollutants from mobile sources. J. Miner. Mater. Charact. Eng. 2018, 6, 105–118. [Google Scholar] [CrossRef][Green Version]
- Mazumder, J.T.; Pandey, S.; Jha, R.K. Homoatomic flatlands beyond graphene: A new avenue for gas sensors. Coord. Chem. Rev. 2024, 507, 215747. [Google Scholar] [CrossRef]
- Bai, N.; Chen, X.; Wang, H.; Zhang, C.; Zhu, J.; Wang, W.; Kang, C.; Tang, Y.; Li, Z.; Cui, B.; et al. High-sensitivity piezoresistive sensors based on functionalized carbon nanotube/TPU composite for human motion detection. Polymer 2025, 322, 128156. [Google Scholar] [CrossRef]
- Rantataro, S.; Gustafsson, E.; Varjos, I. Next-generation carbon nanotube electrochemical sensors for liquid biopsy applications. Electrochem. Commun. 2025, 177, 107967. [Google Scholar] [CrossRef]
- Boudiba, A.; Zhang, C.; Bittencourt, C.; Umek, P.; Olivier, M.-G.; Snyders, R.; Debliquy, M. SO2 gas sensors based on WO3 nanostructures with different morphologies. Procedia Eng. 2012, 47, 1033–1036. [Google Scholar] [CrossRef]
- Wang, X.; Cui, F.; Lin, J.; Ding, B.; Yu, J.; Al-Deyab, S.S. Functionalized nanoporous TiO2 fibers on quartz crystal microbalance platform for formaldehyde sensor. Sens. Actuators B Chem. 2012, 171–172, 658–665. [Google Scholar] [CrossRef]
- Zhao, B.; Yan, Y.; Huang, Y.; Chen, Z.; Liang, Y.; Luo, Z.; Gao, C.; Wang, L. Achievement of high n-type thermoelectric performance and stretchability in single-walled carbon nanotubes/polyethyleneimine /polyvinylpyrrolidone composites: Enabling self-healing and sensitive self-powered temperature sensors. J. Power Sources 2025, 631, 236300. [Google Scholar] [CrossRef]
- Tereshkov, M.; Dontsova, T.; Saruhan, B.; Krüger, S. Metal oxide-based sensors for ecological monitoring: Progress and perspectives. Chemosensors 2024, 12, 42. [Google Scholar] [CrossRef]
- Weng, J.; Zhang, J.; Zhang, C.; Lv, J.; Liu, J.; Zhou, C.; Yuan, J.; Wang, M.; Xu, D.; Zhong, Y.; et al. Effective detection of early Citrus Huanglongbing by polyethyleneimine modified multi-walled carbon nanotubes gas sensor. Sens. Actuators B Chem. 2022, 371, 132508. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering biomimetic superhydrophobic surfaces of electrospun nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Yoosefian, M.; Raissi, H.; Mola, A. The hybrid of Pd and SWCNT (Pd loaded on SWCNT) as an efficient sensor for the formaldehyde molecule detection: A DFT study. Sens. Actuators B Chem. 2015, 212, 55–62. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.M.; Sidqi, M.E.; Radwan, A.; Sayed, M.A.; Aziz, A.A.A. Highly sensitive voltammetric sensor for dopamine based on a novel bimetallic AlZn MOF@ multi-walled carbon nanotubes: Fabrication, electrochemical characterization and applications. Microchem. J. 2025, 212, 113503. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Camlibel, N.O.; Kandola, B.K. Highly sensitive textile pressure sensors with novel hierarchical architecture based on conductive polymers, silver nanoparticles and carbon nanotubes. Sens. Actuators A Phys. 2024, 382, 116166. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Y.; Chen, A.; Yu, W.; Li, Y. Gas sensor based on micro fiber coated with carboxylated carbon nanotubes to detect new environmentally friendly insulating gas C4F7N. Opt. Commun. 2025, 580, 131606. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.; Wang, Y.; Wang, S.; Baughman, R.H. A flexible carbon nanotube yarns based electrochemical sensor for non-enzymatic detection of hydrogen peroxide. Microchem. J. 2025, 214, 114086. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Yuliarto, B. The development of gas sensor based on carbon nanotubes. J. Electrochem. Soc. 2016, 163, B97–B106. [Google Scholar] [CrossRef]
- Song, S.; Chen, D.; Wang, H.; Guo, Q.; Wang, W.; Wu, M.; Yu, W. Film bulk acoustic formaldehyde sensor with polyethyleneimine-modified single-wall carbon nanotubes as sensitive layer. Sens. Actuators B Chem. 2018, 266, 204–212. [Google Scholar] [CrossRef]
- Shi, H.; Chen, J.; Yu, S. DFT study on adsorption of small gas molecules on Pt-capped armchair and zigzag single-walled carbon nanotubes. Mater. Today Commun. 2023, 37, 107200. [Google Scholar] [CrossRef]
- Abe, H.; Kimura, Y.; Ma, T.; Tadaki, D.; Hirano-Iwata, A.; Niwano, M. Response characteristics of a highly sensitive gas sensor using a titanium oxide nanotube film decorated with platinum nanoparticles. Sens. Actuators B Chem. 2020, 321, 959–965. [Google Scholar] [CrossRef]
- Penza, M.; Rossi, R.; Alvisi, M.; Cassano, G.; Signore, M.; Serra, E.; Giorgi, R. Pt- and Pd-nanoclusters functionalized carbon nanotubes networked films for sub-ppm gas sensors. Sens. Actuators B Chem. 2008, 135, 289–297. [Google Scholar] [CrossRef]
- Lee, M.-S.; Lee, S.-Y.; Park, S.-J. Preparation and characterization of multi-walled carbon nanotubes impregnated with polyethyleneimine for carbon dioxide capture. Int. J. Hydrogen Energy 2015, 40, 3415–3421. [Google Scholar] [CrossRef]
- Folke, M.; Cernerud, L.; Ekström, M.; Hök, B. Critical review of non-invasive respiratory monitoring in medical care. Med. Biol. Eng. Comput. 2003, 41, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Duan, Y. Breath analysis: Potential for clinical diagnosis and exposure assessment. Clin. Chem. 2006, 52, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, A.; Turner, A. Sniffing out the truth: Clinical diagnosis using the electronic nose. Clin. Chem. Lab. Med. 2000, 38, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Burdett-Smith, P. A patient who changed my practice: Always check the respiratory rate. BMJ 1997, 314, 7093. [Google Scholar] [CrossRef]
- Lovett, P.B.; Buchwald, J.M.; Stürmann, K.; Bijur, P. The vexatious vital: Neither clinical measurements by nurses nor an electronic monitor provides accurate measurements of respiratory rate in triage. Ann. Emerg. Med. 2005, 45, 68–76. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory; John Wiley & Sons: Hoboken, NJ, USA, 2015; 306р. [Google Scholar]
- Tabtimsai, C.; Ruangpornvisuti, V.; Wanno, B. Density functional theory investigation of the VIIIB transition metal atoms deposited on (5,5) single-walled carbon nanotubes. Phys. E Low-Dimens. Syst. Nanostruct. 2013, 49, 61–67. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]

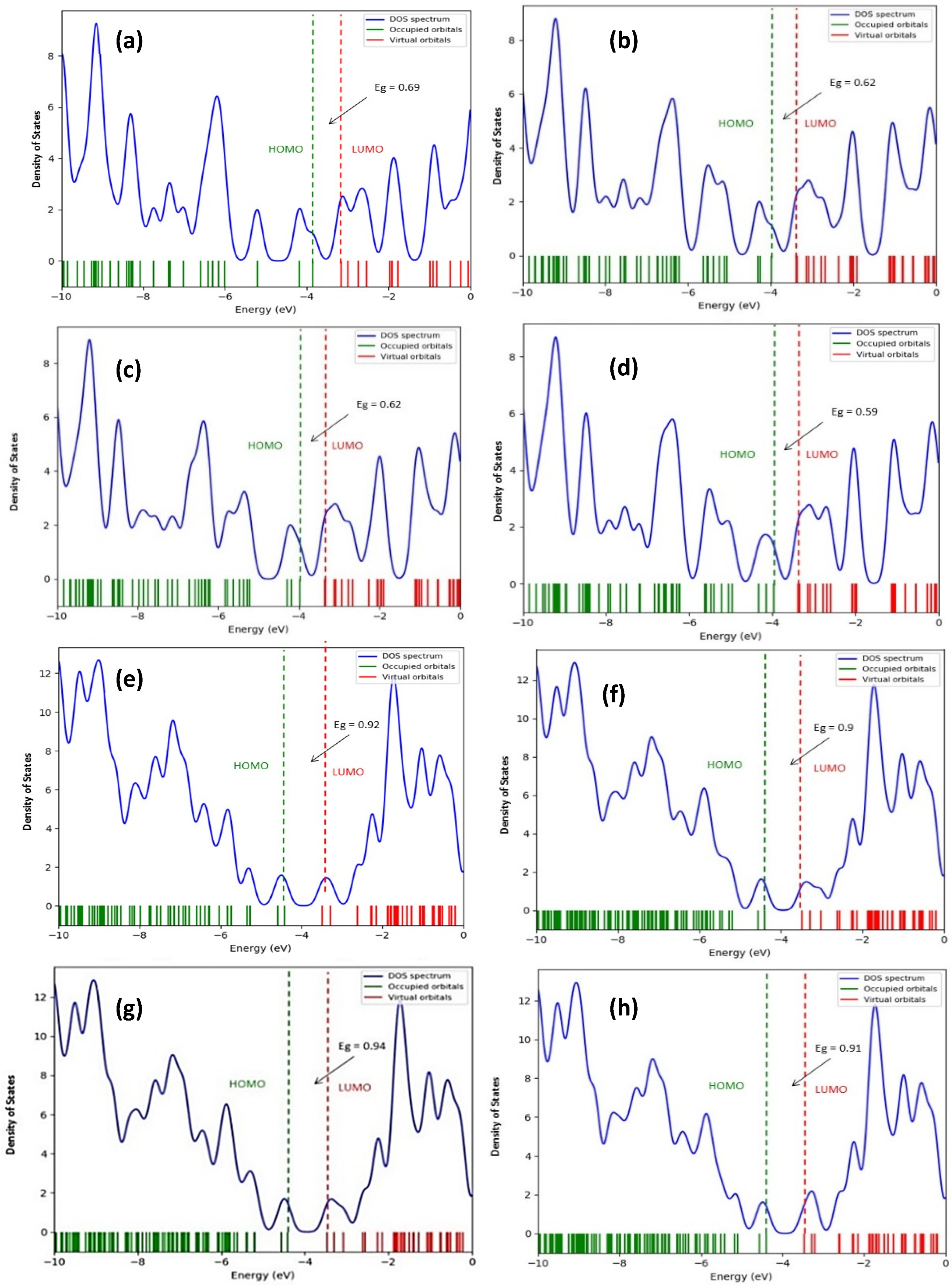


| Type of Structure | Interaction Distance, nm | Energy of Interaction (eV) | The Width of the Forbidden Zone (eV) |
|---|---|---|---|
| «Clean» nanotube type (6.0) | - | - | 0.69 |
| SWCNT (6.0)—Pt on the C atom | 0.21 | −1.57 | 0.62 |
| SWCNT (6.0)—Pt on the C-C center | 0.20 | −1.82 | 0.62 |
| SWCNT (6.0)—Pt on the center of the hexagon | 0.20 | −0. 71 | 0.59 |
| «Clean» nanotube type (6.6) | 0.92 | ||
| SWCNT (6.6)—Pt on the C atom | 0.21 | −1.16 | 0.90 |
| SWCNT (6.6)—Pt on the C-C center | 0.22 | −1.18 | 0.94 |
| SWCNT (6.6)—Pt on the center of the hexagon | 0.20 | −0.52 | 0.91 |
| Variant of Pt Atom Adsorption | The Value of the Charge on the Metal Atom Before Attachment | The Value of the Charge on the Metal Atom After Attachment | The Average Value of the Charge of Carbon Atoms on the Surface of the Nanotube Before the Addition of the Pt Atom | The Average Value of the Charge of the Nearest Atomic Neighbors on the Surface of a Nanotube After the Addition of a Pt Atom |
|---|---|---|---|---|
| SWCNT (6.0)—Pt on the C atom | 0 | 0.015 | −0.010 | −0.077 |
| SWCNT (6.0)—Pt on the C-C center | 0 | 0.014 | 0.009 | −0.382 |
| SWCNT (6.0)—Pt on the center of the hexagon | 0 | 0.017 | −0.011 | −0.085 |
| SWCNT (6.6)—Pt on the C atom | 0 | 0.049 | −0.130 | −0.033 |
| SWCNT (6.6)—Pt on the C-C center | 0 | 0.048 | −0.020 | −0.860 |
| SWCNT (6.6)—Pt on the center of the hexagon | 0 | 0.049 | 0.005 | −0.128 |
| SWCNT Type (6.0) | SWCNT Type (6.6) | |
|---|---|---|
| Ead, eV | −0.60 | −0.85 |
| Rad, nm | 0.22 | 0.21 |
| ΔEg, eV SWCNT + CO systems | 0.69 | - |
| ΔEg, eV SWCNT + Pt systems | 0.62 | 0.94 |
| ΔEg, eV SWCNT +Pt + CO systems | 0.63 | 0.94 |
| SWCNT Type (6.0) | SWCNT Type (6.6) | |
|---|---|---|
| Ead, eV | −0.43 | −0.36 |
| Rad, nm | 0.22 | 0.23 |
| ΔEg, eV SWCNT + CO2 systems | 0.69 | - |
| ΔEg, eV SWCNT + Pt systems | 0.62 | 0.94 |
| ΔEg, eV SWCNT + Pt + CO2 systems | 0.60 | 0.96 |
| CO | CO2 | |||||||
|---|---|---|---|---|---|---|---|---|
| SWCNT | SWCNT + Pt Systems | SWCNT | SWCNT + Pt Systems | |||||
| Type (6.0) | Type (6.6) | Type (6.0) | Type (6.6) | Type (6.0) | Type (6.6) | Type (6.0) | Type (6.6) | |
| Rad, nm | 0.32 | - | 0.22 | 0.21 | 0.33 | - | 0.22 | 0.23 |
| Ead, eV | 11.30 | - | −0.60 | −0.85 | 11.12 | - | −0.43 | −0.36 |
| ΔEg, eV | - | - | 0.63 | 0.94 | - | - | 0.60 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroznina, N.P.; Boroznin, S.V.; Zaporotskova, I.V.; Zaporotskov, P.A.; Sergeev, D.F.; Murugadoss, G.; Venkatesh, N.; Peera, S.G. Platinum Atom-Functionalized Carbon Nanotubes as Efficient Sensors for CO and CO2: A Theoretical Investigation. Inventions 2025, 10, 86. https://doi.org/10.3390/inventions10050086
Boroznina NP, Boroznin SV, Zaporotskova IV, Zaporotskov PA, Sergeev DF, Murugadoss G, Venkatesh N, Peera SG. Platinum Atom-Functionalized Carbon Nanotubes as Efficient Sensors for CO and CO2: A Theoretical Investigation. Inventions. 2025; 10(5):86. https://doi.org/10.3390/inventions10050086
Chicago/Turabian StyleBoroznina, Natalia P., Sergey V. Boroznin, Irina V. Zaporotskova, Pavel A. Zaporotskov, Dmitry F. Sergeev, Govindhasamy Murugadoss, Nachimuthu Venkatesh, and Shaik Gouse Peera. 2025. "Platinum Atom-Functionalized Carbon Nanotubes as Efficient Sensors for CO and CO2: A Theoretical Investigation" Inventions 10, no. 5: 86. https://doi.org/10.3390/inventions10050086
APA StyleBoroznina, N. P., Boroznin, S. V., Zaporotskova, I. V., Zaporotskov, P. A., Sergeev, D. F., Murugadoss, G., Venkatesh, N., & Peera, S. G. (2025). Platinum Atom-Functionalized Carbon Nanotubes as Efficient Sensors for CO and CO2: A Theoretical Investigation. Inventions, 10(5), 86. https://doi.org/10.3390/inventions10050086







