Biological Properties of a Composite Polymer Material Based on Polyurea and Submicron-Sized Selenium Particles
Abstract
1. Introduction
2. Materials and Methods
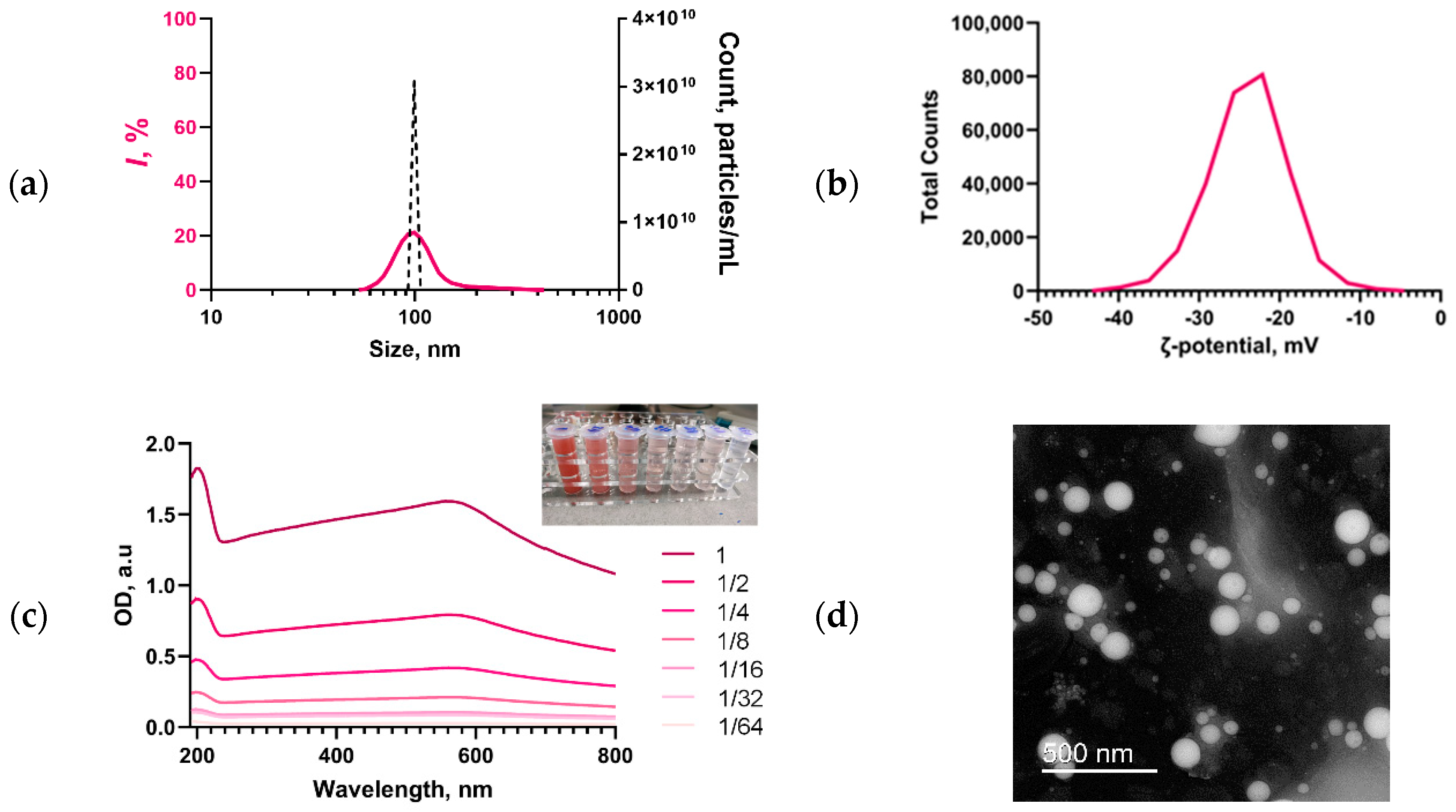
2.1. Synthesis of Se SMPs and Their Characterization
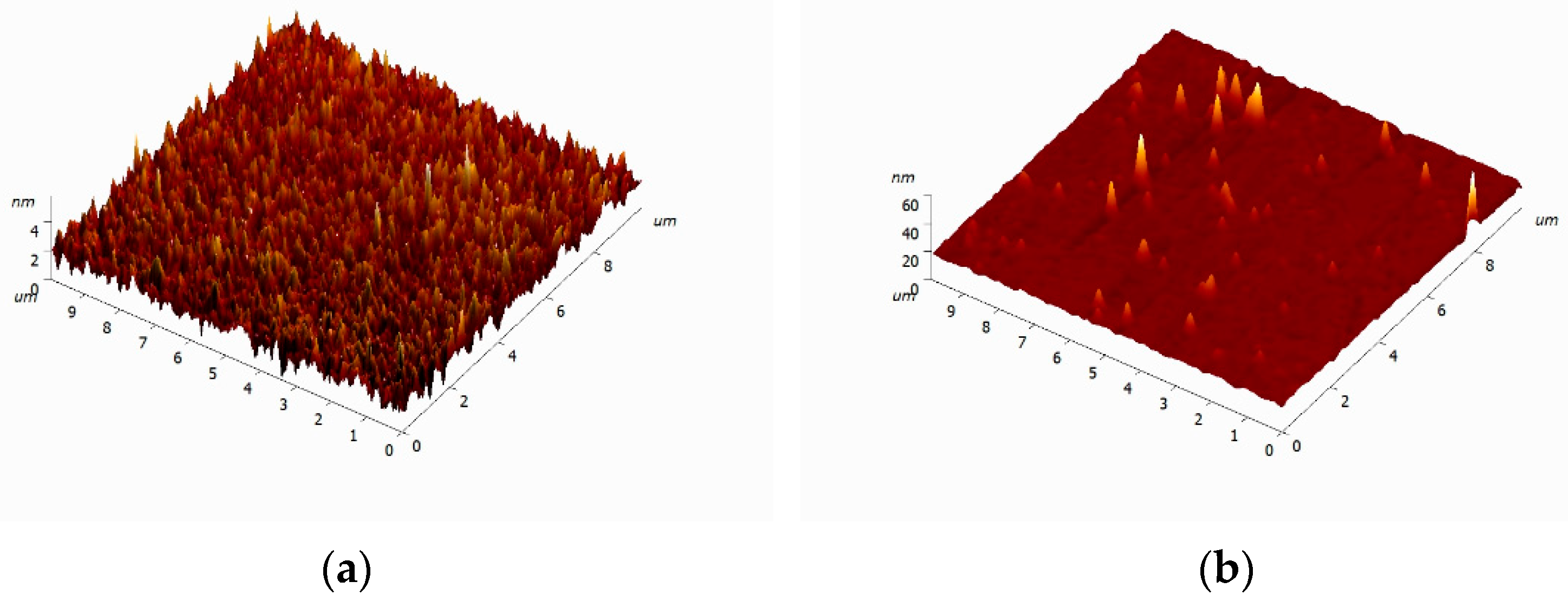
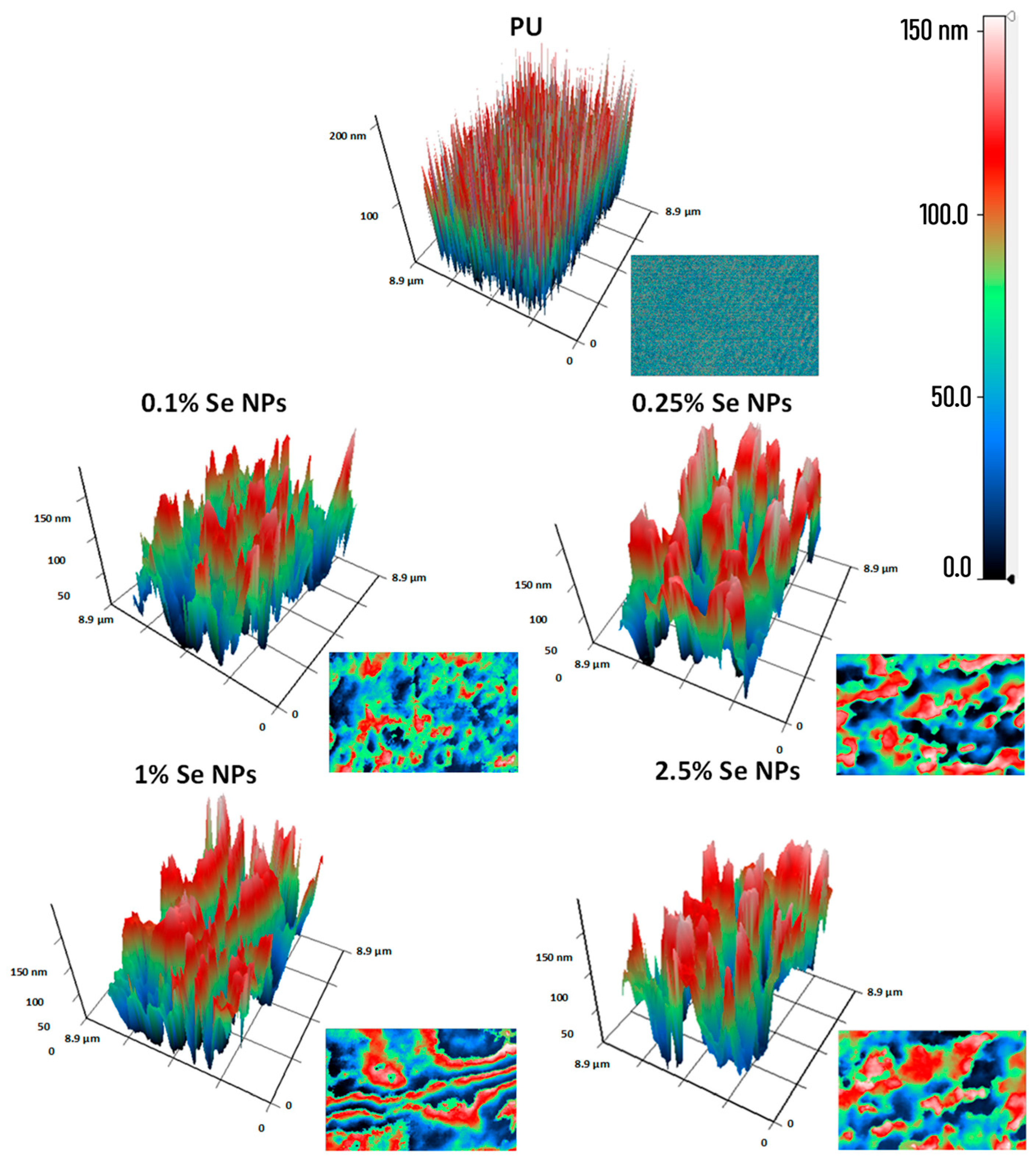
2.2. Preparation and Characterization of Composite Materials Based on Polyurea and Se SMPs
2.3. Measurement of Hydrogen Peroxide Concentration
2.4. Measurement of Hydroxyl Radical Concentration
2.5. Measurement of Level of Long-Lived Reactive Protein Species
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
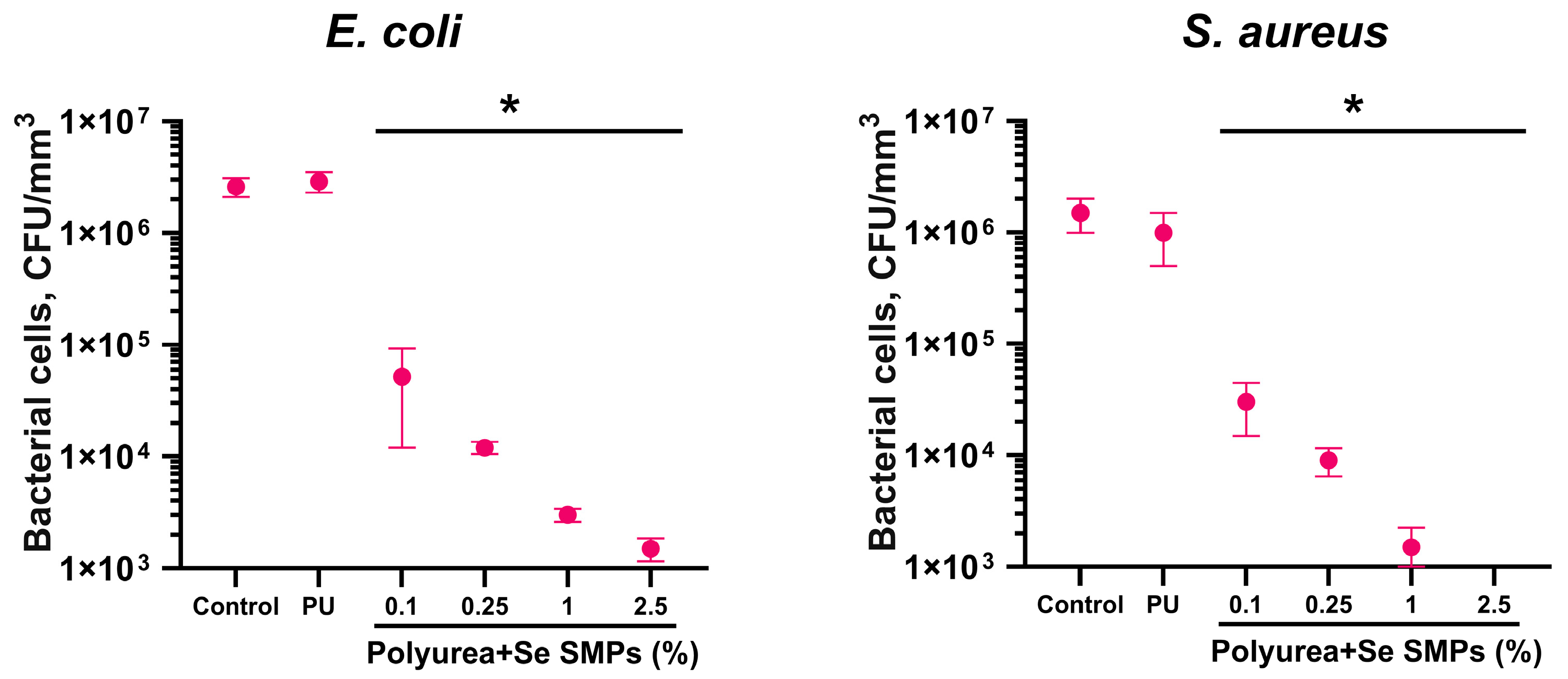
2.7. Analysis of Bacteriostatic Activity
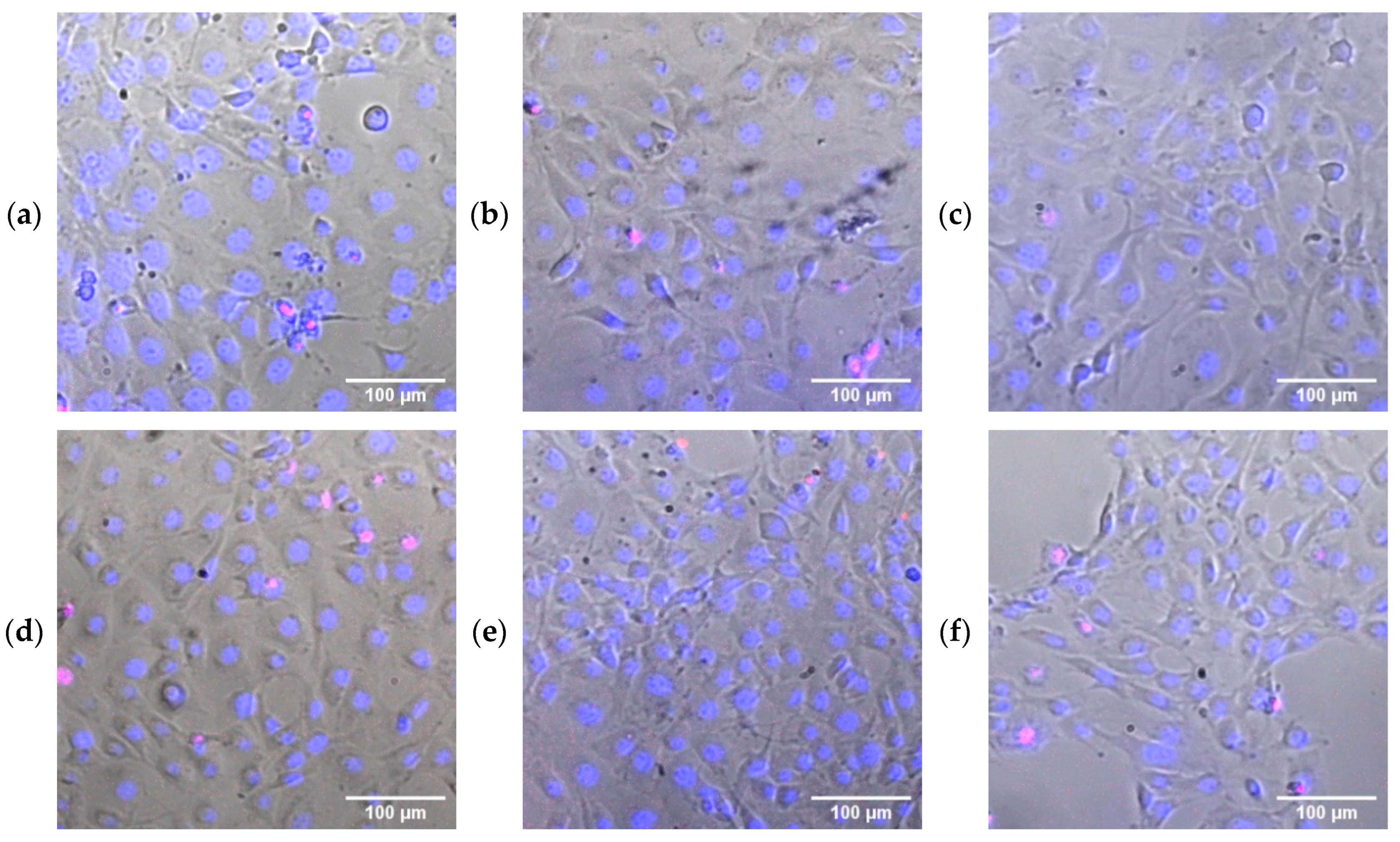
2.8. Cytotoxicity Study
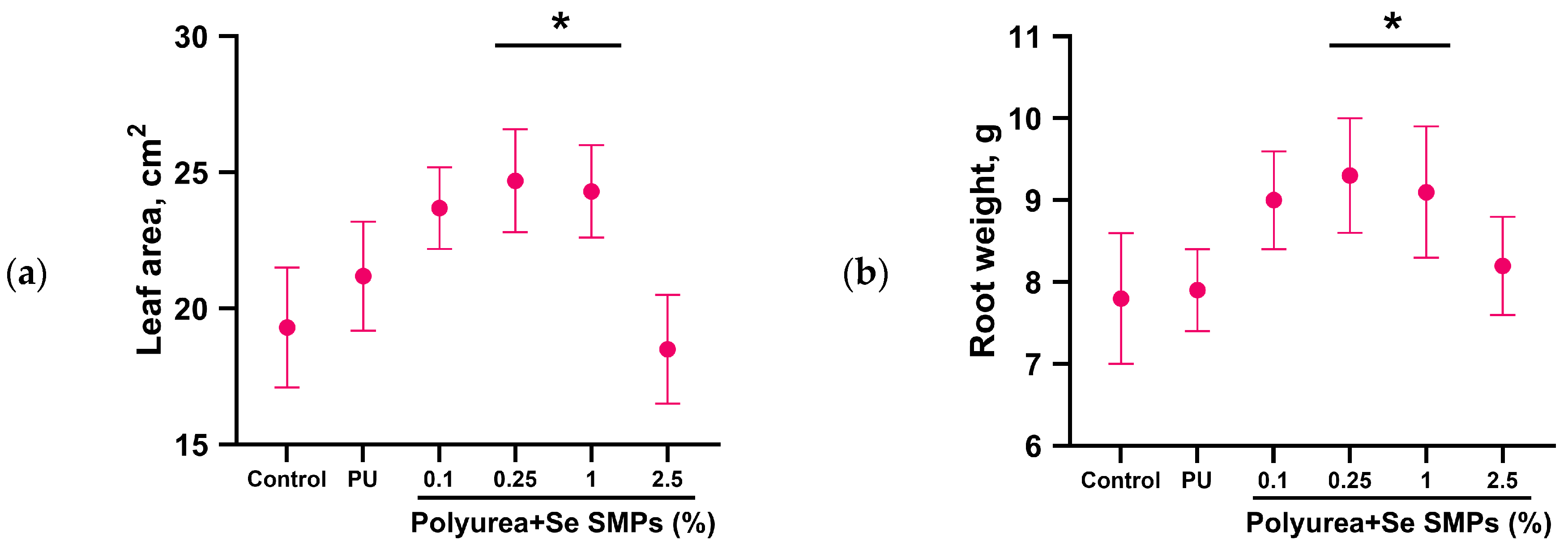
2.9. Study of the Effect of PU-Se SMPs Composites on the Growth and Development of Radish Plants
2.10. Statistical Data Analysis
3. Results
3.1. Physicochemical Characteristics of PU/Se SMPs Composites
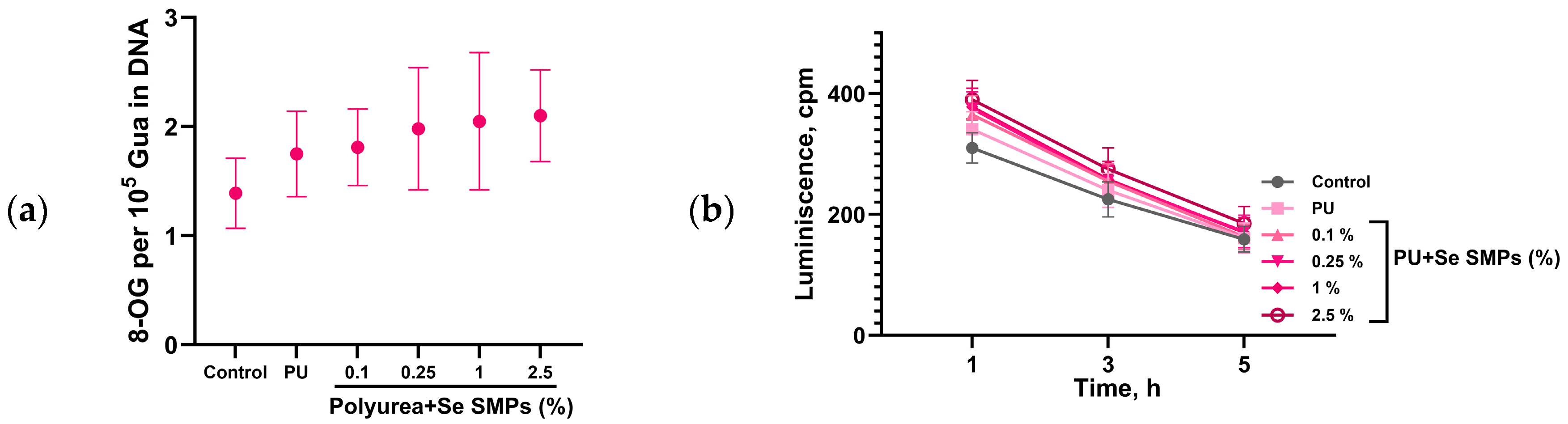
3.2. Biological Activity of PU/Se SMPs Composites
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PU/Se SMP | composite based on PolyUrea and Selenium SubMicron-sized particles |
| PU | PolyUrea |
| Se NPs | Selenium NanoParticles |
| Se SMPs | Selenium SubMicron-sized particles |
| PI | Propidium iodide |
References
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Gur’eva, L.L.; Kuzub, L.I.; Anokhin, D.V.; Badamshina, E.R. From synthesis of silver nanoparticles to polymer nanocomposites. Russ. Chem. Bull. 2025, 74, 281–304. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Kozlov, V.A.; Popov, I.A.; Gudkov, S.V. A Review on Recently Developed Antibacterial Composites of Inorganic Nanoparticles and Non-Hydrogel Polymers for Biomedical Applications. Nanomaterials 2024, 14, 1753. [Google Scholar] [CrossRef]
- Folorunso, O.; Hamam, Y.; Sadiku, R.; Kupolati, W. Effects of Defects on the Properties of Polymer Nanocomposites: A Brief Review. J. Inorg. Organomet. Polym. Mater. 2024, 34, 5667–5690. [Google Scholar] [CrossRef]
- Oladele, I.O.; Adelani, S.O.; Taiwo, A.S.; AkinbamiyorinI, M.; Olanrewaju, O.F.; Orisawayi, A.O. Polymer-based nanocomposites for supercapacitor applications: A review on principles, production and products. RSC Adv. 2025, 15, 7509–7534. [Google Scholar] [CrossRef]
- Satdive, A.; Tayde, S.; Tonde, S.; Hazra, C.; Kundu, D.; Chatterjee, A. Bionanocomposites in the Construction and Building Applications. In Polymer Based Bio-Nanocomposites; Composites Science and Technology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 293–310. [Google Scholar]
- Rajak, D.K.; Wagh, P.H.; Kumar, A.; Behera, A.; Pruncu, C.I. Advanced Polymers in Aircraft Structures. In Materials, Structures and Manufacturing for Aircraft; Sustainable Aviation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 65–88. [Google Scholar]
- Bhat, A.; Budholiya, S.; Aravind Raj, S.; Sultan, M.T.H.; Hui, D.; Md Shah, A.U.; Safri, S.N.A. Review on nanocomposites based on aerospace applications. Nanotechnol. Rev. 2021, 10, 237–253. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Vatankhah, M.; Hassanisaadi, M.; Tamanadar, E.; Skorik, Y. Nanocomposites in agriculture as pesticides for plant protection: A review. Adv. Nat. Sci. Nanosci. Nanotechnol. 2024, 15, 023003. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Kozlov, V.A.; Paskhin, M.O.; Popov, I.A.; Gudkov, S.V. A Mini-Review of Photoconversion Covers for Greenhouses: Assessment Parameters and Plant Experiment Results. Horticulturae 2025, 11, 680. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Gusein-zade Namik Guseynaga, O.; et al. Modern physical methods and technologies in agriculture. Physics-Uspekhi 2023, 67, 194–210. [Google Scholar] [CrossRef]
- Smaoui, S.; Chérif, I.; Ben Hlima, H.; Khan, M.U.; Rebezov, M.; Thiruvengadam, M.; Sarkar, T.; Shariati, M.A.; Lorenzo, J.M. Zinc oxide nanoparticles in meat packaging: A systematic review of recent literature. Food Packag. Shelf Life 2023, 36, 101045. [Google Scholar] [CrossRef]
- Mishra, B.; Panda, J.; Mishra, A.K.; Nath, P.C.; Nayak, P.K.; Mahapatra, U.; Sharma, M.; Chopra, H.; Mohanta, Y.K.; Sridhar, K. Recent advances in sustainable biopolymer-based nanocomposites for smart food packaging: A review. Int. J. Biol. Macromol. 2024, 279, 135583. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Xin, Y.; Yuan, J.; Ji, Y.; Chu, S.; Liu, J.; Luo, Q. Supramolecular Polymer Nanocomposites for Biomedical Applications. Polymers 2021, 13, 513. [Google Scholar] [CrossRef]
- Alosaimi, A.M.; Alorabi, R.O.; Katowah, D.F.; Al-Thagafi, Z.T.; Alsolami, E.S.; Hussein, M.A.; Qutob, M.; Rafatullah, M. Recent Biomedical Applications of Coupling Nanocomposite Polymeric Materials Reinforced with Variable Carbon Nanofillers. Biomedicines 2023, 11, 967. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Shamsabadipour, A.; Mahtabian, S.; Ahmadi, M.; Rahdar, A.; Díez-Pascual, A.M. Role of Iron Oxide (Fe2O3) Nanocomposites in Advanced Biomedical Applications: A State-of-the-Art Review. Nanomaterials 2022, 12, 3873. [Google Scholar] [CrossRef]
- Ray, A.; Das, P.; Chunduri, R.; Kumar, D.; Dulta, K.; Kaushal, A.; Gupta, S.; Rj, S.; Yadav, A.N.; Nagraik, R.; et al. Nanocomposite-based agricultural delivery systems: A sustainable approach to enhanced crop productivity and soil health. J. Nanoparticle Res. 2025, 27, 110. [Google Scholar] [CrossRef]
- Shafiq, F.; Mahmood, A.; Iqbal, M. Agricultural Use of Various Polymeric Nanocomposites and Future Avenues. In Specialty Polymers and Materials; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 21–52. [Google Scholar]
- Javed, F.; Hayat, S.; Aslam, B.; Saqalein, M.; Waseem, M.; Meklat, A.; Muzammil, S. Agricultural applications of bionanocomposites. In Advances in Bionanocomposites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 327–350. [Google Scholar]
- Hoang, N.H.; Thanh, T.L.; Saengchan, C.; Sangpueak, R.; Thepbandit, W.; Zhou, X.; Kamkaew, A.; Buensanteai, K. Application of Nanocomposites-Based Polymers on Managing Fungal Diseases in Crop Production. Plant Pathol. J. 2025, 41, 437–455. [Google Scholar] [CrossRef]
- Rop, K.; Karuku, G.N.; Mbui, D. Polymer Nanocomposite Fertilizers: Effects on Maize, Capsicum, and Kale Growth and Yield. In Handbook of Nanofillers; Springer: Singapore, 2025; pp. 2821–2842. [Google Scholar]
- Prajapat, A.L.; Devre, P.V.; Bhavsar, P.S.; Nille, O.S.; Dongare, P.R.; Gore, A.H. Biopolymer-Based Nanocomposites for Adsorption/Recovery of Fertilizer Nutrients in Agricultural Applications. In Biopolymeric Nanoparticles for Agricultural Applications; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2024; pp. 59–77. [Google Scholar]
- Ghorbani, A.; Emamverdian, A.; Pehlivan, N.; Zargar, M.; Razavi, S.M.; Chen, M. Nano-enabled agrochemicals: Mitigating heavy metal toxicity and enhancing crop adaptability for sustainable crop production. J. Nanobiotechnol. 2024, 22, 91. [Google Scholar] [CrossRef]
- Sepehry Javan, Z.; Razavi, S.M.; Khalofah, A.; Ghorbani, A. The ameliorating effects of cinnamic acid-based nanocomposite against salt stress in peppermint. Environ. Sci. Pollut. Res. 2024, 31, 45055–45073. [Google Scholar] [CrossRef]
- Zhang, D.-D.; Zhao, J.-F.; Tan, L.-Q.; Wu, Q.; Lv, H.-X.; Zhang, Y.-R.; Zhang, M. Effects of zinc oxide nanocomposites on microorganism growth and protection of physicochemical quality during maize storage. Int. J. Food Microbiol. 2024, 411, 110552. [Google Scholar] [CrossRef]
- Asle-Mohammadi, Z.; Razavi, F.; Aghdam, M.S.; Ebrahimi, A. Employing Chitosan-coated Putrescine Nanocomposite for Preserving Kiwifruit Quality During Cold Storage. Appl. Fruit Sci. 2024, 66, 1505–1513. [Google Scholar] [CrossRef]
- Astashev, M.E.; Sarimov, R.M.; Serov, D.A.; Matveeva, T.A.; Simakin, A.V.; Ignatenko, D.N.; Burmistrov, D.E.; Smirnova, V.V.; Kurilov, A.D.; Mashchenko, V.I.; et al. Antibacterial behavior of organosilicon composite with nano aluminum oxide without influencing animal cells. React. Funct. Polym. 2022, 170, 105143. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, W.; Lyu, P.; Yan, S.; Wang, X.; Ju, J. Polyurea for Blast and Impact Protection: A Review. Polymers 2022, 14, 2670. [Google Scholar] [CrossRef]
- Santana, J.S.; Cardoso, E.S.; Triboni, E.R.; Politi, M.J. Polyureas Versatile Polymers for New Academic and Technological Applications. Polymers 2021, 13, 4393. [Google Scholar] [CrossRef]
- Shojaei, B.; Najafi, M.; Yazdanbakhsh, A.; Abtahi, M.; Zhang, C. A review on the applications of polyurea in the construction industry. Polym. Adv. Technol. 2021, 32, 2797–2812. [Google Scholar] [CrossRef]
- Madelatparvar, M.; Hosseini, M.S.; Zhang, C. Polyurea micro-/nano-capsule applications in construction industry: A review. Nanotechnol. Rev. 2023, 12, 20220516. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Lin, J.; Ma, M.; Hu, J.; Qiu, X.; Wu, C.; Feng, C. Recent advances in polyurea elastomers and their applications in blast protection: A review. J. Mater. Sci. 2024, 59, 14893–14923. [Google Scholar] [CrossRef]
- Zhong, J.; Hu, Y.; Wang, D.; Zhou, X.; Yuan, P.; Luo, B.; Li, Y. Enhancing Dental Material Performance: Tung Oil-Infused Polyurea Microcapsule Coatings for Self-Healing and Antimicrobial Applications. Polymers 2024, 16, 918. [Google Scholar] [CrossRef]
- Li, Y.; Woo, Y.; Sekar, M.; Narasimalu, S.; Dong, Z. Effect of Nano-Titanium Dioxide Contained in Titania-Polyurea Coating on Marina Biofouling and Drag Reduction. J. Biomed. Nanotechnol. 2020, 16, 1530–1541. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Selenium Nanoparticles: Green Synthesis and Biomedical Application. Molecules 2023, 28, 8125. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A.; Blinova, E.V. Therapeutic Potential and Main Methods of Obtaining Selenium Nanoparticles. Int. J. Mol. Sci. 2021, 22, 10808. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Baryshev, A.S.; Plotnikov, E.Y. Selenium Nanoparticles in Protecting the Brain from Stroke: Possible Signaling and Metabolic Mechanisms. Nanomaterials 2024, 14, 160. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Mal’tseva, V.N.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V.; Plotnikov, E.Y. Features of the cytoprotective effect of selenium nanoparticles on primary cortical neurons and astrocytes during oxygen–glucose deprivation and reoxygenation. Sci. Rep. 2022, 12, 1710. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Khabatova, V.V.; Gudkov, S.V.; Plotnikov, E.Y.; Turovsky, E.A. Cytoprotective Properties of a New Nanocomplex of Selenium with Taxifolin in the Cells of the Cerebral Cortex Exposed to Ischemia/Reoxygenation. Pharmaceutics 2022, 14, 2477. [Google Scholar] [CrossRef]
- González-Lemus, U.; Medina-Pérez, G.; Espino-García, J.J.; Fernández-Luqueño, F.; Campos-Montiel, R.; Almaraz-Buendía, I.; Reyes-Munguía, A.; Urrutia-Hernández, T. Nutritional Parameters, Biomass Production, and Antioxidant Activity of Festuca arundinacea Schreb. Conditioned with Selenium Nanoparticles. Plants 2022, 11, 2326. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Basuini, M.F.E.; Yilmaz, S.; Abdel-Latif, H.M.R.; Kari, Z.A.; Abdul Razab, M.K.A.; Ahmed, H.A.; Alagawany, M.; Gewaily, M.S. Selenium Nanoparticles as a Natural Antioxidant and Metabolic Regulator in Aquaculture: A Review. Antioxidants 2021, 10, 1364. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Shumeyko, S.A.; Semenova, N.A.; Dorokhov, A.S.; Gudkov, S.V. Selenium Nanoparticles (Se NPs) as Agents for Agriculture Crops with Multiple Activity: A Review. Agronomy 2025, 15, 1591. [Google Scholar] [CrossRef]
- Serov, D.A.; Khabatova, V.V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials 2023, 16, 5363. [Google Scholar] [CrossRef]
- Sans-Serramitjana, E.; Obreque, M.; Muñoz, F.; Zaror, C.; Mora, M.d.L.L.; Viñas, M.; Betancourt, P. Antimicrobial Activity of Selenium Nanoparticles (SeNPs) against Potentially Pathogenic Oral Microorganisms: A Scoping Review. Pharmaceutics 2023, 15, 2253. [Google Scholar] [CrossRef]
- Tran, P.A.; Webster, T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553–1558. [Google Scholar] [CrossRef]
- Salem, S.S.; Badawy, M.S.E.M.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green Biosynthesis of Selenium Nanoparticles Using Orange Peel Waste: Characterization, Antibacterial and Antibiofilm Activities against Multidrug-Resistant Bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef]
- Rangrazi, A.; Bagheri, H.; Ghazvini, K.; Boruziniat, A.; Darroudi, M. Synthesis and antibacterial activity of colloidal selenium nanoparticles in chitosan solution: A new antibacterial agent. Mater. Res. Express 2020, 6, 1250h3. [Google Scholar] [CrossRef]
- Au, A.; Mojadadi, A.; Shao, J.-Y.; Ahmad, G.; Witting, P.K. Physiological Benefits of Novel Selenium Delivery via Nanoparticles. Int. J. Mol. Sci. 2023, 24, 6068. [Google Scholar] [CrossRef]
- Ivanov, V.E.; Usacheva, A.M.; Chernikov, A.V.; Bruskov, V.I.; Gudkov, S.V. Formation of long-lived reactive species of blood serum proteins induced by low-intensity irradiation of helium-neon laser and their involvement in the generation of reactive oxygen species. J. Photochem. Photobiol. B Biol. 2017, 176, 36–43. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Kolmakov, A.G.; Sergiyenko, K.V.; Kaplan, M.A.; Baikin, A.S.; Gudkov, S.V. Mechanical, physical–chemical and biological properties of the new Ti–30Nb–13Ta–5Zr alloy. J. Mater. Sci. 2020, 55, 14516–14529. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shtarkman, I.N.; Chernikov, A.V.; Usacheva, A.M.; Bruskov, V.I. Guanosine and inosine (riboxin) eliminate the long-lived protein radicals induced X-ray radiation. Dokl. Biochem. Biophys. 2007, 413, 50–53. [Google Scholar] [CrossRef]
- Chernikov, A.V.; Gudkov, S.V.; Shtarkman, I.N.; Bruskov, V.I. Oxygen effect in heat-induced DNA damage. Biophysics 2007, 52, 185–190. [Google Scholar] [CrossRef]
- Serov, D.; Burmistrov, D.; Simakin, A.; Astashev, M.; Uvarov, O.; Tolordava, E.; Semenova, A.; Lisitsyn, A.; Gudkov, S. Composite Coating for the Food Industry Based on Fluoroplast and ZnO-NPs: Physical and Chemical Properties, Antibacterial and Antibiofilm Activity, Cytotoxicity. Nanomaterials 2022, 12, 4158. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Bunkin, N.F.; Shafeev, G.A.; Astashev, M.E.; Glinushkin, A.P.; Grinberg, M.A.; Vodeneev, V.A. Development and application of photoconversion fluoropolymer films for greenhouses located at high or polar latitudes. J. Photochem. Photobiol. B Biol. 2020, 213, 112056. [Google Scholar] [CrossRef]
- Cazan, C.; Enesca, A.; Andronic, L. Synergic Effect of TiO2 Filler on the Mechanical Properties of Polymer Nanocomposites. Polymers 2021, 13, 2017. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kotomin, S.V. Effect of Nanoparticles and Their Anisometry on Adhesion and Strength in Hybrid Carbon-Fiber-Reinforced Epoxy Nanocomposites. J. Compos. Sci. 2023, 7, 147. [Google Scholar] [CrossRef]
- Prorokova, N.P.; Odintsova, O.I.; Rumyantseva, V.E.; Rumyantsev, E.V.; Konovalova, V.S. Giving Improved and New Properties to Fibrous Materials by Surface Modification. Coatings 2023, 13, 139. [Google Scholar] [CrossRef]
- Khalid, A.; Tran, P.A.; Norello, R.; Simpson, D.A.; O’Connor, A.J.; Tomljenovic-Hanic, S. Intrinsic fluorescence of selenium nanoparticles for cellular imaging applications. Nanoscale 2016, 8, 3376–3385. [Google Scholar] [CrossRef]
- Tao, X.T.; Watanabe, T.; Zou, D.C.; Shimoda, S.; Sato, H.; Miyata, S. Polyurea with Large Positive Birefringence for Second Harmonic Generation. Macromolecules 2002, 28, 2637–2643. [Google Scholar] [CrossRef]
- Huo, S.; Zhao, P.; Shi, Z.; Zou, M.; Yang, X.; Warszawik, E.; Loznik, M.; Göstl, R.; Herrmann, A. Mechanochemical bond scission for the activation of drugs. Nat. Chem. 2021, 13, 131–139. [Google Scholar] [CrossRef]
- Bailly, M.; Kontopoulou, M.; El Mabrouk, K. Effect of polymer/filler interactions on the structure and rheological properties of ethylene-octene copolymer/nanosilica composites. Polymer 2010, 51, 5506–5515. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Lyakhov, G.A.; Man’ko, V.I.; Shcherbakov, I.A.; Suyazov, N.V. Impact of Classical Vibrations and Magnetic Fields on Quantum Objects. Phys. Wave Phenom. 2024, 32, 67–72. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Kostevich, V.A.; Runova, O.L.; Gorudko, I.V.; Vasilyev, V.B.; Cherenkevich, S.N.; Panasenko, O.M. Proatherogenic modification of LDL by surface-bound myeloperoxidase. Chem. Phys. Lipids 2014, 180, 72–80. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Sharapov, M.G.; Tikhaze, A.K.; Goncharov, R.G.; Antonova, O.A.; Konovalova, G.G.; Novoselov, V.I. Dicarbonyl-Modified Low-Density Lipoproteins Are Key Inducers of LOX-1 and NOX1 Gene Expression in the Cultured Human Umbilical Vein Endotheliocytes. Biochemistry 2023, 88, 2125–2136. [Google Scholar] [CrossRef]
- Kruchinin, A.A.; Kamzeeva, P.N.; Zharkov, D.O.; Aralov, A.V.; Makarova, A.V. 8-Oxoadenine: A «New» Player of the Oxidative Stress in Mammals? Int. J. Mol. Sci. 2024, 25, 1342. [Google Scholar] [CrossRef]
- Dushenko, M.V.; Abdullaev, S.A.; Ignatov, M.A.; Osipov, A.N. Comparative Study of Mitochondrial DNA Abnormalities in Mesenchymal Stem Cells after Exposure to X-Ray Radiation in Low and Medium Doses. Bull. Exp. Biol. Med. 2025, 178, 541–546. [Google Scholar] [CrossRef]
- Quezada-Maldonado, E.M.; Sánchez-Pérez, Y.; Chirino, Y.I.; García-Cuellar, C.M. Airborne particulate matter induces oxidative damage, DNA adduct formation and alterations in DNA repair pathways. Environ. Pollut. 2021, 287, 117313. [Google Scholar] [CrossRef]
- Karmanova, E.E.; Chernikov, A.V.; Popova, N.R.; Sharapov, M.G.; Ivanov, V.E.; Bruskov, V.I. Metformin mitigates radiation toxicity exerting antioxidant and genoprotective properties. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 2449–2460. [Google Scholar] [CrossRef]
- Freire, B.M.; Lange, C.N.; Cavalcanti, Y.T.; Monteiro, L.R.; Pieretti, J.C.; Seabra, A.B.; Batista, B.L. The dual effect of Selenium nanoparticles in rice seedlings: From increasing antioxidant activity to inducing oxidative stress. Plant Stress 2024, 11, 100372. [Google Scholar] [CrossRef]
- Stone, J.R.; Yang, S. Hydrogen Peroxide: A Signaling Messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef]
- Lyublinskaya, O.; Antunes, F. Measuring intracellular concentration of hydrogen peroxide with the use of genetically encoded H2O2 biosensor HyPer. Redox Biol. 2019, 24, 101200. [Google Scholar] [CrossRef]
- Stepanov, E.V.; Shcherbakov, I.A. Physicochemical Methods of Studying Hydrogen Peroxide for Biomedical Applications. Phys. Wave Phenom. 2023, 31, 92–97. [Google Scholar] [CrossRef]
- Kopeikin, V.V.; Valueva, S.V.; Kipper, A.I.; Borovikova, L.N.; Nazarkina, Y.I.; Khlebosolova, E.N.; Filippov, A.I. Adsorption of Hydroxyethyl Cellulose Selenium Nanoparticles during Their Formation in Water. Russ. J. Appl. Chem. 2003, 76, 600–602. [Google Scholar] [CrossRef]
- Klechkovskaya, V.V.; Volkov, V.V.; Shtykova, E.V.; Arkharova, N.A.; Baklagina, Y.G.; Khripunov, A.K.; Smyslov, R.Y.; Borovikova, L.N.; Tkachenko, A.A. Network Model of Acetobacter Xylinum Cellulose Intercalated by Drug Nanoparticles. In Nanomaterials for Application in Medicine and Biology; NATO Science for Peace and Security Series B: Physics and Biophysics; Springer: Dordrecht, The Netherlands, 2008; pp. 165–177. [Google Scholar]
- Valueva, S.V.; Ivanov, I.V.; Volkov, A.Y.; Vylegzhanina, M.E.; Borovikova, L.N.; Kutin, A.A.; Yakimansky, A.V. Selenium Nanoparticles Stabilized by Amphiphilic Molecular Brushes with Varying Degrees of Polymerization of Side Chains: Spectral and Structural-Morphological Characteristics. Russ. J. Phys. Chem. A 2024, 98, 304–313. [Google Scholar] [CrossRef]
- Valueva, S.V.; Krasnopeeva, E.L.; Borovikova, L.N.; Morozova, P.Y.; Sokolova, M.P.; Melenevskaya, E.Y.; Yakimansky, A.V. Triple Nanosystems Based on Amphiphilic Molecular Brushes, Selenium Nanoparticles and Photosensitizer: Synthesis, Spectral, and Morphological Characteristics. Nanobiotechnol. Rep. 2024, 19, 108–115. [Google Scholar] [CrossRef]
- Panov, D.A.; Katsev, A.M.; Omel’chenko, A.V. Synthesis and Properties of Selenium Nanoparticles in a Natural Polysaccharide Matrix. Russ. J. Bioorg. Chem. 2023, 49, 1567–1576. [Google Scholar] [CrossRef]
- Valueva, S.V.; Morozova, P.Y.; Vylegzhanina, M.E.; Ivanov, I.V. Hybrid Nanosystems Based on Selenium Nanoparticles, Radachlorin, and Polymer Carriers (Graft Copolymers): Synthesis, Morphology, and Spectral Characteristics. Nanobiotechnol. Rep. 2025, 19, 959–965. [Google Scholar] [CrossRef]
- Valueva, S.V.; Vylegzhanina, M.E.; Mitusova, K.A.; Bezrukova, M.A.; Nazarova, O.V.; Zolotova, Y.I.; Panarin, E.F. Structural, Morphological, and Spectral Characteristics of Hybrid Bioactive Copper-, Selenium-, and Silver-Containing Nanosystems Based on Poly-4-Acryloylmorpholine. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2021, 15, 110–120. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Tretyakova, M.S.; Graskova, I.A.; Klimenkov, I.V.; Sudakov, N.P.; Alexandrova, G.P.; Sukhov, B.G. Biological Activity and Environmental Safety of Selenium Nanoparticles Encapsulated in Starch Macromolecules. Nanotechnol. Russ. 2020, 15, 96–104. [Google Scholar] [CrossRef]
- ElSheikh, S.K.; Eid, E.-S.G.; Abdelghany, A.M.; Abdelaziz, D. Physical/mechanical and antibacterial properties of composite resin modified with selenium nanoparticles. BMC Oral Health 2024, 24, 1245. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Reynolds, E.C.; Pantarat, N.; Biswas, D.P.; O’Connor, A.J. Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology 2016, 27, 045101. [Google Scholar] [CrossRef]
- Filipović, N.; Veselinović, L.; Ražić, S.; Jeremić, S.; Filipič, M.; Žegura, B.; Tomić, S.; Čolić, M.; Stevanović, M. Poly (ε-caprolactone) microspheres for prolonged release of selenium nanoparticles. Mater. Sci. Eng. C 2019, 96, 776–789. [Google Scholar] [CrossRef]
- Abdelaziz, H.T.O.; Seif Mohamed, E.M.; Younis, S.K.A.; Ahmed, N.; Michaeel, M.N.; Abu-Hussien, S.H.; Bakry, A.; Ebeed, N.M.; Nasser, M.A.; El-Nasr, M.K.A.; et al. Selenium nanoparticle loaded on PVA/chitosan biofilm synthesized from orange peels: Antimicrobial and antioxidant properties for plum preservation. BMC Chem. 2025, 19, 245. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Dai, C.; Wang, P.; Fan, S.; Yu, B.; Qu, Y. Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX. Environ. Res. 2021, 194, 110630. [Google Scholar] [CrossRef]
- Shakibaie, M.; Forootanfar, H.; Golkari, Y.; Mohammadi-Khorsand, T.; Shakibaie, M.R. Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J. Trace Elem. Med. Biol. 2015, 29, 235–241. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef] [PubMed]
- Grudniak, A.; Folcik, J.; Szmytke, J.; Sentkowska, A. Mechanism of Antioxidant Activity of Selenium Nanoparticles Obtained by Green and Chemical Synthesis. Int. J. Nanomed. 2025, 20, 2797–2811. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, X.; Chen, Q.; Yu, Q.; Sun, D.; Liu, J. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016, 30, 397–407. [Google Scholar] [CrossRef]
- Hernández-Díaz, J.A.; Garza-García, J.J.O.; León-Morales, J.M.; Zamudio-Ojeda, A.; Arratia-Quijada, J.; Velázquez-Juárez, G.; López-Velázquez, J.C.; García-Morales, S. Antibacterial Activity of Biosynthesized Selenium Nanoparticles Using Extracts of Calendula officinalis against Potentially Clinical Bacterial Strains. Molecules 2021, 26, 5929. [Google Scholar] [CrossRef]
- Apryatina, K.V.; Murach, E.I.; Amarantov, S.V.; Erlykina, E.I.; Veselov, V.S.; Smirnova, L.A. Synthesis of a Bioactive Composition of Chitosan–Selenium Nanoparticles. Appl. Biochem. Microbiol. 2022, 58, 126–131. [Google Scholar] [CrossRef]
- Hamdy, S.M.; Alshaya, D.S.; Alomari, K.B.; Felemban, M.F.; Ashour, A.A.; Alqarni, A.; Attia, K.A.; El-Rab, S.M.F.G. Biofabrication, Cytotoxicity, and Biofunctionalities of Polyhydroxbutrate-Selenium-Cefepime as Controlled-Release Smart Nanodrug Delivery System. J. Inorg. Organomet. Polym. Mater. 2025. [Google Scholar] [CrossRef]
- Boroumand, S.; Safari, M.; Shaabani, E.; Shirzad, M.; Faridi-Majidi, R. Selenium nanoparticles: Synthesis, characterization and study of their cytotoxicity, antioxidant and antibacterial activity. Mater. Res. Express 2019, 6, 0850d8. [Google Scholar] [CrossRef]
- Webster, T.J.; Ramos, J.F. Cytotoxicity of selenium nanoparticles in rat dermal fibroblasts. Int. J. Nanomed. 2012, 7, 3907–3914. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Mal’tseva, V.N.; Turovsky, E.A.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V. Mechanisms of the Cytotoxic Effect of Selenium Nanoparticles in Different Human Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants 2022, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Wu, H.; Yuan, Q.; Wang, J.; Cui, J.; Lin, A. Effects of selenium fertilizer application and tomato varieties on tomato fruit quality: A meta-analysis. Sci. Hortic. 2022, 304, 111242. [Google Scholar] [CrossRef]
- Wang, L.; Gao, F.; Zhang, L.; Zhao, L.; Deng, Y.; Guo, H.; Qin, L.; Wang, C. Effects of Basal Selenium Fertilizer Application on Agronomic Traits, Yield, Quality, and Se Content of Dryland Maize. Plants 2022, 11, 3099. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Liu, J.; Li, X.; Yue, L.; Cao, X.; Li, J.; Wang, C.; Wang, Z. Bioavailability of selenium nanoparticles in soil and plant: The role of particle size. Environ. Exp. Bot. 2024, 220, 105682. [Google Scholar] [CrossRef]
- Samynathan, R.; Venkidasamy, B.; Ramya, K.; Muthuramalingam, P.; Shin, H.; Kumari, P.S.; Thangavel, S.; Sivanesan, I. A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance. Plants 2023, 12, 853. [Google Scholar] [CrossRef]
- Perfileva, A.; Nozhkina, O.; Graskova, I.; Sukhov, B.; Trofimov, B. Carrageenan as polymer matrix for selenium nanocomposites. Limnol. Freshw. Biol. 2020, 4, 876–878. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Khan, Z.; Duan, S.; Li, Z.; Shen, H. Algal polysaccharides–Selenium nanoparticles regulate the uptake and distribution of selenium in rice plants. Front. Plant Sci. 2023, 14, 1135080. [Google Scholar] [CrossRef]
- Khan, Z.; Chowdhury, D.; Upadhyaya, H. Application of the composite nanoparticles of selenium and chitosan for ameliorating arsenic stress in rice seedlings. Plant Physiol. Biochem. 2025, 220, 109470. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Blinov, A.V.; Serov, A.V.; Gvozdenko, A.A.; Kravtsov, A.A.; Nagdalian, A.A.; Raffa, V.V.; Maglakelidze, D.G.; Blinova, A.A.; Kobina, A.V.; et al. Effect of Selenium Nanoparticles on Germination of Hordéum Vulgáre Barley Seeds. Coatings 2021, 11, 862. [Google Scholar] [CrossRef]
- Qu, L.; Xu, J.; Dai, Z.; Elyamine, A.M.; Huang, W.; Han, D.; Dang, B.; Xu, Z.; Jia, W. Selenium in soil-plant system: Transport, detoxification and bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumeyko, S.A.; Burmistrov, D.E.; Yanykin, D.V.; Baimler, I.V.; Simakin, A.V.; Astashev, M.E.; Dubinin, M.V.; Pishchalnikov, R.Y.; Sarimov, R.M.; Kozlov, V.A.; et al. Biological Properties of a Composite Polymer Material Based on Polyurea and Submicron-Sized Selenium Particles. Inventions 2025, 10, 82. https://doi.org/10.3390/inventions10050082
Shumeyko SA, Burmistrov DE, Yanykin DV, Baimler IV, Simakin AV, Astashev ME, Dubinin MV, Pishchalnikov RY, Sarimov RM, Kozlov VA, et al. Biological Properties of a Composite Polymer Material Based on Polyurea and Submicron-Sized Selenium Particles. Inventions. 2025; 10(5):82. https://doi.org/10.3390/inventions10050082
Chicago/Turabian StyleShumeyko, Sergey A., Dmitriy E. Burmistrov, Denis V. Yanykin, Ilya V. Baimler, Alexandr V. Simakin, Maxim E. Astashev, Mikhail V. Dubinin, Roman Y. Pishchalnikov, Ruslan M. Sarimov, Valeriy A. Kozlov, and et al. 2025. "Biological Properties of a Composite Polymer Material Based on Polyurea and Submicron-Sized Selenium Particles" Inventions 10, no. 5: 82. https://doi.org/10.3390/inventions10050082
APA StyleShumeyko, S. A., Burmistrov, D. E., Yanykin, D. V., Baimler, I. V., Simakin, A. V., Astashev, M. E., Dubinin, M. V., Pishchalnikov, R. Y., Sarimov, R. M., Kozlov, V. A., Dorokhov, A. S., & Izmailov, A. Y. (2025). Biological Properties of a Composite Polymer Material Based on Polyurea and Submicron-Sized Selenium Particles. Inventions, 10(5), 82. https://doi.org/10.3390/inventions10050082









