Functionalized Graphene Quantum Dots for Thin-Film Illuminator and Cell Dyeing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvothermal Synthesis of PEG- and NaOH-Modified GQD Clusters
2.2. Materials Characterization of the Modified GQD Clusters
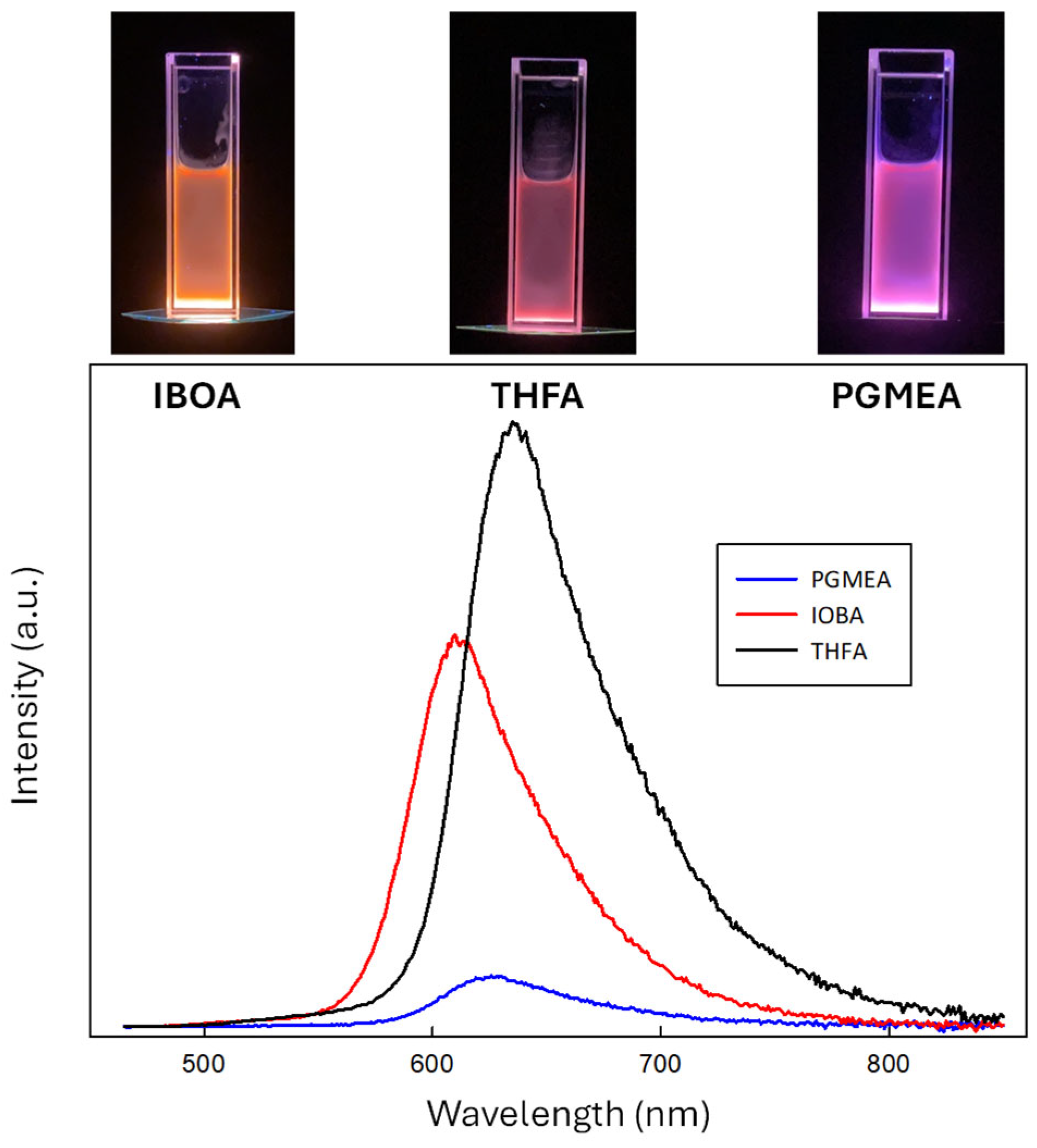
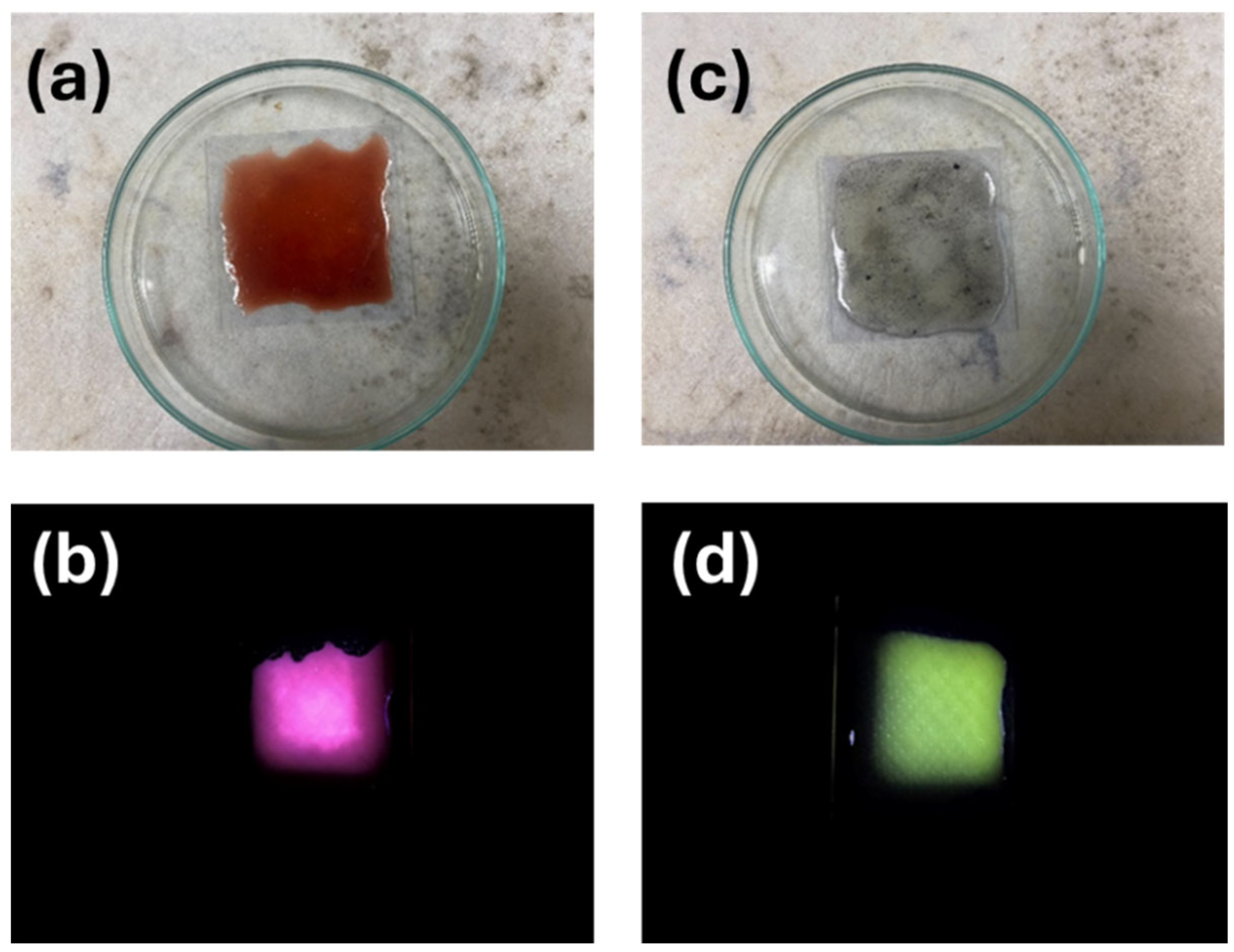
2.3. Thin-Film Illuminators Using the Modified GQD Clusters
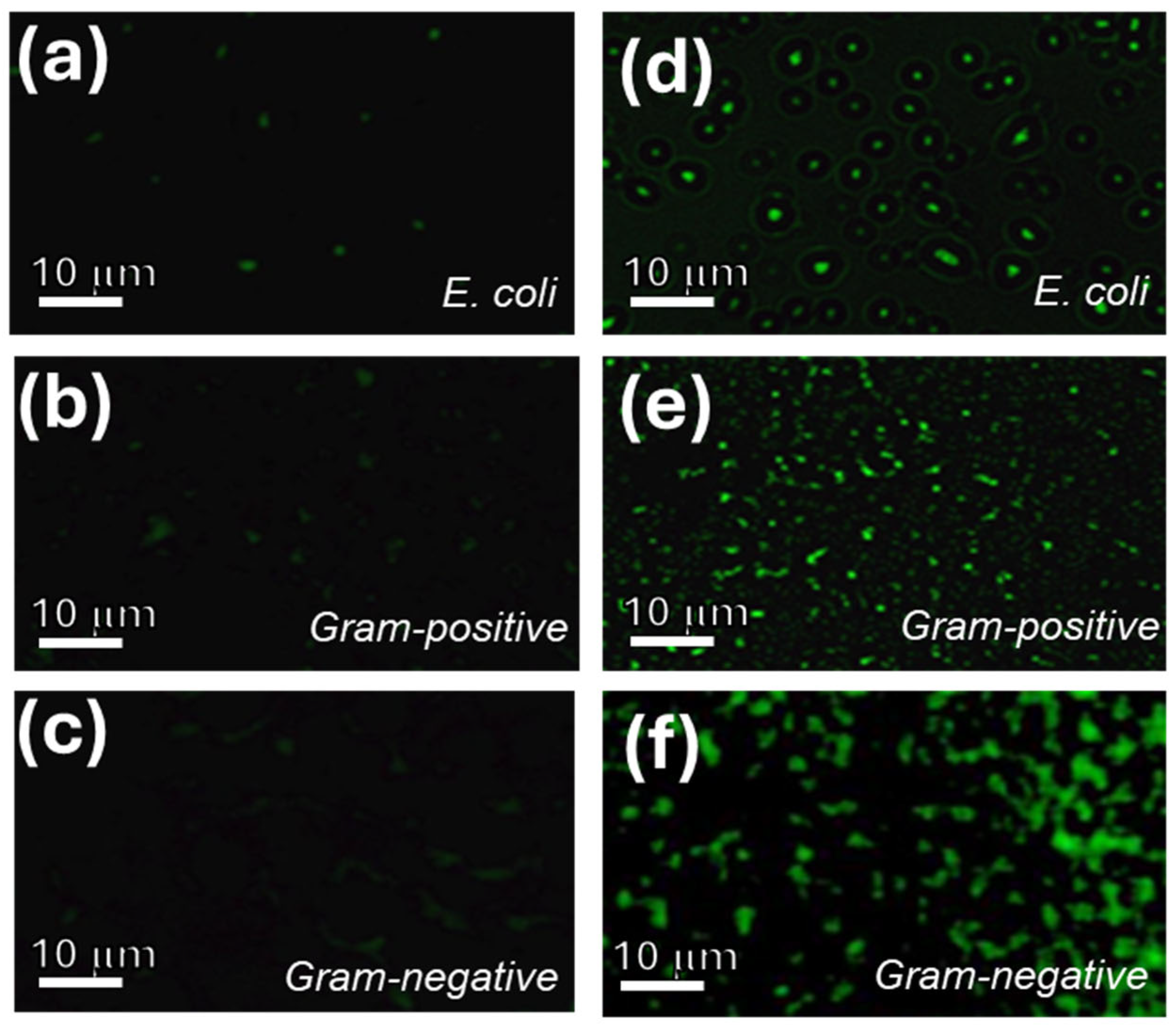
2.4. Cell Imaging Using the Modified GQD Clusters
3. Results and Discussion
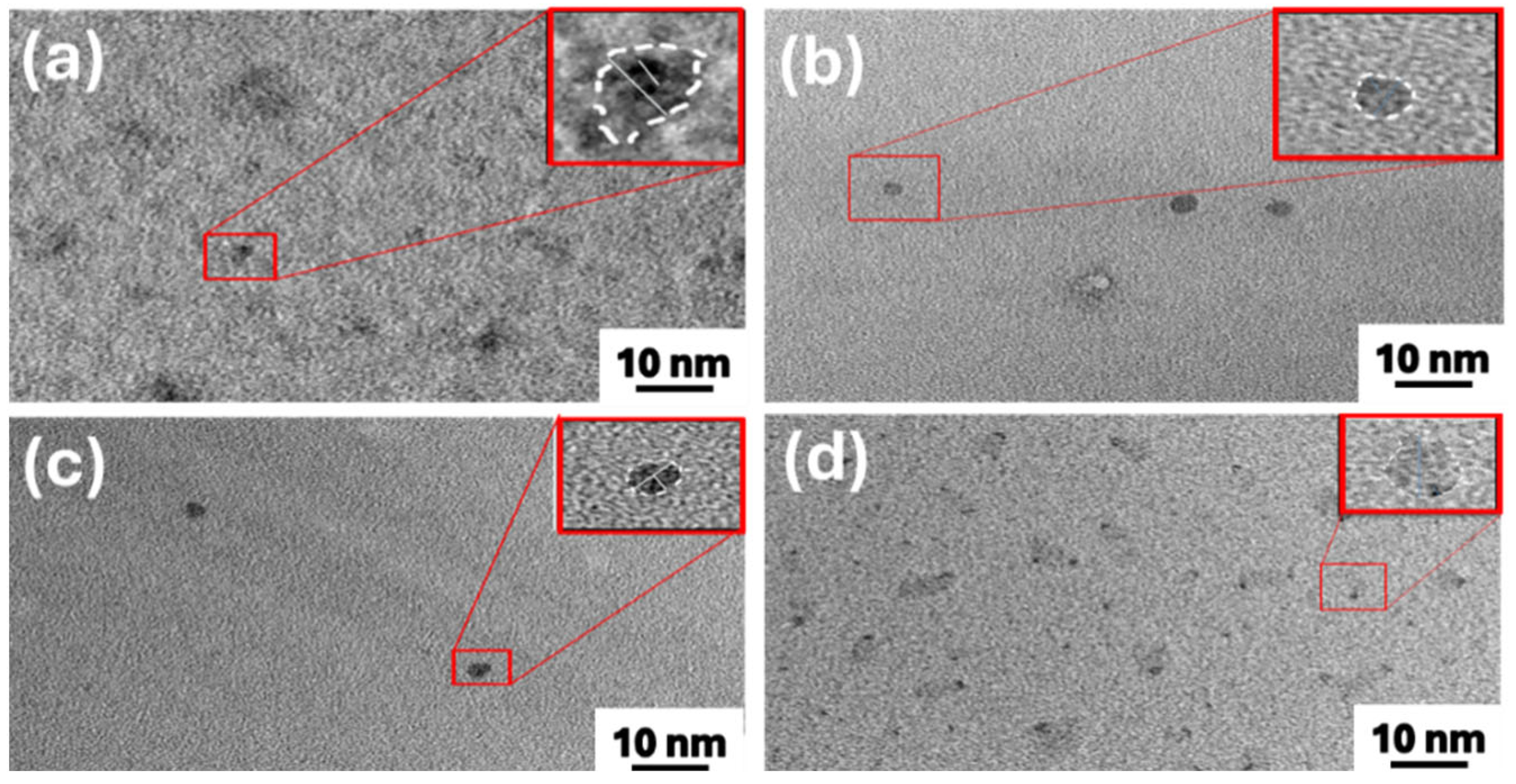
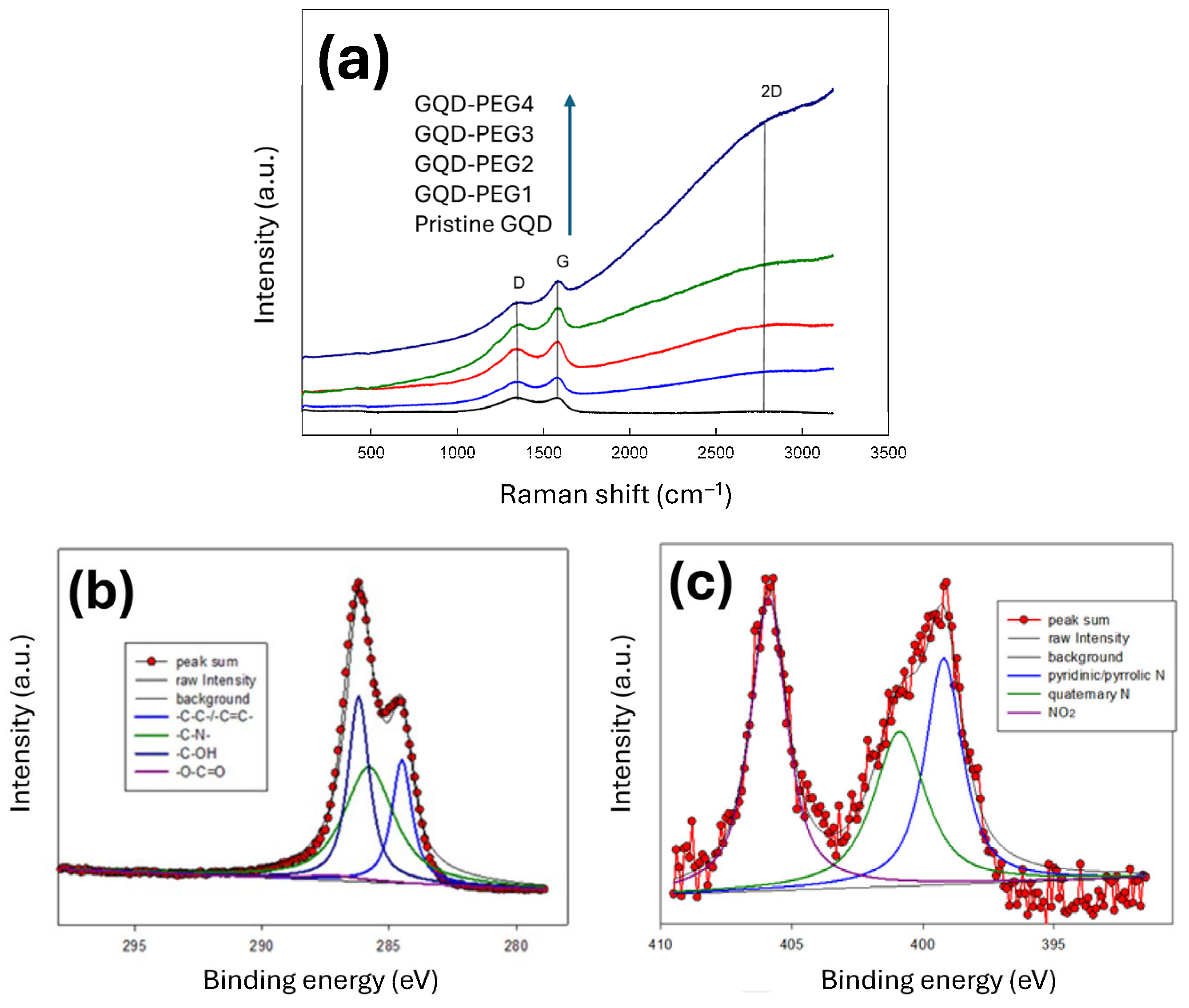
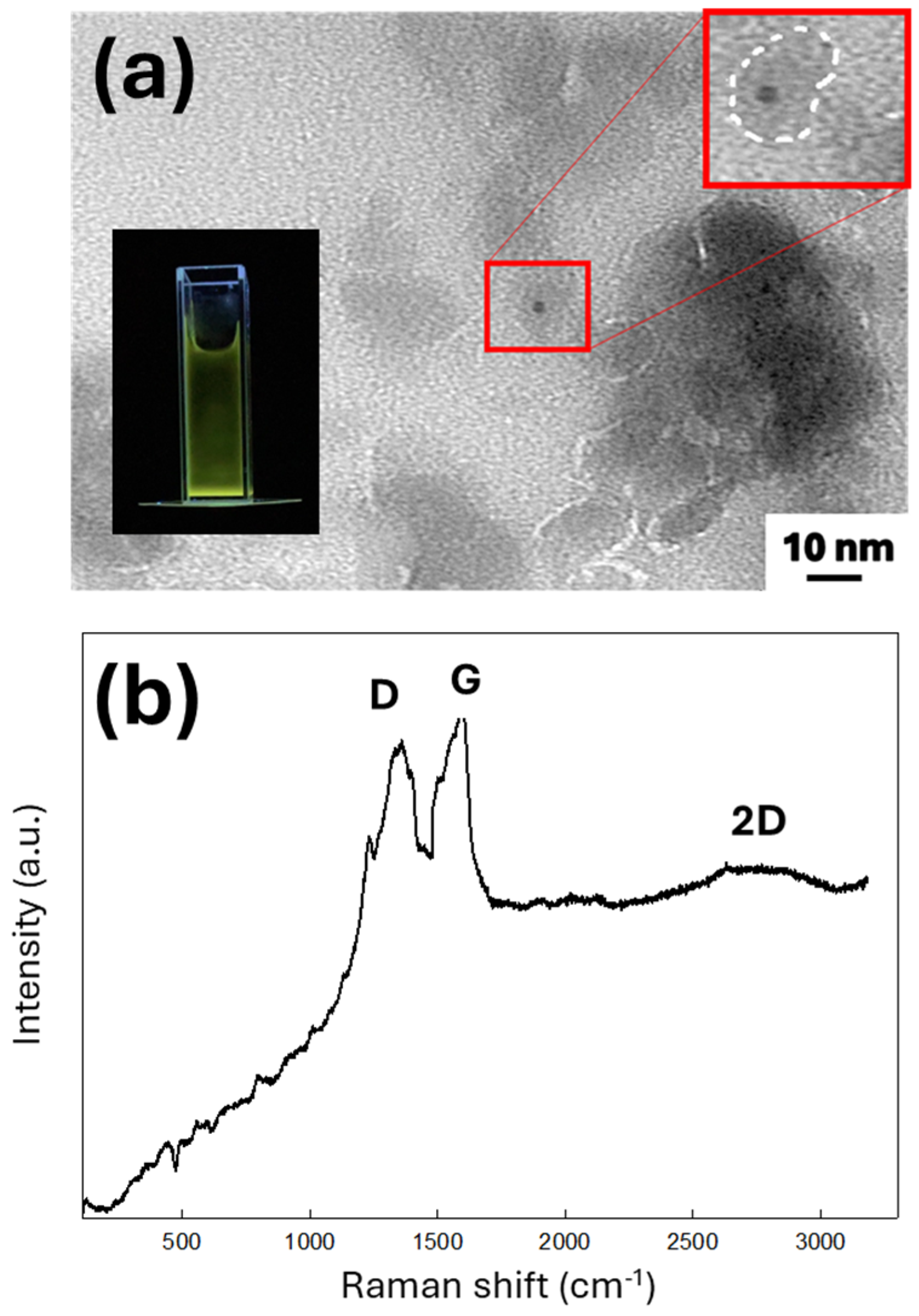
3.1. Morphology and Structural Characteristics of PEG-Modified GQD Clusters
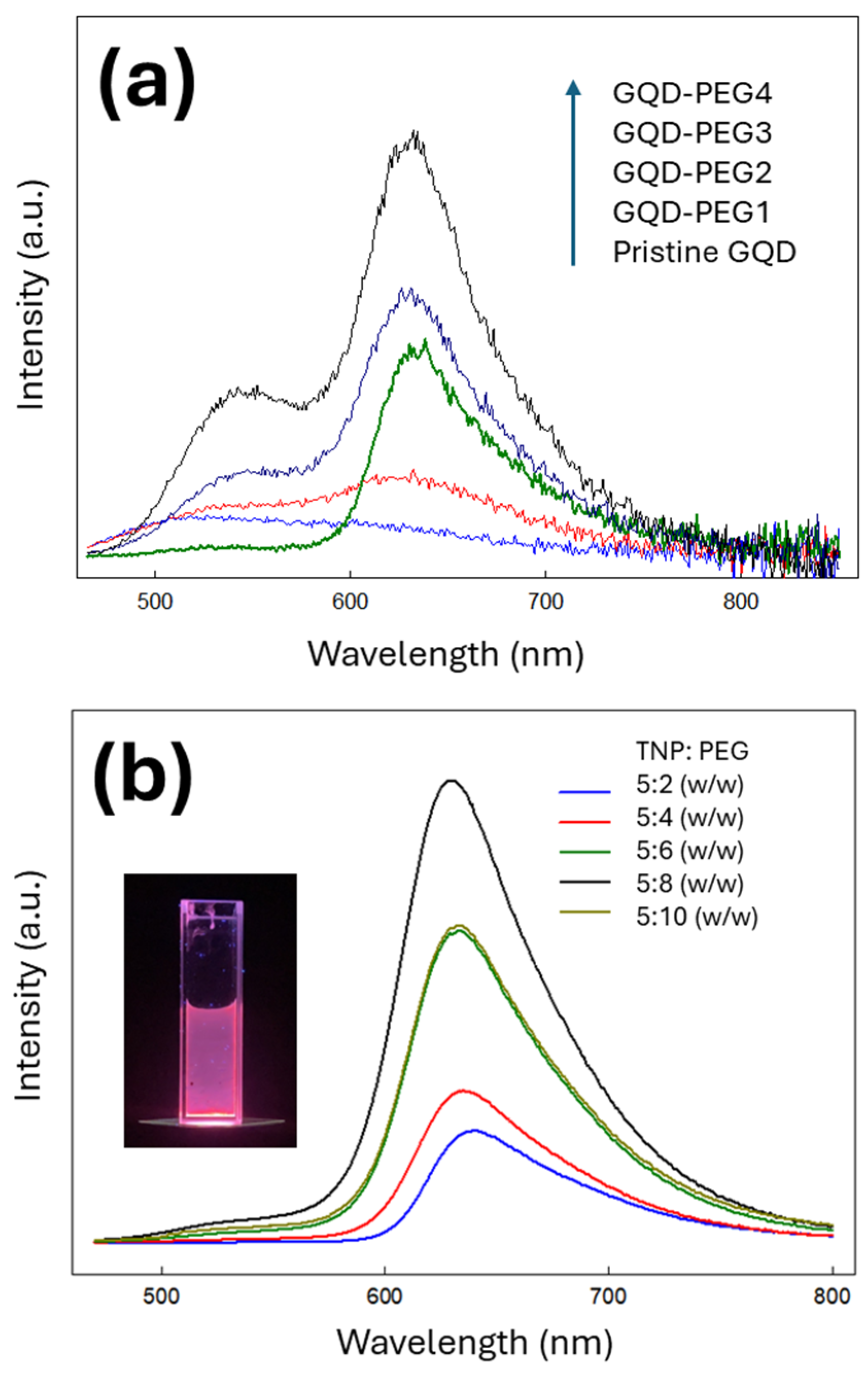
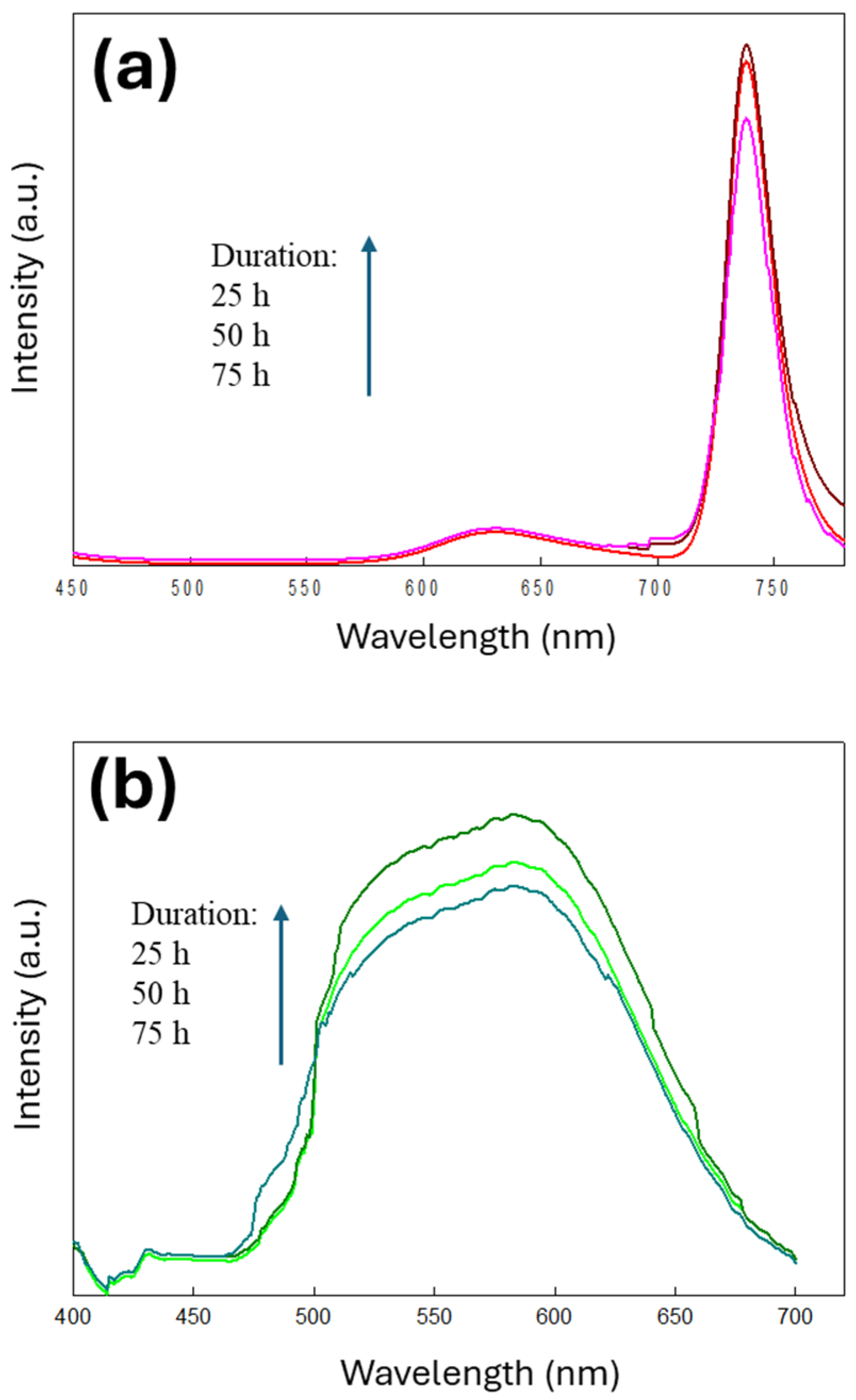
3.2. PL Emission from PEG-Modified GQD Clusters
3.3. Structural and Optical Characterization of GQD-KOH and GQD-NaOH Samples
3.4. Cell Labeling Using GQD-KOH and GQD-NaOH Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. Engl. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kalytchuk, S.; Zhang, Y.; Shi, H.; Kershaw, S.V.; Rogach, A.L. Thickness-Dependent Full-Color Emission Tunability in a Flexible Carbon Dot Ionogel. J. Phys. Chem. Lett. 2014, 5, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Bin Yang, H.; Guo, C.; Shao, J.; Chi, Y.; Chen, G. Carbon-Based Dots Co-doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. Angew. Chem. Int. Ed. Engl. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, C.; Yan, X.; Wu, M.; Zhang, H. Observation of pH-, solvent-, spin-, and excitation-dependent blue photoluminescence from carbon nanoparticles. Chem. Commun. 2010, 46, 3681–3683. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Z.; Zhang, H.; Liu, C.; Zhang, Y. Highly luminescent organosilane-functionalized carbon dots for multicolor patterning, sensors, and bioimaging. Adv. Funct. Mater. 2011, 21, 1027–1031. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. Engl. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Kohli, H.K.; Parab, D. Green synthesis of carbon quantum dots and applications: An insight. Next Mater. 2025, 8, 100527. [Google Scholar] [CrossRef]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. An Electrochemical Avenue to Blue Luminescent Nanocrystals from Multiwalled Carbon Nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Central Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Juang, R.-S.; Fu, C.-C.; Hsieh, C.-T.; Gu, S.; Gandomi, Y.A.; Liu, S.-H. Highly luminescent aggregate-induced emission from polyethylene glycol-coated carbon quantum dot clusters under blue light illumination. J. Mater. Chem. C 2020, 8, 16569–16576. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. Engl. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Ou, S.-F.; Zheng, Y.-Y.; Lee, S.-J.; Chen, S.-T.; Wu, C.-H.; Hsieh, C.-T.; Juang, R.-S.; Peng, P.-Z.; Hsueh, Y.-H. N-Doped Carbon Quantum Dots as Fluorescent Bioimaging Agents. Crystals 2021, 11, 789. [Google Scholar] [CrossRef]
- Yang, S.-T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Sun, Y.P. Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.-T.; Gandomi, Y.A.; Chang, J.-K.; Li, J.; Li, J.; Zhang, H.; Guo, Q.; Lau, K.C.; Pandey, R. Microwave growth and tunable photoluminescence of nitrogen-doped graphene and carbon nitride quantum dots. J. Mater. Chem. C 2019, 7, 5468–5476. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Lee, C.-E.; Chen, Y.-F.; Chang, J.-K.; Teng, H.-S. Thermal conductivity from hierarchical heat sinks using carbon nanotubes and graphene nanosheets. Nanoscale 2015, 7, 18663–18670. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ji, C.; Zhou, Y.; Zhao, T.; Leblanc, R.M. Polyethylene glycol (PEG) derived carbon dots: Preparation and applications. Appl. Mater. Today 2020, 20, 100677. [Google Scholar] [CrossRef]
- Lou, Y.; Ji, J.; Qin, A.; Liao, L.; Li, Z.; Chen, S.; Zhang, K.; Ou, J. Cane Molasses Graphene Quantum Dots Passivated by PEG Functionalization for Detection of Metal Ions. ACS Omega 2020, 5, 6763–6772. [Google Scholar] [CrossRef]
- Chua, C.K.; Sofer, Z.; Luxa, J.; Pumera, M. Selective Nitrogen Functionalization of Graphene by Bucherer-Type Reaction. Chem.–A Eur. J. 2015, 21, 8090–8095. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Zhang, Q.; Zhao, X.-C.; Zhang, B.; Kong, Q.-Q.; Yang, M.-G.; Yang, Q.-H.; Wang, M.-Z.; Yang, Y.-G.; Schlögl, R.; et al. Hierarchically aminated graphene honeycombs for electrochemical capacitive energy storage. J. Mater. Chem. 2012, 22, 14076–14084. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Yang, B. Surface Chemistry Routes to Modulate the Photoluminescence of Graphene Quantum Dots: From Fluorescence Mechanism to Up-Conversion Bioimaging Applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P.; Shih, C.K. Deep Ultraviolet to Near-Infrared Emission and Photoresponse in Layered N-Doped Graphene Quantum Dots. ACS Nano 2014, 8, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, C.K.; Seo, J.G.; Hong, S.J.; Song, G.; Lee, J.; Ahn, C.; Lee, D.J.; Song, S.H. Highly luminescent polyethylene glycol-passivated graphene quantum dots for light emitting diodes. RSC Adv. 2020, 10, 27418–27423. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Hao, Y.; Gan, Z.; Xu, J.; Wu, X.; Chu, P.K. Poly(ethylene glycol)/carbon quantum dot composite solid films exhibiting intense and tunable blue–red emission. Appl. Surf. Sci. 2014, 311, 490–497. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.-T.; Yuan, C.-Y.; Gandomi, Y.A.; Chang, J.-K.; Fu, C.-C.; Yang, J.-W.; Juang, R.-S. Fluorescence of functionalized graphene quantum dots prepared from infrared-assisted pyrolysis of citric acid and urea. J. Lumin. 2020, 217, 116774. [Google Scholar] [CrossRef]
- Rathee, G.; Puertas-Segura, A.; Blair, J.; Rathee, J.; Tzanov, T. Quantum dots@layered double hydroxides: Emerging nanocomposites for multifaceted applications. Prog. Mater. Sci. 2025, 150, 101403. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.H. Review on Interfacial Bonding Mechanism of Functional Polymer Coating on Glass in Atomistic Modeling Perspective. Polymers 2021, 13, 2244. [Google Scholar] [CrossRef]
- Kalluri, A.; Dharmadhikari, B.; Debnath, D.; Patra, P.; Kumar, C.V. Advances in Structural Modifications and Properties of Graphene Quantum Dots for Biomedical Applications. ACS Omega 2023, 8, 21358–21376. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent Advances on Graphene Quantum Dots for Bioimaging Applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal′Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Marconcini, P.; Macucci, M. Transport Simulation of Graphene Devices with a Generic Potential in the Presence of an Orthogonal Magnetic Field. Nanomaterials 2022, 12, 1087. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juang, R.-S.; Li, Y.-R.; Fu, C.-C.; Hsieh, C.-T. Functionalized Graphene Quantum Dots for Thin-Film Illuminator and Cell Dyeing Applications. Inventions 2025, 10, 81. https://doi.org/10.3390/inventions10050081
Juang R-S, Li Y-R, Fu C-C, Hsieh C-T. Functionalized Graphene Quantum Dots for Thin-Film Illuminator and Cell Dyeing Applications. Inventions. 2025; 10(5):81. https://doi.org/10.3390/inventions10050081
Chicago/Turabian StyleJuang, Ruey-Shin, Yi-Ru Li, Chun-Chieh Fu, and Chien-Te Hsieh. 2025. "Functionalized Graphene Quantum Dots for Thin-Film Illuminator and Cell Dyeing Applications" Inventions 10, no. 5: 81. https://doi.org/10.3390/inventions10050081
APA StyleJuang, R.-S., Li, Y.-R., Fu, C.-C., & Hsieh, C.-T. (2025). Functionalized Graphene Quantum Dots for Thin-Film Illuminator and Cell Dyeing Applications. Inventions, 10(5), 81. https://doi.org/10.3390/inventions10050081







