Evidence for the Root-Uptake of Arsenite at Lateral Root Junctions and Root Apices in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Results
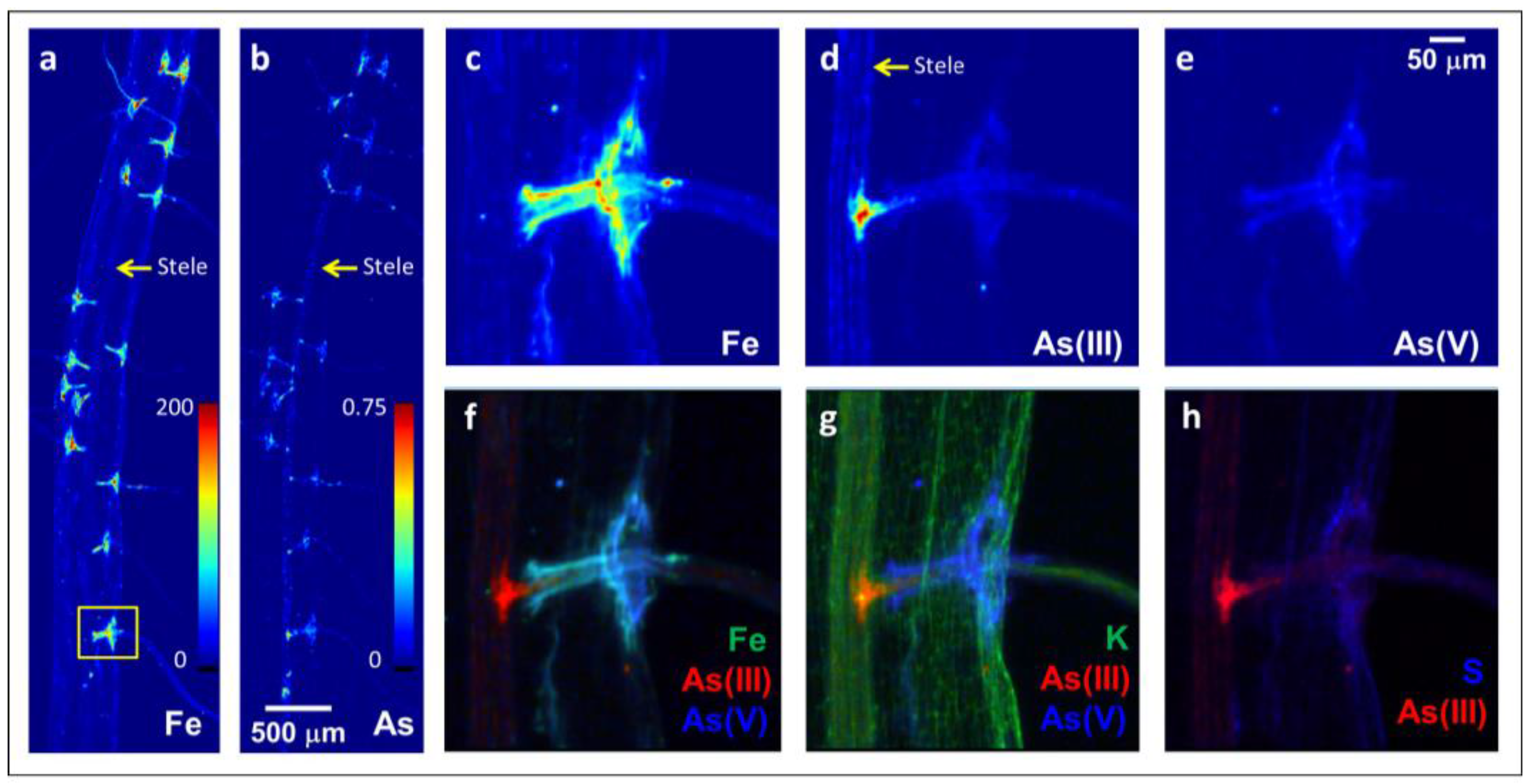
2.1. Arsenite Uptake at Lateral Root Junctions
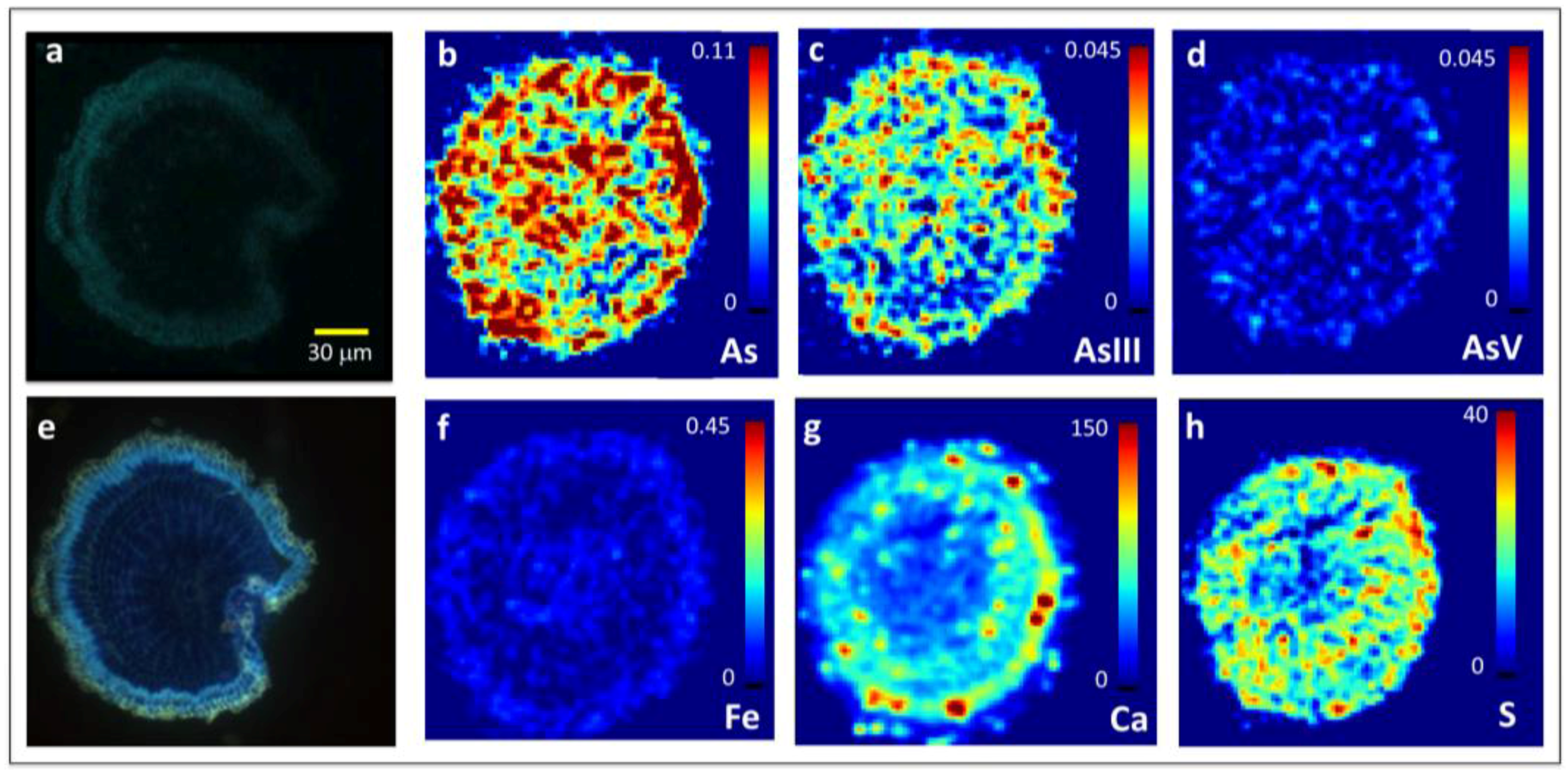
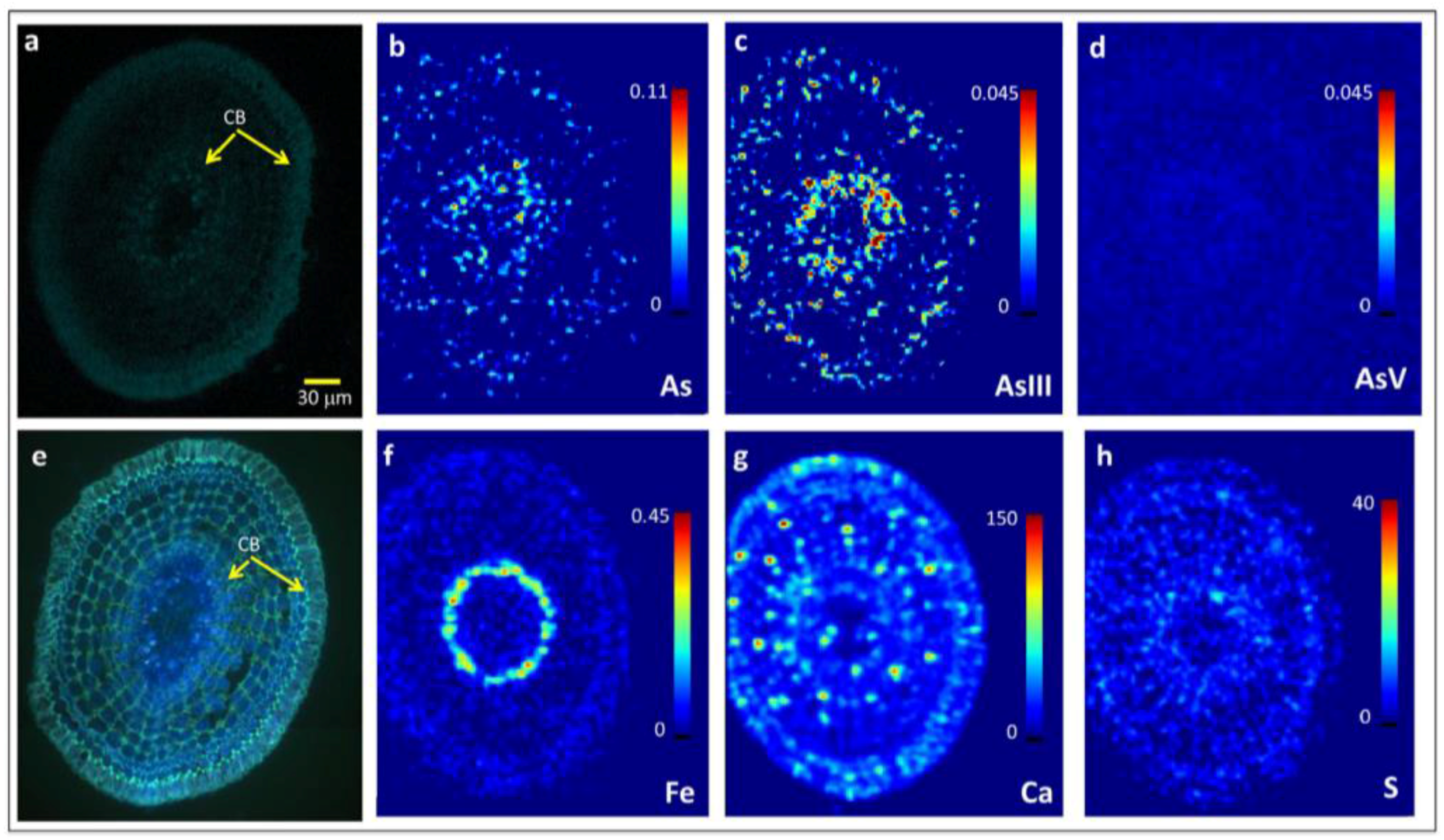
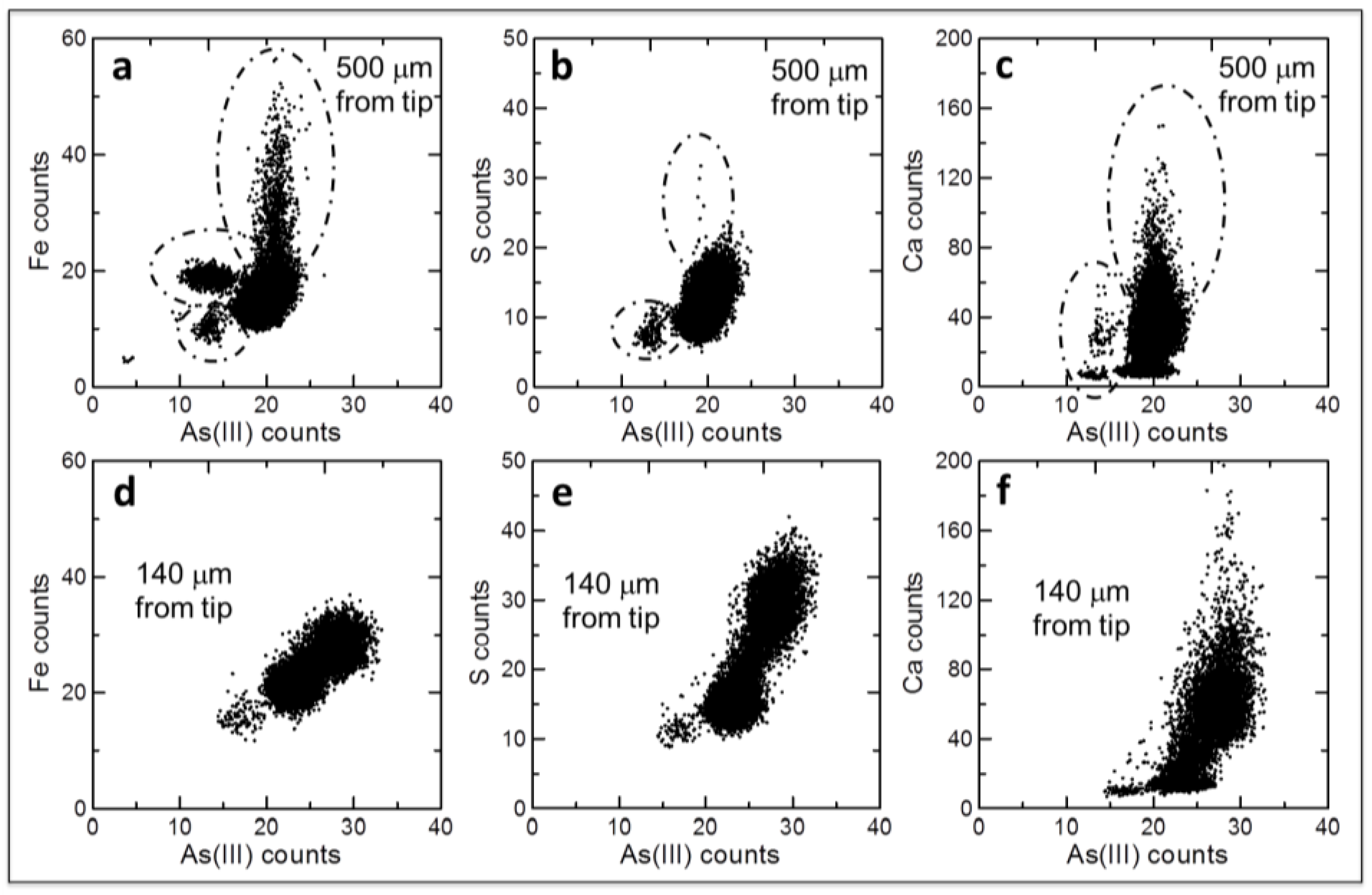
2.2. Arsenite Uptake Rice Root Apices
3. Discussion
4. Materials and Methods
4.1. Soil Experiment
4.2. Hydroponic Experiment and Root Sectioning
4.3. Light Microscopy, Section Staining, and Confocal Imaging
4.4. μXRF Imaging and Spectroscopy
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rahman, M.A.; Rahman, M.M.; Hasegawa, H. Arsenic-induced straighthead: An impending threat to sustainable rice production in south and south-east asia. Bull. Environ. Contam. Toxicol. 2012, 88, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Zhu, Y.G.; Meharg, A.A. Methylated arsenic species in rice: Geographical variation, origin, and uptake mechanisms. Environ. Sci. Technol. 2013, 47, 3957–3966. [Google Scholar] [CrossRef] [PubMed]
- Zavala, Y.J.; Duxbury, J.M. Arsenic in rice: I. Estimating normal levels of total arsenic in rice grain. Environ. Sci. Technol. 2008, 42, 3856–3860. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Price, A.H.; Raab, A.; Hossain, S.A.; Feldmann, J.; Meharg, A.A. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ. Sci. Technol. 2005, 39, 5531–5540. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Macnair, M.R. Suppression of the high-affinity phosphate-uptake system—A mechanism of arsenate tolerance in Holcus lanatus L. J. Exp. Bot. 1992, 43, 519–524. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.-Y.; Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Ago, Y.; Liu, W.J.; Mitani, N.; Feldmann, J.; McGrath, S.P.; Ma, J.F.; Zhao, F.J. The rice aquaporin lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009, 150, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, A.L.; Morris, A.H.; Gill, R.; Kearns, K.A.; Mann, J.N.; Paukett, M.; Leskanic, C. Soil incorporation of silica-rich rice husk decreases inorganic arsenic in rice grain. J. Agric. Food Chem. 2016, 64, 3760–3766. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Shen, J.L.; Wu, J.S.; Tang, Z.; Shen, Q.R.; Zhao, F.J. Impact of agronomic practices on arsenic accumulation and speciation in rice grain. Environ. Pollut. 2014, 194, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Minamikawa, R.; Hattori, K.H.; Kurishima, K.; Kihou, N.; Yuita, K. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ. Sci. Technol. 2004, 38, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: New York, NY, USA, 2003. [Google Scholar]
- Ma, F.S.; Peterson, C.A. Current insights into the development, structure, and chemistry of the endodermis and exodermis of roots. Can. J. Bot. 2003, 81, 405–421. [Google Scholar] [CrossRef]
- Enstone, D.E.; Peterson, C.A. The apoplastic permeability of root apices. Can. J. Bot. 1992, 70, 1502–1512. [Google Scholar] [CrossRef]
- Hochholdinger, F.; Zimmermann, R. Conserved and diverse mechanisms in root development. Curr. Opin. Plant Biol. 2008, 11, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Vansteveninck, R.F.M. The role of particular pericycle cells in apoplastic transport in root meristems of barley. J. Plant Physiol. 1990, 135, 554–558. [Google Scholar] [CrossRef]
- Peterson, R.L.; Peterson, C.A. Ontogeny and anatomy of lateral roots. In New Root Formation in Plants and Cuttings; Jackson, M.B., Ed.; Springer: Dordrecht, The Netherlands, 1986; pp. 1–30. [Google Scholar]
- Williams, P.N.; Santner, J.; Larsen, M.; Lehto, N.J.; Oburger, E.; Wenzel, W.; Glud, R.N.; Davison, W.; Zhang, H. Localized flux maxima of arsenic, lead, and iron around root apices in flooded lowland rice. Environ. Sci. Technol. 2014, 48, 8498–8506. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, A.L.; Webb, S.M.; Andrews, J.C.; Fendorf, S. Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L.) roots having variable fe coatings. Environ. Sci. Technol. 2010, 44, 8108–8113. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; de Jonge, M.D.; Wang, P.; McKenna, B.A.; Lombi, E.; Paterson, D.J.; Howard, D.L.; James, S.A.; Spiers, K.M.; Ryan, C.G.; et al. Laterally resolved speciation of arsenic in roots of wheat and rice using fluorescence-xanes imaging. New Phytol. 2014, 201, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Ohkura, T.; Takahashi, Y.; Maejima, Y.; Arao, T. Arsenic distribution and speciation near rice roots influenced by iron plaques and redox conditions of the soil matrix. Environ. Sci. Technol. 2014, 48, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Menzies, N.W.; Lombi, E.; McKenna, B.A.; James, S.; Tang, C.X.; Kopittke, P.M. Synchrotron-based X-ray absorption near-edge spectroscopy imaging for laterally resolved speciation of selenium in fresh roots and leaves of wheat and rice. J. Exp. Bot. 2015, 66, 4795–4806. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; de Jonge, M.D.; Menzies, N.W.; Wang, P.; Donner, E.; McKenna, B.A.; Paterson, D.; Howard, D.L.; Lombi, E. Examination of the distribution of arsenic in hydrated and fresh cowpea roots using two- and three-dimensional techniques. Plant Physiol. 2012, 159, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Goto, S.; Tamai, K.; Ichii, M. Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiol. 2001, 127, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, S.; Tang, Z.; Liu, G.; Moore, K.L.; Maathuis, F.J.M.; Miller, A.J.; McGrath, S.P.; Zhao, F.J. The nodulin 26-like intrinsic membrane protein osnip3;2 is involved in arsenite uptake by lateral roots in rice. J. Exp. Bot. 2017, 68, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Hering, J.G. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.C.; Lopes, G.; Guilherme, L.R.G.; Seyfferth, A.L. A new approach to sampling intact Fe plaque reveals Si-induced changes in Fe mineral composition and shoot as in rice. Environ. Sci. Technol. 2017, 51, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, A.L. Abiotic effects of dissolved oxyanions on iron plaque quantity and mineral composition in a simulated rhizosphere. Plant Soil 2015, 397, 43–61. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; Webb, S.M.; Andrews, J.C.; Fendorf, S. Defining the distribution of arsenic species and plant nutrients in rice (Oryza sativa L.) from the root to the grain. Geochim. Cosmochim. Acta 2011, 75, 6655–6671. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Sanderson, J. Sites of absorption and translocation of iron in barley roots—Tracer and microautoradiographic studies. Plant Physiol. 1978, 61, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Romheld, V.; Kissel, M. Localization of phytosiderophore release and of iron uptake along intact barley roots. Physiol. Plant 1987, 71, 157–162. [Google Scholar]
- Wang, P.; Menzies, N.W.; Lombi, E.; McKenna, B.A.; de Jonge, M.D.; Paterson, D.J.; Howard, D.L.; Glover, C.J.; James, S.; Kappen, P.; et al. In situ speciation and distribution of toxic selenium in hydrated roots of cowpea. Plant Physiol. 2013, 163, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E.; Peterson, C.A. How does water get through roots? J. Exp. Bot. 1998, 49, 775–788. [Google Scholar] [CrossRef]
- Yamaji, N.; Mitatni, N.; Ma, J.F. A transporter regulating silicon distribution in rice shoots. Plant Cell 2008, 20, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, U209–U212. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Schroder, M.; Wu, Z.C.; Martin, B.G.H.; Hawes, C.R.; McGrath, S.P.; Hawkesford, M.J.; Ma, J.F.; Zhao, F.J.; Grovenor, C.R.M. High-resolution secondary ion mass spectrometry reveals the contrasting subcellular distribution of arsenic and silicon in rice roots. Plant Physiol. 2011, 156, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Chen, Y.; van de Meene, A.M.L.; Hughes, L.; Liu, W.J.; Geraki, T.; Mosselmans, F.; McGrath, S.P.; Grovenor, C.; Zhao, F.J. Combined nanosims and synchrotron X-ray fluorescence reveal distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytol. 2014, 201, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.M.; Scheckel, K.G.; Lombi, E.; Newville, M.; Choi, Y.; Norton, G.J.; Charnock, J.M.; Feldmann, J.; Price, A.H.; Meharg, A.A. Grain unloading of arsenic species in rice. Plant Physiol. 2010, 152, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, A.L.; Fendorf, S. Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 13176–13183. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.C.; Hug, S.J.; Dittmar, J.; Voegelin, A.; Saha, G.C.; Ali, M.A.; Badruzzanian, A.B.M.; Kretzschniar, R. Spatial distribution and temporal variability of arsenic in irrigated rice fields in bangladesh. 1. Irrigation water. Environ. Sci. Technol. 2007, 41, 5960–5966. [Google Scholar] [CrossRef] [PubMed]
- Pedler, J.F.; Parker, D.R.; Crowley, D.E. Zinc deficiency-induced phytosiderophore release by the triticaceae is not consistently expressed in solution culture. Planta 2000, 211, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, A.L.; Parker, D.R. Effects of genotype and transpiration rate on the uptake and accumulation of perchlorate (CLO4−) in lettuce. Environ. Sci. Technol. 2007, 41, 3361–3367. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Enstone, D.E.; Peterson, C.A. A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant-tissue. Protoplasma 1988, 146, 133–142. [Google Scholar] [CrossRef]
- Webb, S.M. The Microanalysis Toolkit: X-ray Fluorescence Image Processing Software. Am. Inst. Phys. Conf. Proc. 2011, 1365, 196–199. [Google Scholar]


| Hydroponic Component | Concentration in 8 L pot (µM) |
|---|---|
| Ca(NO3)2·4H2O | 1900.0 |
| KNO3 | 1000.0 |
| MES | 1000.0 |
| MgSO4·7H2O | 500.0 |
| NH4NO3 | 100.0 |
| KH2PO4 | 80.0 |
| HEDTA | 57.7 |
| H4SiO4 | 50.0 |
| FeCl3·6H2O | 20.0 |
| H3BO3 | 10.0 |
| ZnCl2 | 8.0 |
| CuCl2·2H2O | 2.0 |
| MnCl2·4H2O | 0.6 |
| NiCl2·6H2O | 0.1 |
| Na2MoO4·2H2O | 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyfferth, A.L.; Ross, J.; Webb, S.M. Evidence for the Root-Uptake of Arsenite at Lateral Root Junctions and Root Apices in Rice (Oryza sativa L.). Soils 2017, 1, 3. https://doi.org/10.3390/soils1010003
Seyfferth AL, Ross J, Webb SM. Evidence for the Root-Uptake of Arsenite at Lateral Root Junctions and Root Apices in Rice (Oryza sativa L.). Soils. 2017; 1(1):3. https://doi.org/10.3390/soils1010003
Chicago/Turabian StyleSeyfferth, Angelia L., Jean Ross, and Samuel M. Webb. 2017. "Evidence for the Root-Uptake of Arsenite at Lateral Root Junctions and Root Apices in Rice (Oryza sativa L.)" Soils 1, no. 1: 3. https://doi.org/10.3390/soils1010003
APA StyleSeyfferth, A. L., Ross, J., & Webb, S. M. (2017). Evidence for the Root-Uptake of Arsenite at Lateral Root Junctions and Root Apices in Rice (Oryza sativa L.). Soils, 1(1), 3. https://doi.org/10.3390/soils1010003






