Legacy of Rice Roots as Encoded in Distinctive Microsites of Oxides, Silicates, and Organic Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site and Soil Material
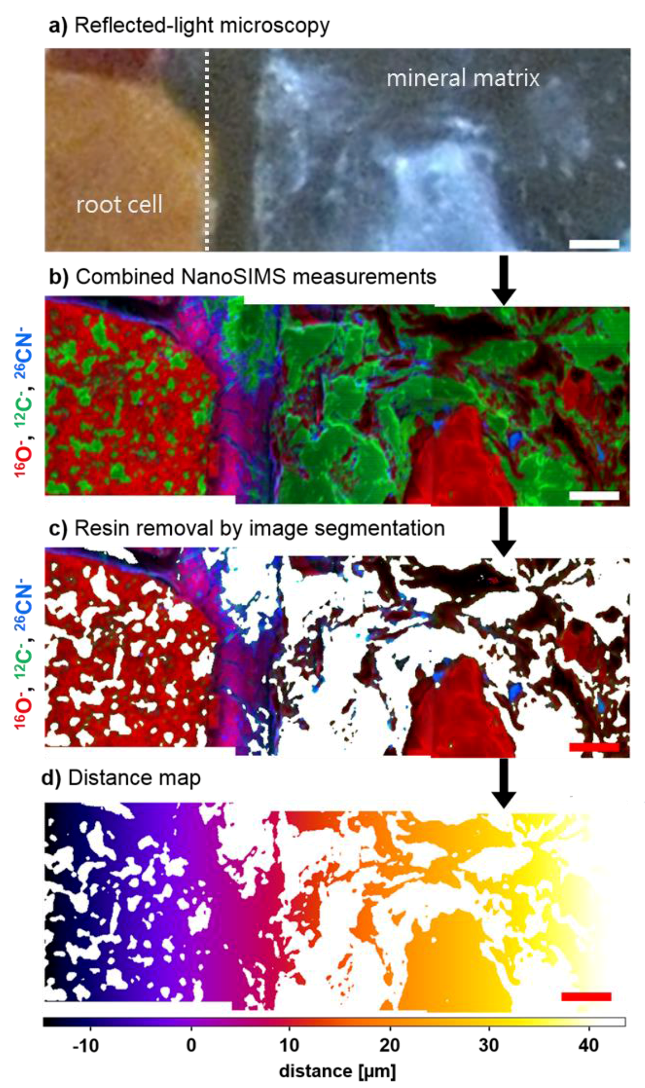
2.2. Sample Preparation, Microscopy, and NanoSIMS Analysis
2.3. Image and Statistical Analyses
3. Results
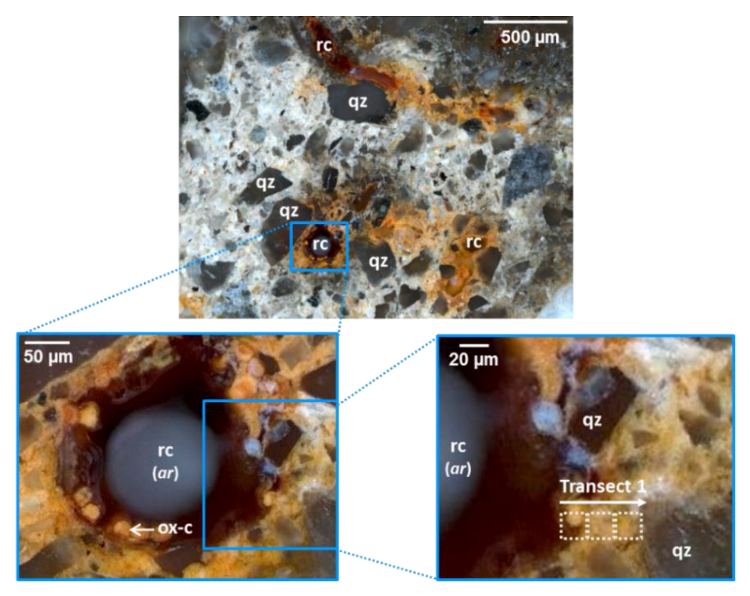
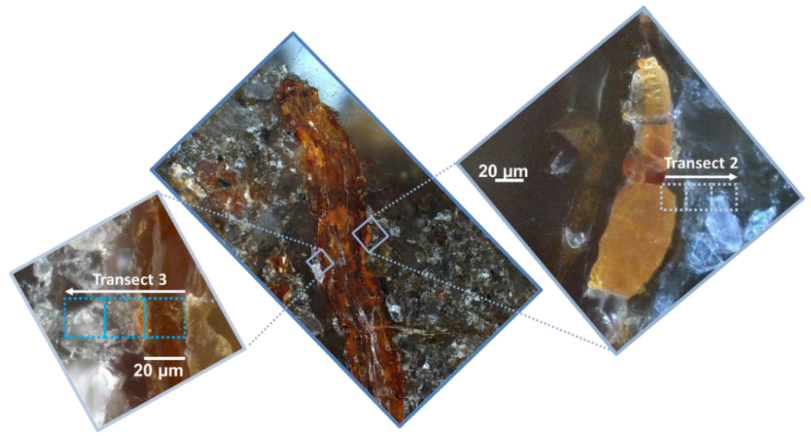
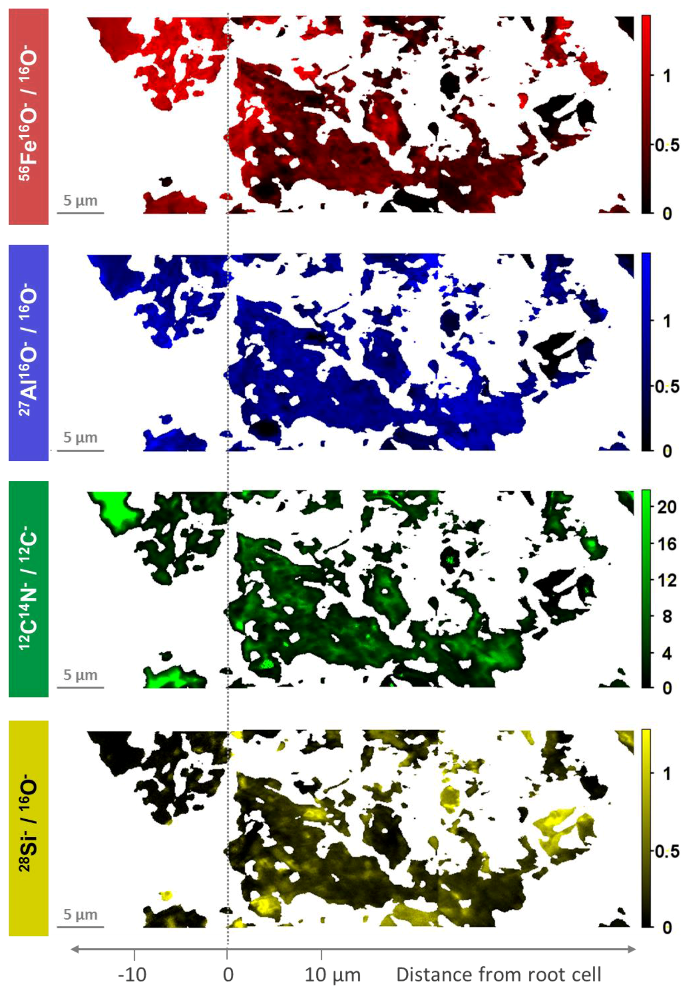
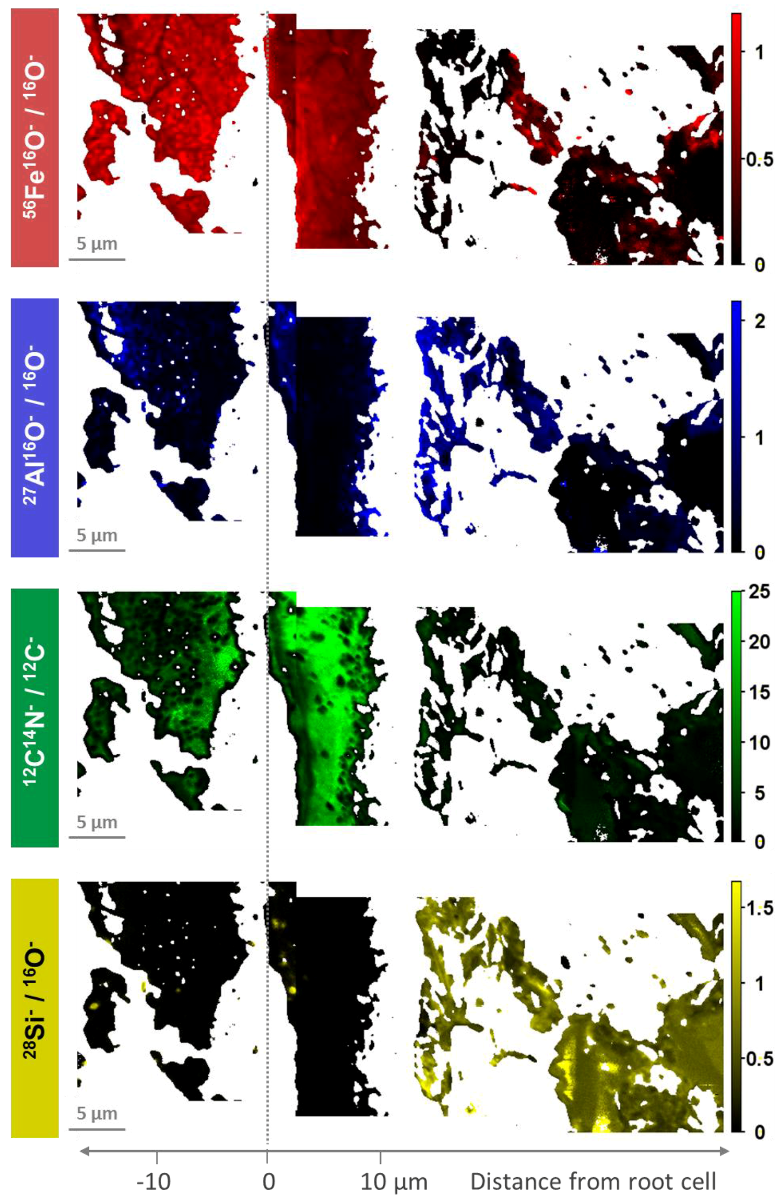
3.1. Distribution of Fe, Al, Si, and Organic C in Transects from Root Channels into the Soil Matrix
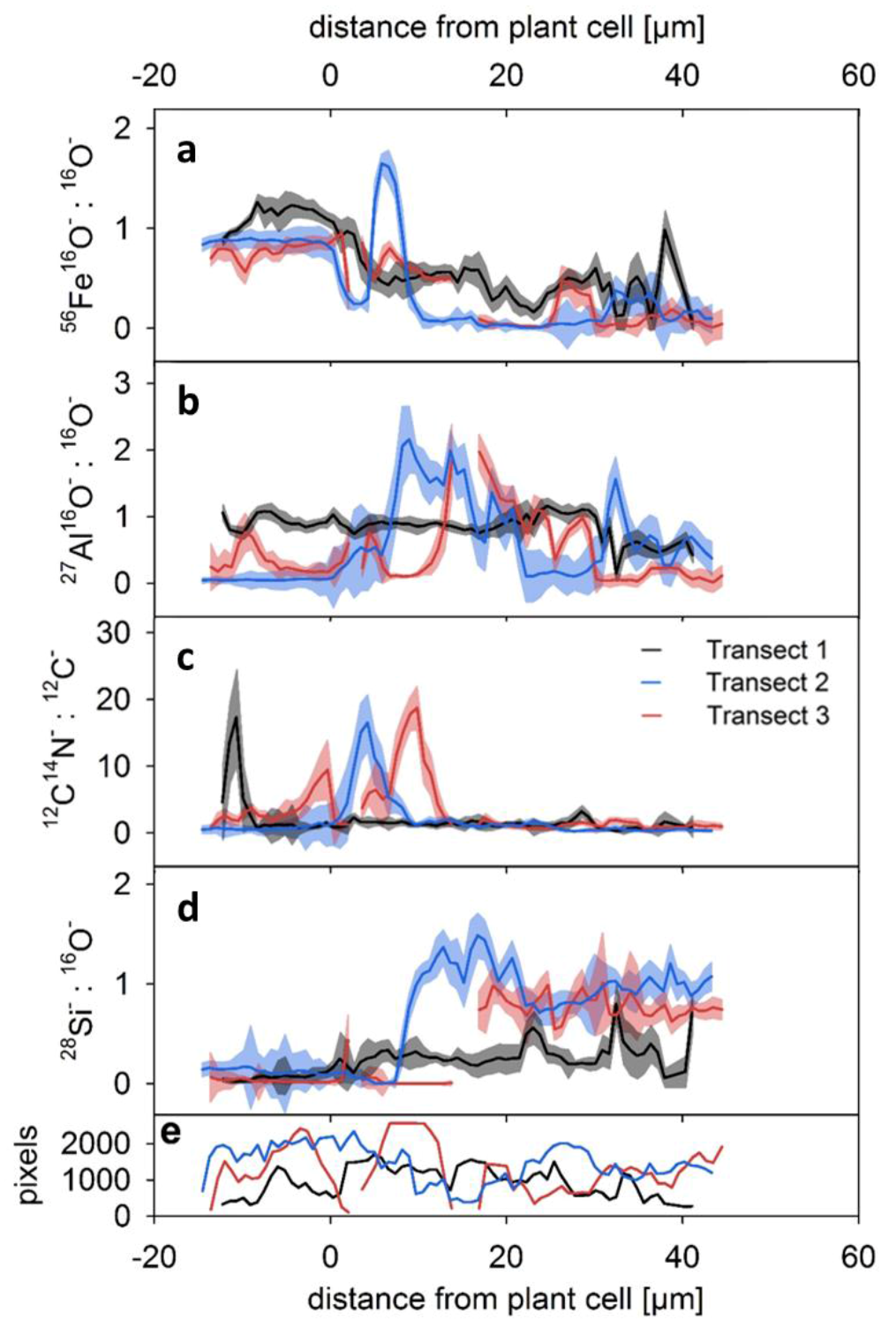
3.2. Average Element Occurrences with Distance from Root Channels
4. Discussion
4.1. Oxide-Encrusted Root Cells
4.2. Element Distribution from the Periphery of the Root into the Soil Matrix
4.3. Distinctive Zones of Oxides and Silicates
5. Conclusions
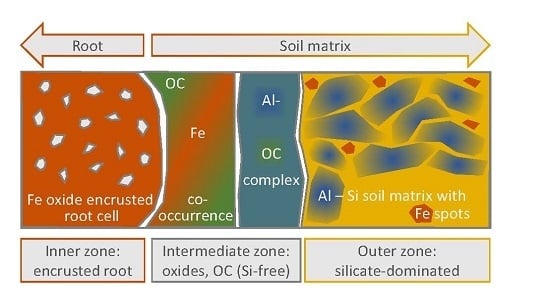
- The inner zone is composed of oxide-encrusted residues of root cells (rhizodermis cells). This zone shows no occurrence of 28Si−/16O−. High occurrences of 56Fe16O−/16O− and clear positive relations between 27Al16O−/16O− and 12C14N−/12C− revealed coexisting regions of Fe (hydr)oxides and Al–organic complexes.
- An intermediate zone was identified in two of the three transects (approx. 0–10 µm from the outermost root cell) and comprised not only high occurrences of precipitated Fe (hydr)oxides, but also rhizodeposits with Al compounds retained therein. This supports the ability of rice roots to protect the rice plants against phytotoxic concentrations of reduced substances.
- Contiguous to the intermediate zone, the outer zone is characterized by an abrupt change in element composition. 28Si−/16O− and 27Al16O−/16O− in distances of > 10 µm from the edge of the root channel revealed the occurrence of silicates in this zone. Lower 56Fe16O−/16O− ratios with a different spatial pattern as compared to 27Al16O−/16O− indicated interspersed Fe (hydr)oxides.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ponnamperuma, F.N. The chemistry of submerged soils. Adv. Agron. 1972, 24, 29–96. [Google Scholar]
- Armstrong, W.; Justin, S.; Beckett, P.M.; Lythe, S. Root adaptation to soil waterlogging. Aquat. Bot. 1991, 39, 57–73. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Papen, H.; Rennenberg, H. Scanning electron microscopy analysis of the aerenchyma in two rice cultivars. Phyton-Ann. Rei Bot. 2000, 40, 43–55. [Google Scholar]
- Armstrong, W. Radial Oxygen Losses from Intact Rice Roots as Affected by Distance from the Apex, Respiration and Waterlogging. Physiol. Plantarum 1971, 25, 192–197. [Google Scholar] [CrossRef]
- Kumazawa, K. Physiological specificity of rice root in relation to oxidizing power and nutrient uptake. In Biology of Rice; Elsevier Science Publishers: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Flessa, H.; Fischer, W.R. Plant-induced changes in the redox potentials of rice rhizospheres. Plant Soil 1992, 143, 55–60. [Google Scholar] [CrossRef]
- Wu, C.; Ye, Z.H.; Li, H.; Wu, S.C.; Deng, D.; Zhu, Y.G.; Wong, M.H. Do radial oxygen loss and external aeration affect iron plaque formation and arsenic accumulation and speciation in rice? J. Exp. Bot. 2012, 63, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Eickhorst, T.; Tippkoetter, R. Monitoring of root growth and redox conditions in paddy soil rhizotrons by redox electrodes and image analysis. Plant Soil 2011, 341, 221–232. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; Webb, S.M.; Andrews, J.C.; Fendorf, S. Arsenic Localization, Speciation, and Co-Occurrence with Iron on Rice (Oryza sativa L.) Roots Having Variable Fe Coatings. Environ. Sci. Technol. 2010, 44, 8108–8113. [Google Scholar] [CrossRef] [PubMed]
- Doran, G.; Eberbach, P.; Helliwell, S. The impact of rice plant roots on the reducing conditions in flooded rice soils. Chemosphere 2006, 63, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Trolldenier, G. Visualisation of oxidizing power of rice roots and of possible participation of bacteria in iron deposition. Z. Pflanzenern. Bodenk. 1988, 151, 117–121. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Spence, A.; Robinson, C.; Hanson, R.E. The effects of microstructural changes on montmorillonite-microbial interactions. J. Mol. Struct. 2014, 1056, 157–165. [Google Scholar] [CrossRef]
- Frommer, J.; Voegelin, A.; Dittmar, J.; Marcus, M.A.; Kretzschmar, R. Biogeochemical processes and arsenic enrichment around rice roots in paddy soil: Results from micro-focused X-ray spectroscopy. Eur. J. Soil Sci. 2011, 62, 305–317. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Ohkura, T.; Takahashi, Y.; Maejima, Y.; Arao, T. Arsenic Distribution and Speciation near Rice Roots Influenced by Iron Plaques and Redox Conditions of the Soil Matrix. Environ. Sci. Technol. 2014, 48, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Heister, K.; Hoeschen, C.; Pronk, G.J.; Mueller, C.W.; Koegel-Knabner, I. NanoSIMS as a tool for characterizing soil model compounds and organomineral associations in artificial soils. J. Soils Sediment 2012, 12, 35–47. [Google Scholar] [CrossRef]
- Mueller, C.W.; Remusat, L.; Rumpel, C. Characterization of biogeochemical processes at the microscale: Concepts and applications of NanoSIMS. In Terrestrial Ecosystem Research Infrastructures; Chabbi, A., Loescher, H.W., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Clode, P.L.; Kilburn, M.R.; Jones, D.L.; Stockdale, E.A.; Cliff, J.B.; Herrmann, A.M.; Murphy, D.V. In Situ Mapping of Nutrient Uptake in the Rhizosphere Using Nanoscale Secondary Ion Mass Spectrometry. Plant Physiol. 2009, 151, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, First Update 2007; World Soil Resources Reports 103; FAO: Rome, Italy, 2007. [Google Scholar]
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for Chemical Analysis of Soils. New Zealand Soil Bureau Scientific Report 1987; New Zealand Soil Bureau: Lower Hutt, New Zealand, 1987; Volume 80. [Google Scholar]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate buffer. Clays Clay Miner. 1960, 7, 317–327. [Google Scholar] [CrossRef]
- Mueller, C.W.; Weber, P.K.; Kilburn, M.R.; Hoeschen, C.; Kleber, M.; Pett-Ridge, J. Advances in the Analysis of Biogeochemical Interfaces: NanoSIMS to Investigate Soil Microenvironments. Adv. Agron. 2013, 121, 1–46. [Google Scholar]
- Herrmann, A.M.; Clode, P.L.; Fletcher, I.R.; Nunan, N.; Stockdale, E.A.; O’Donnell, A.G.; Murphy, D.V. A novel method for the study of the biophysical interface in soils using nano-scale secondary ion mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.W.; Hoeschen, C.; Steffens, M.; Buddenbaum, H.; Hinkel, K.; Bockheim, J. Microscale soil structures foster organic matter stabilization in permafrost soils. Geoderma 2017, 293, 44–53. [Google Scholar] [CrossRef]
- Thévenaz, P.; Unser, M. User-friendly semiautomated assembly of accurate image mosaics in microscopy. Microsc. Res. Tech. 2007, 70, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Strähle, C.; Köthe, U.; Hamprecht, F.A. Ilastik: Interactive Learning and Segmentation Toolkit. Eighth IEEE Int. Symp. Biomed. Imaging (ISBI) 2011, 230–233. [Google Scholar]
- Khan, N.; Seshadri, B.; Bolan, N.; Saint, C.P.; Kirkham, M.B.; Chowdhury, S.; Yamaguchi, N.; Lee, D.Y.; Li, G.; Kunhikrishnan, A.; et al. Root Iron Plaque on Wetland Plants as a Dynamic Pool of Nutrients and Contaminants. Adv. Agron. 2016, 138, 1–96. [Google Scholar]
- Green, M.S.; Etherington, J.R. Oxidation of Ferrous Iron by Rice (Oryza sativa L.) Roots: A Mechanism for Waterlogging Tolerance? J. Exp. Bot. 1977, 28, 678–689. [Google Scholar] [CrossRef]
- April, R.; Keller, D. Mineralogy of the rhizosphere in forest soils of the eastern United States. Biogeochemistry 1990, 9, 1–18. [Google Scholar] [CrossRef]
- Cloutier-Hurteau, B.; Turmel, M.-C.; Sauve, S.; Courchesne, F. The speciation of water-soluble Al and Zn in the rhizosphere of forest soils. J. Environ. Monit. 2010, 12, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Violante, A.; Caporale, A.G. Biogeochemical processes at soil-root interface. J. Soil Sci. Plant Nutr. 2015, 15, 422–448. [Google Scholar] [CrossRef]
- Batty, L.C.; Baker, A.J.M.; Wheeler, B.D. Aluminium and phosphate uptake by Phragmites australis: The role of Fe, Mn and Al root plaques. Ann. Bot. 2002, 89, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Klotzbucher, T.; Marxen, A.; Jahn, R.; Vetterlein, D. Silicon cycle in rice paddy fields: Insights provided by relations between silicon forms in topsoils and plant silicon uptake. Nutr. Cycl. Agroecosyst. 2016, 105, 157–168. [Google Scholar] [CrossRef]
- McNear, D.H., Jr. The rhizosphere—roots, soil and everything in between. Nat. Educ. Knowl. 2013, 4, 1. [Google Scholar]



| Parameter | Unit | Value |
|---|---|---|
| Bulk density | g·cm−3 | 1.82 |
| pHCaCl2 1 | 5.1 | |
| OC 2 | mg·g−1 | 6.20 |
| Nt2 | mg·g−1 | 0.68 |
| Alox 3 | mg·g−1 | 0.53 |
| Feox 3 | mg·g−1 | 2.48 |
| Mnox 3 | mg·g−1 | 0.01 |
| AlDCB 4 | mg·g−1 | 0.47 |
| FeDCB 4 | mg·g−1 | 4.02 |
| MnDCB 4 | mg·g−1 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kölbl, A.; Schweizer, S.A.; Mueller, C.W.; Höschen, C.; Said-Pullicino, D.; Romani, M.; Lugmeier, J.; Schlüter, S.; Kögel-Knabner, I. Legacy of Rice Roots as Encoded in Distinctive Microsites of Oxides, Silicates, and Organic Matter. Soils 2017, 1, 2. https://doi.org/10.3390/soils1010002
Kölbl A, Schweizer SA, Mueller CW, Höschen C, Said-Pullicino D, Romani M, Lugmeier J, Schlüter S, Kögel-Knabner I. Legacy of Rice Roots as Encoded in Distinctive Microsites of Oxides, Silicates, and Organic Matter. Soils. 2017; 1(1):2. https://doi.org/10.3390/soils1010002
Chicago/Turabian StyleKölbl, Angelika, Steffen A. Schweizer, Carsten W. Mueller, Carmen Höschen, Daniel Said-Pullicino, Marco Romani, Johann Lugmeier, Steffen Schlüter, and Ingrid Kögel-Knabner. 2017. "Legacy of Rice Roots as Encoded in Distinctive Microsites of Oxides, Silicates, and Organic Matter" Soils 1, no. 1: 2. https://doi.org/10.3390/soils1010002
APA StyleKölbl, A., Schweizer, S. A., Mueller, C. W., Höschen, C., Said-Pullicino, D., Romani, M., Lugmeier, J., Schlüter, S., & Kögel-Knabner, I. (2017). Legacy of Rice Roots as Encoded in Distinctive Microsites of Oxides, Silicates, and Organic Matter. Soils, 1(1), 2. https://doi.org/10.3390/soils1010002