Molecular and Microscopic Insights into the Formation of Soil Organic Matter in a Red Pine Rhizosphere
Abstract
1. Introduction
2. Materials and Methods
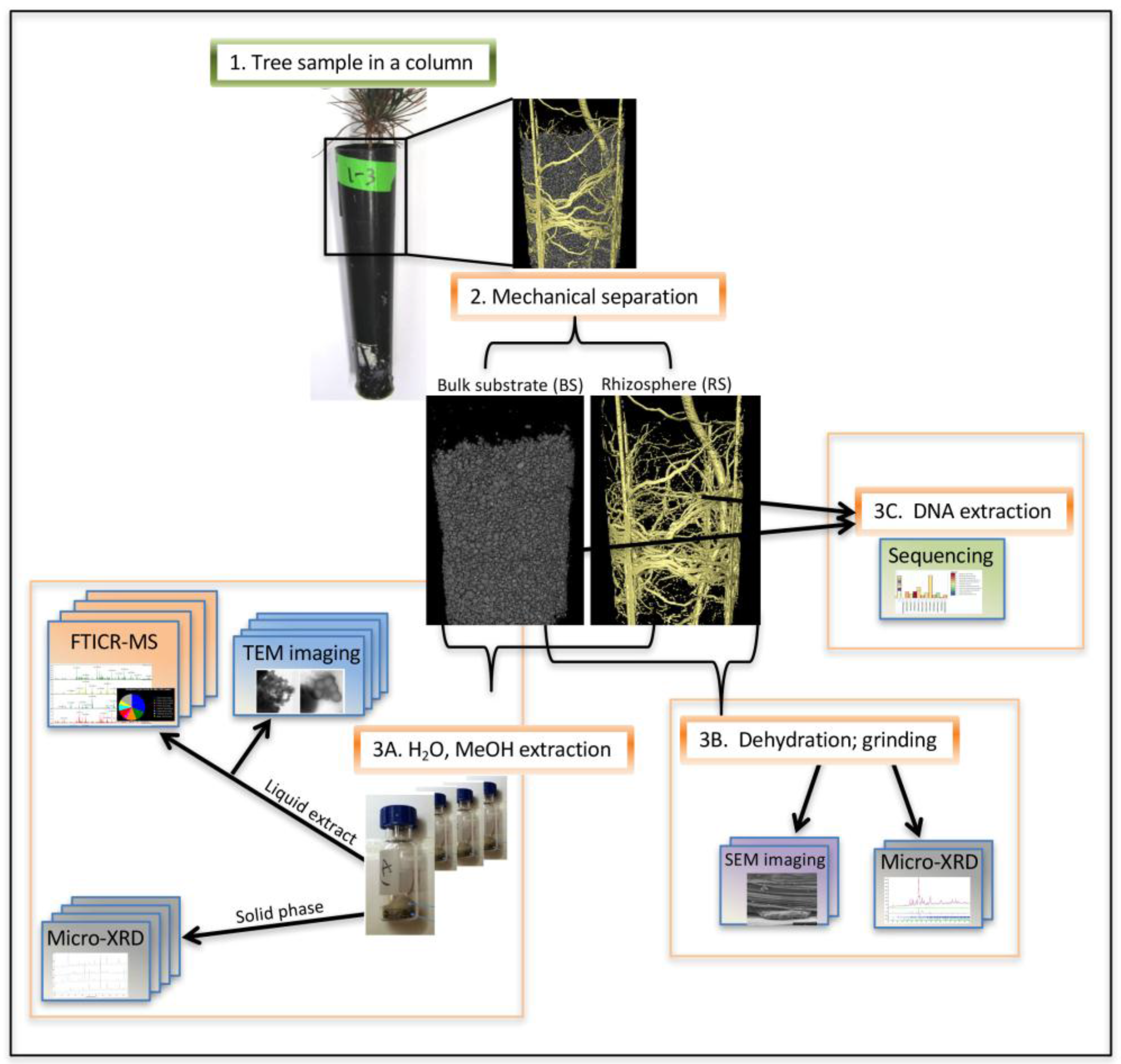
2.1. Experimental Approach
2.2. Microbial DNA Extraction, Sequencing, and Data Analysis
2.3. Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FTICR-MS)
2.4. Transmission Electron Microscopy (TEM)
2.5. Scanning Ion Microscopy—Helium Ion Microscopy (HeIM)
2.6. XRD, Micro-XRD
3. Results
3.1. Column Growth Experiment
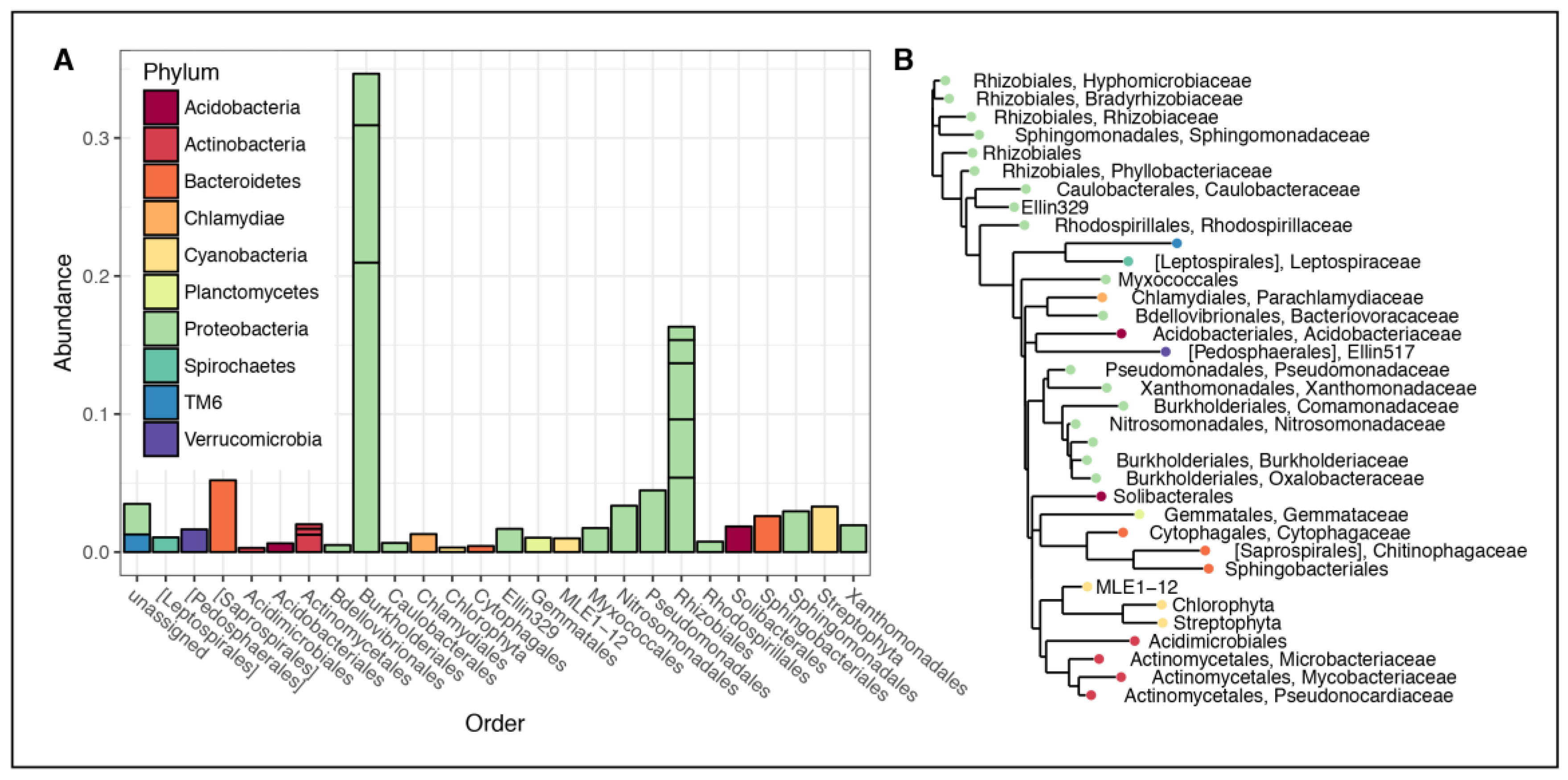
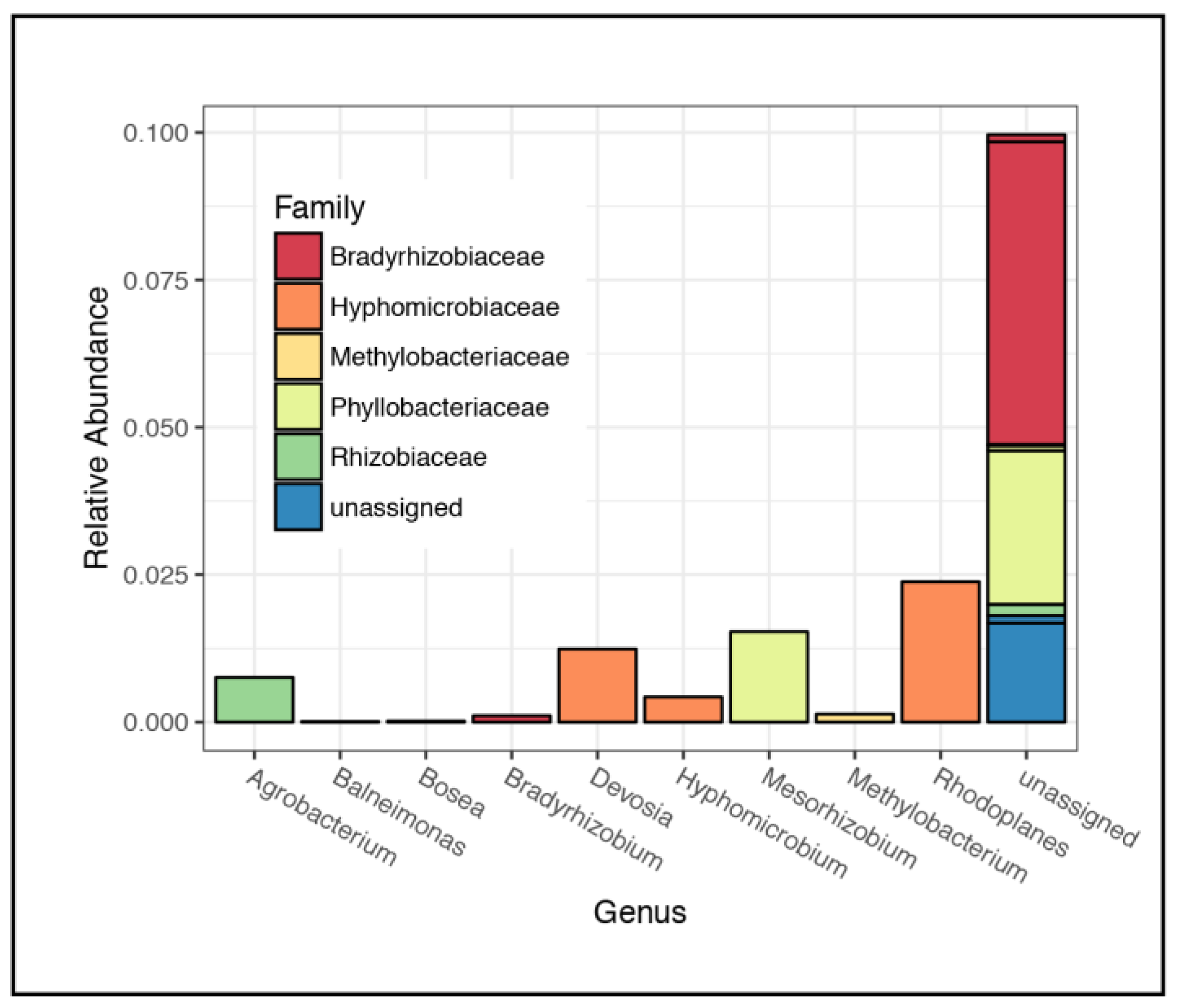
3.2. Microbial Community Composition
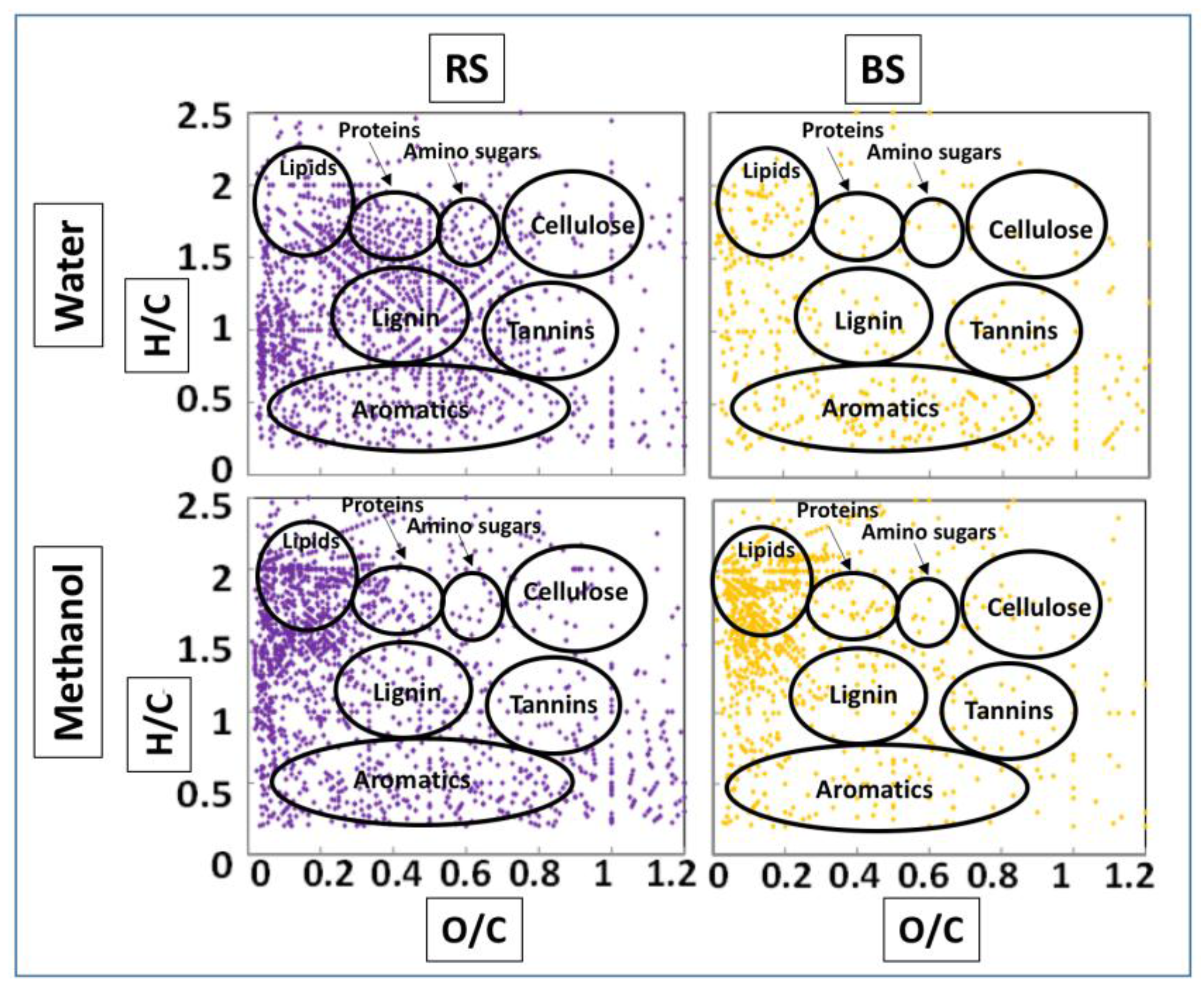
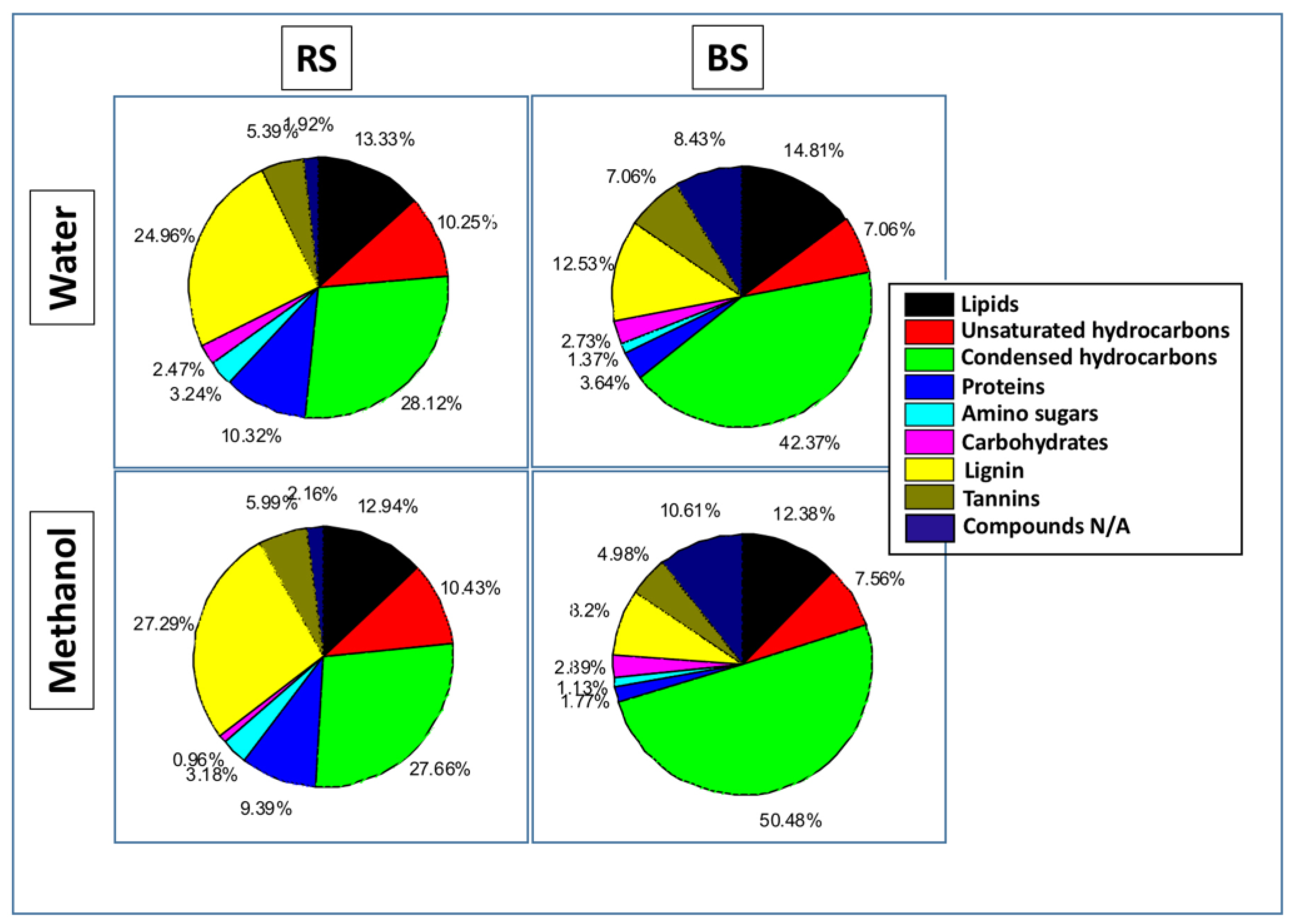
3.3. FTICR-MS
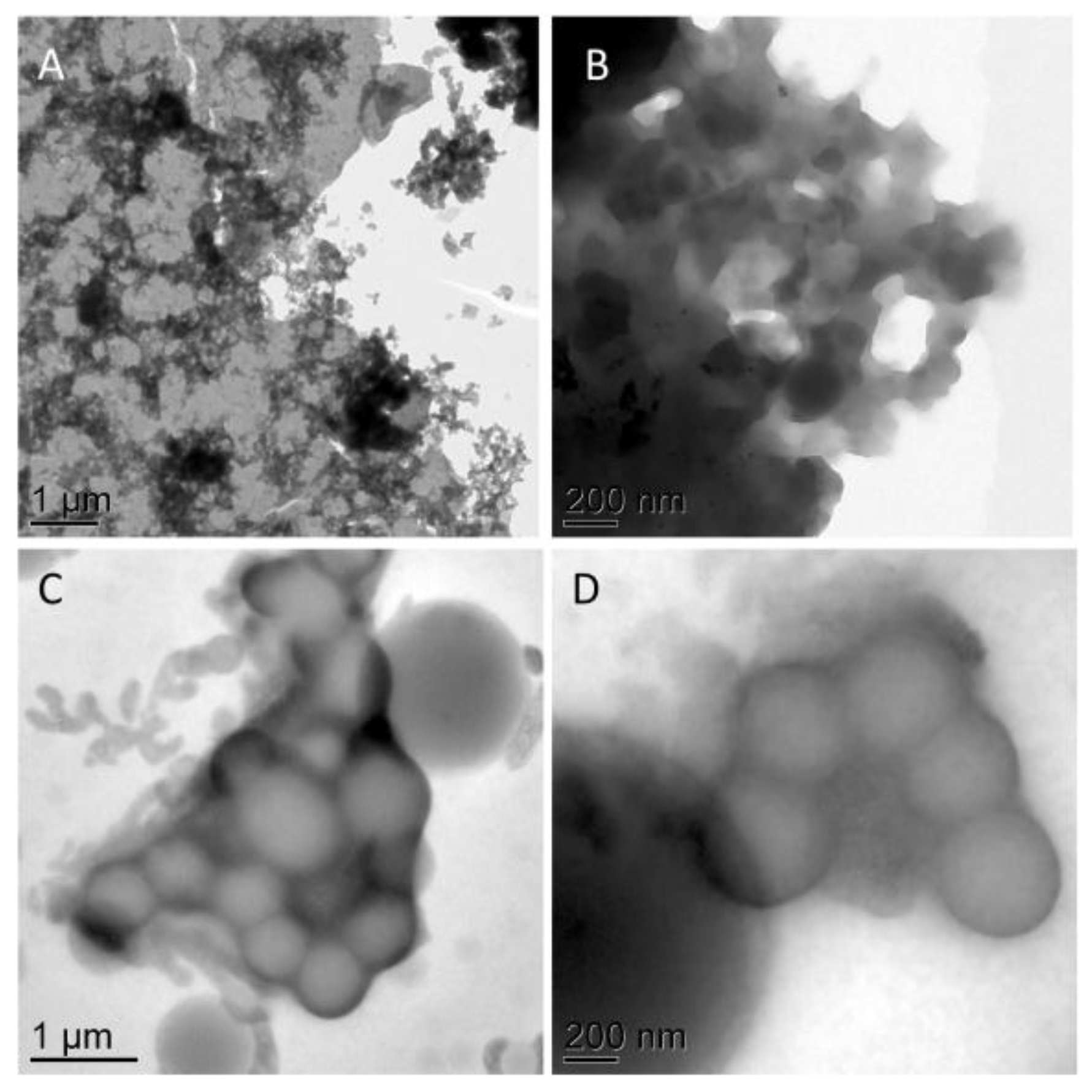
3.4. TEM
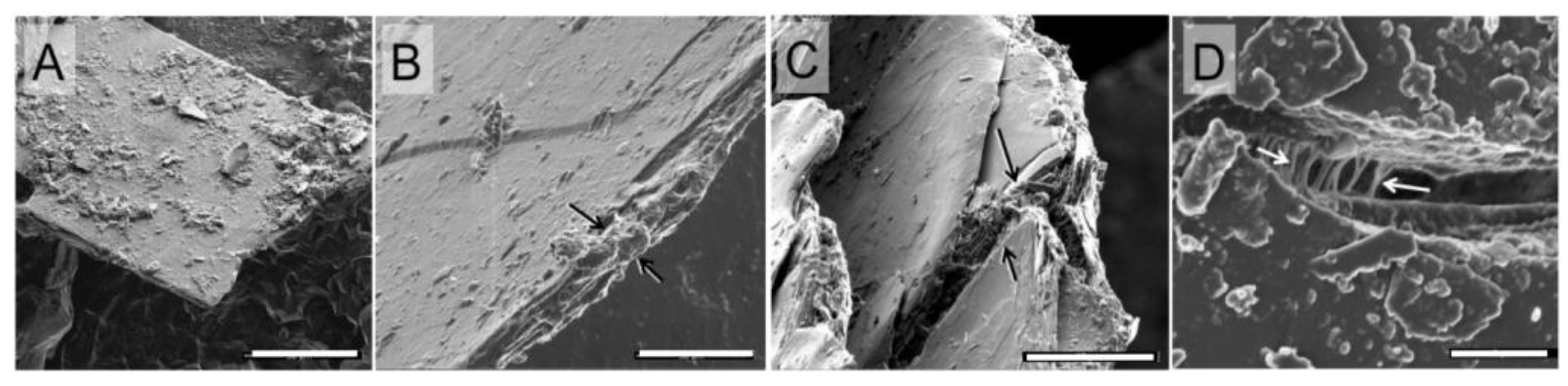
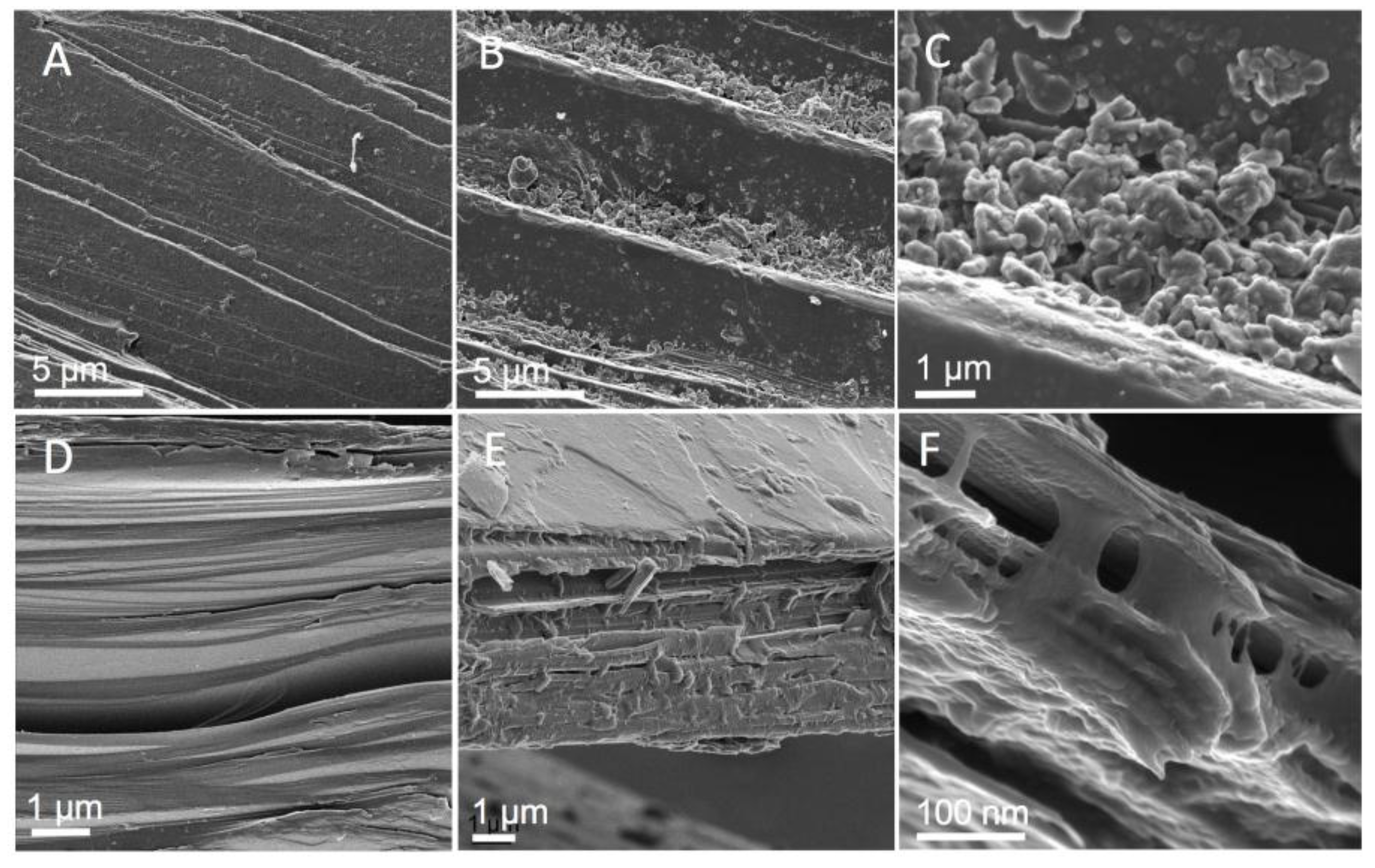
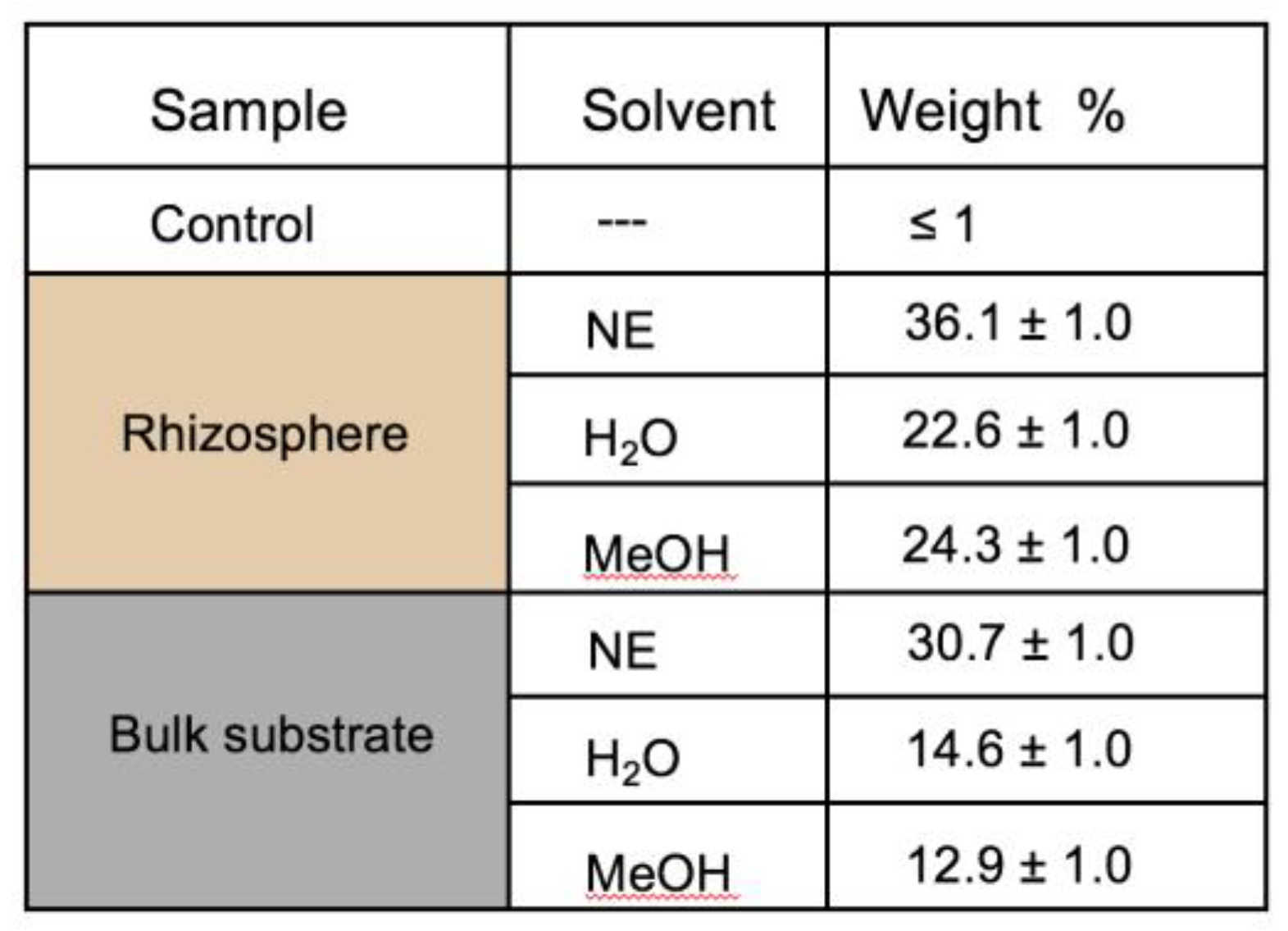
3.5. HeIM
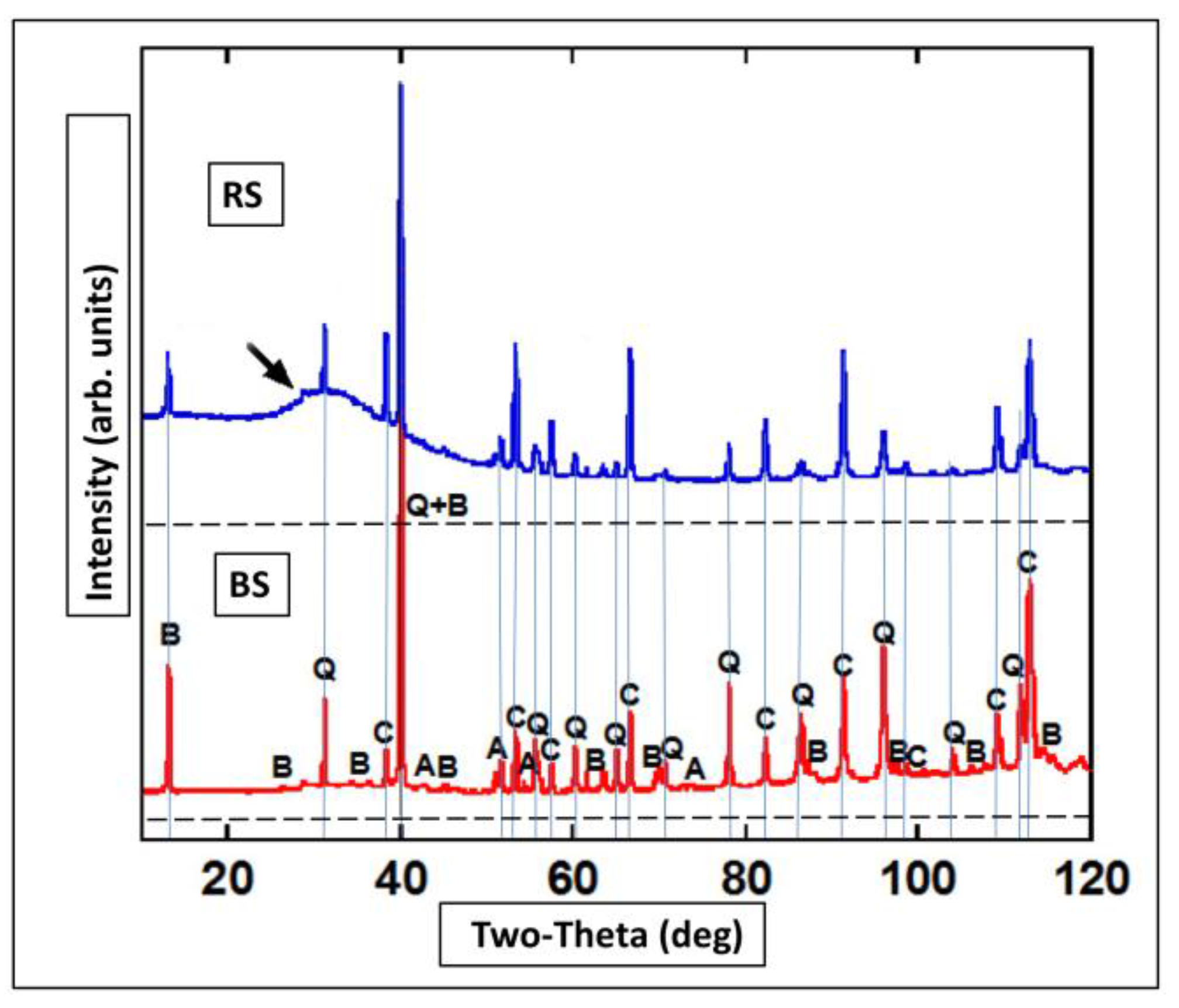
3.6. XRD and Micro-XRD
4. Discussion
4.1. Rhizospheric Microbiome
4.2. Molecular Characterization of Rhizospheric Biomass
4.3. Correlated Imaging of Extracted Material
4.4. SOM Association with Mineral Surfaces
4.5. XRD of Minerals with Amorphous SOM
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Houghton, R.A. Balancing the global carbon budget. Annu Rev. Earth Planet. Sci. 2007, 35, 313–347. [Google Scholar] [CrossRef]
- Köchy, M.; Hiederer, R.; Freibauer, A. Global distribution of soil organic carbon—Part 1: Masses and frequency distributions of SOC stocks for the tropics, permafrost regions, wetlands, and the world. SOIL 2015, 1, 351–365. [Google Scholar] [CrossRef]
- Jiao, N.; Herndl, G.J.; Hansell, D.A.; Benner, R.; Kattner, G.; Wilhelm, S.W.; Kirchman, D.L.; Weinbauer, M.G.; Luo, T.W.; Chen, F.; et al. Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 2010, 8, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Balser, T.C. Microbial production of recalcitrant organic matter in global soils: Implications for productivity and climate policy. Nat. Rev. Microbiol. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Benner, R. Biosequestration of carbon by heterotrophic microorganisms. Nat. Rev. Microbiol. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M.; Johnson, M.G. Advances in Understanding the Molecular Structure of Soil Organic Matter: Implications for Interactions in the Environment. Adv. Agron. 2010, 106, 77–142. [Google Scholar] [CrossRef]
- Marschner, B.; Brodowski, S.; Dreves, A.; Gleixner, G.; Gude, A.; Grootes, P.M.; Hamer, U.; Heim, A.; Jandl, G.; Ji, R.; et al. How relevant is recalcitrance for the stabilization of organic matter in soils? J. Plant Nutr. Soil Sci. 2008, 171, 91–110. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.H.; Joergensen, R.G. Relationship between SIR and FE estimates of microbial biomass C in deciduous forest soils at different pH. Soil Biol. Biochem. 1997, 29, 1033–1042. [Google Scholar] [CrossRef]
- Kindler, R.; Miltner, A.; Richnow, H.H.; Kastner, M. Fate of gram-negative bacterial biomass in soil—Mineralization and contribution to SOM. Soil Biol. Biochem. 2006, 38, 2860–2870. [Google Scholar] [CrossRef]
- Kindler, R.; Miltner, A.; Thullner, M.; Richnow, H.H.; Kastner, M. Fate of bacterial biomass derived fatty acids in soil and their contribution to soil organic matter. Org. Geochem. 2009, 40, 29–37. [Google Scholar] [CrossRef]
- Potthoff, M.; Dyckmans, J.; Flessa, H.; Beese, F.; Joergensen, R.G. Decomposition of maize residues after manipulation of colonization and its contribution to the soil microbial biomass. Biol. Fertil. Soils 2008, 44, 891–895. [Google Scholar] [CrossRef]
- Simpson, A.J.; Simpson, M.J.; Smith, E.; Kelleher, B.P. Microbially derived inputs to soil organic matter: Are current estimates too low? Environ. Sci. Technol. 2007, 41, 8070–8076. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J. Soil Carbon Microbes and Global Carbon. Nat. Clim. Chang. 2013, 3, 867–868. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Oburger, E.; Schmidt, H. New Methods To Unravel Rhizosphere Processes. Trends Plant Sci. 2016, 21, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of soil organic matter: Association with minerals or chemical recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]
- Pett-Ridge, J.; Firestone, M.K. Using stable isotopes to explore root-microbe-mineral interactions in soil. Rhizosphere 2017, 3, 244–253. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Yang, C.H.; Crowley, D.E. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl. Environ. Microbiol. 2000, 66, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Balogh-Brunstad, Z.; Keller, C.K.; Bormann, B.T.; O’Brien, R.; Wang, D.; Hawley, G. Chemical weathering and chemical denudation dynamics through ecosystem development and disturbance. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R.; Preston, C.M.; Nierop, K.G.J. Strengthening the soil organic carbon pool by increasing contributions from recalcitrant aliphatic bio(macro)molecules. Geoderma 2007, 142, 1–10. [Google Scholar] [CrossRef]
- von Lutzow, M.; Kogel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Balogh-Brunstad, Z.; Keller, C.K.; Gill, R.A.; Bormann, B.T.; Li, C.Y. The effect of bacteria and fungi on chemical weathering and chemical denudation fluxes in pine growth experiments. Biogeochemistry 2008, 88, 153–167. [Google Scholar] [CrossRef]
- Koide, R.T.; Kabir, Z. Nutrient economy of red pine is affected by interactions between Pisolithus tinctorius and other forest-floor microbes. New Phytol. 2001, 150, 179–188. [Google Scholar] [CrossRef]
- Shi, Z.Q.; Balogh-Brunstad, Z.; Grant, M.; Harsh, J.; Gill, R.; Thomashow, L.; Dohnalkova, A.; Stacks, D.; Letourneau, M.; Keller, C.K. Cation uptake and allocation by red pine seedlings under cation-nutrient stress in a column growth experiment. Plant Soil 2014, 378, 83–98. [Google Scholar] [CrossRef]
- Bormann, B.T.; Wang, D.; Bormann, F.H.; Benoit, G.; April, R.; Snyder, M.C. Rapid, plant-induced weathering in an aggrading experimental ecosystem. Biogeochemistry 1998, 43, 129–155. [Google Scholar] [CrossRef]
- Tfaily, M.M.; Chu, R.K.; Tolic, N.; Roscioli, K.M.; Anderton, C.R.; Pasa-Tolic, L.; Robinson, E.W.; Hess, N.J. Advanced Solvent Based Methods for Molecular Characterization of Soil Organic Matter by High-Resolution Mass Spectrometry. Anal. Chem. 2015, 87, 5206–5215. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Aronesty, E. EA-Utils: Command-Line Tools for Processing Biological Sequencing Data. Available online: https://expressionanalysis.github.io/ea-utils/ (accessed on 22 February 2017).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing Large Minimum Evolution Trees with Profiles instead of a Distance Matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Kujawinski, E.B.; Behn, M.D. Automated analysis of electrospray ionization Fourier transform ion cyclotron resonance mass spectra of natural organic matter. Anal. Chem. 2006, 78, 4363–4373. [Google Scholar] [CrossRef] [PubMed]
- Kujawinski, E.B.; Longnecker, K.; Blough, N.V.; Del Vecchio, R.; Finlay, L.; Kitner, J.B.; Giovannoni, S.J. Identification of possible source markers in marine dissolved organic matter using ultrahigh resolution mass spectrometry. Geochim. Cosmochim. Acta 2009, 73, 4384–4399. [Google Scholar] [CrossRef]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef] [PubMed]
- Madsen, I.C.; Scarlett, N.V.Y. Quantitative Phase Analysis. In Powder Diffraction: Theory and Practice; Dinnabier, R.E., Ed.; Royal Society of Chemistry: London, UK, 2008. [Google Scholar]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. Fems Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.; Bouziri, L.; Nicolardot, B.; Ranjard, L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Eskelinen, A.; Stark, S.; Mannisto, M. Links between plant community composition, soil organic matter quality and microbial communities in contrasting tundra habitats. Oecologia 2009, 161, 113–123. [Google Scholar] [CrossRef]
- De Souza, J.A.M.; Alves, L.M.C.; de Mello Varani, A.; de Macedo Lemos, E.G. The Family Bradyrhizobiaceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 135–154. [Google Scholar]
- Gliesche, E.A. Genus Hyphomicrobium Stutzer and Hartleb 1898, 76AL. In Bergey’s Manual of Systematic Bacteriology; Williams and Wilkins: Baltimore, MD, USA, 2005; pp. 476–494. [Google Scholar]
- Oren, E.A. The family Hyphomicrobiaceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 247–281. [Google Scholar]
- Okamura, K.; Kanbe, T.; Hiraishi, A. Rhodoplanes serenus sp nov, a purple non-sulfur bacterium isolated from pond water. Int. J. Syst Evol. Microbiol. 2009, 59, 531–535. [Google Scholar] [CrossRef]
- Uebayasi, M.; Tomizuka, N.; Kamibayashi, A.; Tonomura, K. Autotrophic Growth of a Hyphomicrobium Sp and Its Hydrogenase Activity. Agric. Biol. Chem. 1981, 45, 1783–1790. [Google Scholar] [CrossRef]
- Sy, A.; Timmers, A.C.J.; Knief, C.; Vorholt, J.A. Methylotrophic metabolism is advantageous for Methylobacterium extorquens during colonization of Medicago truncatula under competitive conditions. Appl. Environ. Microbiol. 2005, 71, 7245–7252. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Kang, B.G.; Lee, Y.J.; Chung, J.B.; Sa, T.M. Effect of co-inoculation of methylotrophic Methylobacterium oryzae with Azospirillum brasilense and Burkholderia pyrrocinia on the growth and nutrient uptake of tomato, red pepper and rice. Plant Soil 2010, 328, 71–82. [Google Scholar] [CrossRef]
- Phillips, L.A.; Ward, V.; Jones, M.D. Ectomycorrhizal fungi contribute to soil organic matter cycling in sub-boreal forests. ISME J. 2014, 8, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Nicolas, C.; Bentzer, J.; Ellstrom, M.; Smits, M.; Rineau, F.; Canback, B.; Floudas, D.; Carleer, R.; Lackner, G.; et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 2016, 209, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.M.; Xing, B.S. Organic matter-surface area relationships in acid soils. Soil Sci. Soc. Am. J. 2001, 65, 250–258. [Google Scholar] [CrossRef]
- Leifeld, J.; Kogel-Knabner, I. Organic carbon and nitrogen in fine soil fractions after treatment with hydrogen peroxide. Soil Biol. Biochem. 2001, 33, 2155–2158. [Google Scholar] [CrossRef]
- Tian, J.; Lu, S.H.; Fan, M.S.; Li, X.L.; Kuzyakov, Y. Labile soil organic matter fractions as influenced by non-flooded mulching cultivation and cropping season in rice-wheat rotation. Eur. J. Soil Biol. 2013, 56, 19–25. [Google Scholar] [CrossRef]
- Eusterhues, K.; Rumpel, C.; Kleber, M.; Kogel-Knabner, I. Stabilisation of soil organic matter by interactions with minerals as revealed by mineral dissolution and oxidative degradation. Org. Geochem. 2003, 34, 1591–1600. [Google Scholar] [CrossRef]
- Siregar, A.; Kleber, M.; Mikutta, R.; Jahn, R. Sodium hypochlorite oxidation reduces soil organic matter concentrations without affecting inorganic soil constituents. Eur. J. Soil Sci. 2005, 56, 481–490. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Farrar, J.; Hawes, M.; Jones, D.; Lindow, S. How roots control the flux of carbon to the rhizosphere. Ecology 2003, 84, 827–837. [Google Scholar] [CrossRef]
- Thurman, E.M. Organic Geochemistry of Natural Waters; Kluwer Academic Publishers: Hingham, MA, USA, 1985. [Google Scholar]
- Urbanek, E.; Hallett, P.; Feeney, D.; Horn, R. Water repellency and distribution of hydrophilic and hydrophobic compounds in soil aggregates from different tillage systems. Geoderma 2007, 140, 147–155. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral-Organic Associations: Formation, Properties, and Relevance in Soil Environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar] [CrossRef]
- Torn, M.S.; Trumbore, S.E.; Chadwick, O.A.; Vitousek, P.M.; Hendricks, D.M. Mineral control of soil organic carbon storage and turnover. Nature 1997, 389, 170–173. [Google Scholar] [CrossRef]
- Baisden, W.T.; Amundson, R.; Cook, A.C.; Brenner, D.L. Turnover and storage of C and N in five density fractions from California annual grassland surface soils. Glob. Biogeochem. Cycles 2002, 16, 64-1–64-19. [Google Scholar] [CrossRef]
- Krull, E.S.; Baldock, J.A.; Skjemstad, J.O. Importance of mechanisms and processes of the stabilisation of soil organic matter for modelling carbon turnover. Funct. Plant Biol. 2003, 30, 207–222. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Zachara, J.M. Electron transfer at the microbe-mineral interface: A grand challenge in biogeochemistry. Geobiology 2008, 6, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.L.; Dohnalkova, A.C.; Nachimuthu, P.; Kennedy, D.W.; Saffarini, D.A.; Arey, B.W.; Shi, L.; Wang, Z.; Moore, D.; Mclean, J.S.; et al. Role of outer-membrane cytochromes MtrC and OmcA in the biomineralization of ferrihydrite by Shewanella oneidensis MR-1. Geobiology 2010, 8, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Wieder, W.R.; Bonan, G.B.; Allison, S.D. Global soil carbon projections are improved by modelling microbial processes. Nat. Clim. Chang. 2013, 3, 909–912. [Google Scholar] [CrossRef]
- Lawrence, C.R.; Neff, J.C.; Schimel, J.P. Does adding microbial mechanisms of decomposition improve soil organic matter models? A comparison of four models using data from a pulsed rewetting experiment. Soil Biol. Biochem. 2009, 41, 1923–1934. [Google Scholar] [CrossRef]
- Wieder, W.R.; Grandy, A.S.; Kallenbach, C.M.; Bonan, G.B. Integrating microbial physiology and physio-chemical principles in soils with the MIcrobial-MIneral Carbon Stabilization (MIMICS) model. Biogeosciences 2014, 11, 3899–3917. [Google Scholar] [CrossRef]
- Kleber, M.; Mikutta, R.; Torn, M.S.; Jahn, R. Poorly crystalline mineral phases protect organic matter in acid subsoil horizons. Eur. J. Soil Sci. 2005, 56, 717–725. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dohnalkova, A.C.; Tfaily, M.M.; Smith, A.P.; Chu, R.K.; Crump, A.R.; Brislawn, C.J.; Varga, T.; Shi, Z.; Thomashow, L.S.; Harsh, J.B.; et al. Molecular and Microscopic Insights into the Formation of Soil Organic Matter in a Red Pine Rhizosphere. Soils 2017, 1, 4. https://doi.org/10.3390/soils1010004
Dohnalkova AC, Tfaily MM, Smith AP, Chu RK, Crump AR, Brislawn CJ, Varga T, Shi Z, Thomashow LS, Harsh JB, et al. Molecular and Microscopic Insights into the Formation of Soil Organic Matter in a Red Pine Rhizosphere. Soils. 2017; 1(1):4. https://doi.org/10.3390/soils1010004
Chicago/Turabian StyleDohnalkova, Alice C., Malak M. Tfaily, A. Peyton Smith, Rosalie K. Chu, Alex R. Crump, Colin J. Brislawn, Tamas Varga, Zhenqing Shi, Linda S. Thomashow, James B. Harsh, and et al. 2017. "Molecular and Microscopic Insights into the Formation of Soil Organic Matter in a Red Pine Rhizosphere" Soils 1, no. 1: 4. https://doi.org/10.3390/soils1010004
APA StyleDohnalkova, A. C., Tfaily, M. M., Smith, A. P., Chu, R. K., Crump, A. R., Brislawn, C. J., Varga, T., Shi, Z., Thomashow, L. S., Harsh, J. B., & Keller, C. K. (2017). Molecular and Microscopic Insights into the Formation of Soil Organic Matter in a Red Pine Rhizosphere. Soils, 1(1), 4. https://doi.org/10.3390/soils1010004




