Abstract
The coronavirus disease 2019 (COVID-19) is an emerging pandemic caused by a novel coronavirus (SARS-CoV-2). Since December 2019 the new virus has spread all over the world and has become a major health problem also because of the public measures that could affect people’s sexual activity. We report a case of a 35-year-old married male admitted to the andrology outpatient clinic in July 2021 because of sudden onset erectile dysfunction (ED). The diagnostic workup showed no risk factor for ED, normal levels of testosterone, increased levels of endothelial dysfunction markers, such as CRP (C-Reactive Protein) and Endothelin-1, and reduced Vitamin D (VD) levels. Dynamic penile duplex ultrasound (D-PDU) revealed dysfunctional penile arterial flow. The five-item International Index of Erectile Function (IIEF-5) and the Short-Form Health Survey (SF-36) showed a reduction in all domain scores. The patient, initially unresponsive to the high dose oral phosphodiesterase 5 inhibitors (PDE-5is), was treated with vitamin-D and then submitted to LI-SWT (low intensity shockwave treatment), with a progressive clinical benefit at the 12-month follow-up. After 18 months, hormone levels persisted in normal ranges, with a consistent reduction in CRP and Endothelin-1. Additionally, IIEF-5, SF-36 and arterial flow significantly improved over the follow-up period. Thus far, the erectile function was restored and the patient is no more treated with PDE-5i.
1. Introduction
Since 2019, COVID-19 has become a major public health concern. In most cases the respiratory symptoms of COVID-19 include fever, cough and shortness of breath [1]. The disease could involve the lungs and progress to severe forms, including acute respiratory distress syndrome (ARDS) and respiratory failure [2].
COVID-19 has been related to many long-term consequences due to endothelial dysfunction [3]. The pathophysiological mechanisms behind endothelial dysfunction in COVID-19 are still not entirely understood. Viral infection by SARS-CoV-2 occurs first in airway epithelial cells and vascular endothelial cells, causing endothelial dysfunction either directly through endothelial cell infection, or indirectly through other susceptible types of cell infection, which provoke hyperinflammation [4]. Endothelial dysregulation promotes vasoconstriction, vascular leakage, thrombosis and hyperinflammation, involving, consequently, an elevated prothrombin time, increased D-dimers and fibrin degradation products, augmented levels of C-reactive protein (CRP) and interleukin-6 (IL-6) [5].
Thrombotic and inflammatory processes are involved in developing ARDS and extrapulmonary complications. However, even asymptomatic forms of COVID-19 could lead to microvascular involvement [6].
In 2020, due to the absence of vaccines and effective treatments, social distancing and self-isolation were implemented in many countries in order to minimize disease transmission. During the pandemic, many people experienced psychological discomfort and symptoms such as depression and anxiety [7]. Moreover, because of these unusual circumstances and emotional damage, people changed their sexual behaviors [8,9,10].
Erectile dysfunction (ED) is defined as the inability to attain or maintain an erection sufficient to allow sexual satisfaction [11]. Even if ED could be determined by psychogenic disorders, in most cases it recognizes an organic etiology [12].
Data from recent studies suggest a statistical association between COVID-19 and ED [13,14,15,16]. These results also suggest the possible role of the infection in the development of ED: the removal of the possible influence of anxiety and depression confirmed that the prevalence of ED among COVID-19 patients is not only a consequence of stress and psychological factors due to the lockdown, but it is also related to other organic factors [17]. On the other hand, it is now clear that it is hard to fully separate organic and psychogenic conditions, which in most cases coexist and contribute to the pathogenesis of ED [18].
A potential association between organic ED and COVID-19 has been postulated since the beginning of the pandemic [8] and a 2021 study found SARS-CoV-2 in the vascular endothelial cells of the corpus cavernosum in penis biopsies in men who developed severe ED, with a reduced expression of endothelial nitric oxide synthase (eNOS) [5,14].
Endothelial dysfunction is one of the most frequent mechanisms that can lead to ED [19]. The vascular tone results from the balance between vasoconstrictors (i.e., endothelin-1 or angiotensin 2), and vasodilators (i.e., nitric oxide (NO) or prostacyclin) agents. In the latter, NO is synthesized by eNOS [20]. The damaged endothelium attracts pro-inflammatory cells and expresses factors limiting NO availability (causing vessel and smooth muscle contraction). Another pathway involved is the augmented production of tumor necrosis factor alpha (TNF-α) in response to increased inflammatory conditions, which induces reactive oxygen species (ROS) generation and a drop in NO levels, apart from suppressing (eNOS) expression [21]. For these reasons, ED is considered a characteristic consequence of endothelial dysfunction.
In addition to endothelial damage, other mechanisms resulting from the COVID-19 infection could determine ED. SARS-CoV-2 may affect the testicles because the Leydig cells express ACE2, possibly leading to the development of a form of hypergonadotropic hypogonadism [22,23]. Moreover, complications of the post-acute phase of COVID-19 (the so-called “long COVID”) that interests many patients should not be overlooked. These include dyspnea, fatigue, anxiety, sleep disorders, myocarditis and cardiomyopathy [24] and pulmonary fibrosis (which causes hypoxia in the penile vascular bed) [25]; all factors contribute to potentially determining ED [26]. As observed by Romano et al., sexual function could be impaired in patients affected by chronic conditions such as gastrointestinal diseases [27].
Major possible underlying mechanisms involved in COVID-19-related erectile dysfunction are summarized in Figure 1.
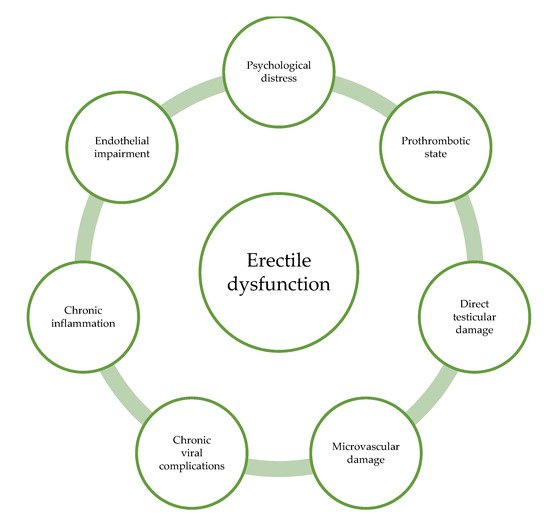
Figure 1.
Different pathogenetic mechanisms by which COVID-19 could affect erectile function.
2. Patients and Methods
A 35-year-old nonsmoker man was admitted in January 2021 to our andrology day hospital for newly onset ED. He had been married since 2015 and never suffered ED. A review of his medical history did not reveal any disorder. There were no prior surgeries or accidents. Moreover, the patient did not take any drug and his BMI was 22.4 kg/m2.
He reported poorly symptomatic COVID-19 four months before. He only suffered cough and fever (lower than 38 °C). Olfaction and taste were not involved (data not shown). Paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs) were used to treat the SARS-CoV-2 infection. Three weeks after his remission he reported his first sudden episode of ED with inability to achieve erection during climax (three out four sexual intercourses during the last 8 weeks). During the first admittance a complete clinical and biochemical exam was carried out, with no significant findings except the detection of Vitamin D deficiency (VDD) and the presence of positive markers of inflammation and endothelial damage; endocrinological laboratory testing was executed, showing a state of eugonadism. Results are summarized in Table 1 and Table 2.

Table 1.
Hormone levels during follow-up period.

Table 2.
Inflammatory markers during follow-up period.
To complete our diagnostic process, two different questionnaires were administered to the patient: SF-36 [28], IIEF-5 [29,30] (results are summarized in Table 3).

Table 3.
Questionnaires scores during follow-up period.
Oral supplementation with cholecalciferol 25,000 UI every two weeks and sildenafil 50 mg on demand were initially started. After 4 weeks, the patient reported modest clinical benefits so that the dose of sildenafil was titrated to 100 mg on demand. Although increasing the dose of sildenafil can ameliorate both the sexual success rate and the sexual attempts’ frequency [31], the patient still failed with sexual attempts.
Because of a persisting incomplete response to PDE-5i, after 30 days with sildenafil 100 mg on demand, a penile duplex ultrasound was performed (8 weeks since the patient’s first visit) to assess potential penile vascular impairment [32]. After injection of Alprostadil 20 mcg, a mild reduction in systolic peak flow velocity was found (Figure 2).
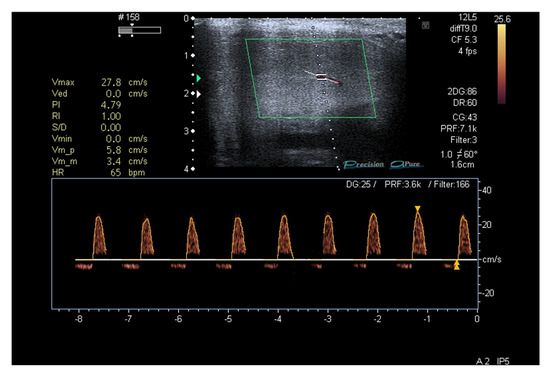
Figure 2.
Penile duplex ultrasound at baseline (2 months since patient’s first visit).
However, after 60 days, the patient still reported no improvement in his sexual intercourses.
Since the patient firmly refused to use a vacuum device and second-line therapies (intra-cavernous injections) to improve his erectile function, we decided to apply low intensity shockwave treatment (LI-SWT).
The patient underwent six consecutive weekly LI-SWT sessions of 20 min consisting of 4000 pulses with an energy density of 0.09 mJ/mm2 and a frequency of 8 Hz. We used linear-focused piezo shockwaves (Linear Shockwave Tissue Coverage-Erectile Dysfunction, LSTC-ED, Richard Wolf GmbH, Knittlingen, Germany). Therapy with tadalafil daily 5 mg was started for four months according to a previously published procedure [33].
3. Results
After 6 months from the first visit (T1 in the tables), the patient came back to our outpatient clinic and reported no adverse events and a good improvement in the frequency and quality of his erections (as highlighted by the better scores on the questionnaires administered), even though he did not turn back to his pre-COVID state (see Table 1; IIEF5 +4 points). The hormonal balance at T1 remained normal, while we observed a reduction in the Endothelin-1 and CRP levels (see Table 1 and Table 2). Therapy with tadalafil was carried on. An improvement in Vitamin D levels was found so we decided to shift Cholecalciferol supplementation to a monthly dose of 25,000 UI.
After a 12-month follow-up (T2 in the tables), the subject significantly improved his IIEF5 score (IIEF5 + 3 points). Vitamin D and inflammation indices were within the normal ranges. In agreement with the patient, we proposed to start daily tadalafil titration at an alternate-day intake until the suspension of the drug two months later.
In July 2022 (T3, four months after tadalafil was withdrawn), the patient was re-evaluated, confirming the clinical remission of ED (IIEF5 +2 points) without therapy and the normalization of the inflammation and vascular damage indices. Lastly, a penile duplex ultrasound was repeated after 18 months and a recovery of the penile vascular function was found (Figure 3).
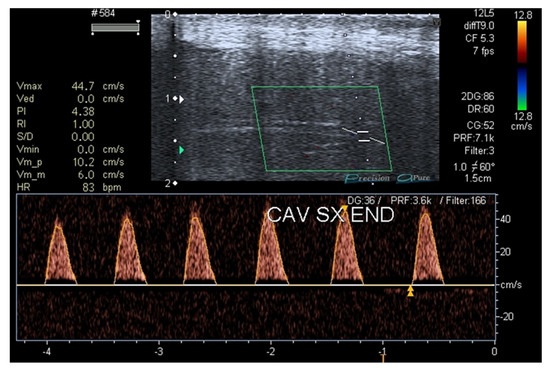
Figure 3.
Penile duplex ultrasound at T3 (18 months after patient’s first visit).
4. Discussion
Because of COVID-19’s continuous spreading, clinicians should be prepared to treat long COVID and its consequences that are common in recovered patients [34]. In particular, the understanding of the physio-pathological mechanisms ranging from SARS-CoV-2 infection to its poly-district clinical manifestations is increasingly necessary to be able to orient, even if empirically, on their therapeutic management.
With relation to the new evident emergence of ED as a potential complication, although rare, the most plausible etiological hypothesis seems to be the vascular one [5]. Moreover, physical and mental stress related to quarantine might worsen ED [9].
In the case here reported by our group, at first, we had no clinical explanation for the etiology of ED other than the possibility of endothelial damage in the penile vascular tissue. This hypothesis was confirmed by the first penile ultrasound performed after two months of poor responsiveness to PDE-5is.
Even if PDE-5is are recognized as a first-line treatment for ED [35], approximately 30–35% of patients are considered non-responders [36]. Many comorbidities could affect the efficacy of PDE-5is [37]. These patients suffer from histopathological changes in penile tissues due to persistent hypoxia with related arterial insufficiency [38]. It has been suggested to optimize treatment with increasing doses of medications [39]. About 50–70% of patients still do not have an adequate response after restoring correct use of PDE-5is [40]. However, 8–12% of PDE-5is non-responders could benefit from a second PDE-5i so we decided to shift sildenafil to tadalafil 5 mg daily [41].
Considering its long half-life (18h) [42], tadalafil could be taken long before sexual intercourse increasing spontaneity [43] and compared to on-demand sildenafil, daily tadalafil has a broader period of opportunity for sexual activity, more sexual attempts, reducing sex time concerns and a more satisfying erection hardness [44]. Moreover, the daily dosing regimen of tadalafil could be considered a salvage therapy in previous PDE-5is non-responders [45].
In recent decades, “penile rehabilitation” (restoring penile function) has been debated, even if only minor studies suggest clinical benefit [46]. It has been postulated that the chronic daily use of tadalafil could contribute in repairing endothelial injury. Moreover, daily tadalafil leads to significantly lower levels in C-reactive protein and endothelin-1, which are known to be associated with inflammation and also to COVID-19 [47].
Shockwave therapy has been proposed as a therapeutic option in patients with vasculogenic ED who are poor responders to PDE-5is and who are not fit for second-line therapies [11,33]. LI-SWT proved to be safe and effective with a significant improvement of penile hardness. An improvement in cell proliferation and NO release has been observed because of nitric oxide synthase (eNOS) activation [48].
Regardless of the pathophysiological mechanism behind endothelial damage, there is no question that multiorgan endothelial dysfunction is a characteristic of COVID-19, especially in its severe form [49]. Endothelial damage in acute COVID-19 causes endothelin-1, acute phase proteins (such as CRP) and inflammatory cytokine elevation [50]; the endothelial cell involvement seems to be able to continue even beyond the acute phase of illness [51]. Moreover, in a recent study by Perri et al., it has been suggested that SARS-CoV-2 could activate the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome determining a chronic pro-inflammatory state [52].
In fact, as seen in many other studies about COVID-19 [50,53], in the case here reported, inflammation markers, such as Endothelin-1 and CRP, were elevated at baseline (four months after COVID-19 remission). We observed a consistent reduction in Endothelin-1 and CRP values after six months and throughout the follow-up period (Table 2). The same improvement was observed in IIEF-5 and SF-36 scores (Table 3) and in the dynamic penile ultrasound performed after 18 months from the first visit (Figure 3).
A recent study by Harirugsakul et al. investigated erectile function three months after COVID-19 in a cohort of 129 subjects. They concluded that the ED course was persistent and self-remitted, similar to other COVID-19 sequelae. Particularly, improvement of anosmia and ageusia could explain transitory ED [54]. Anosmia and ageusia may contribute to the reduction of sexual interest, probably because of the reduced perception of pheromones [55,56,57].
Vitamin D, a steroidal hormone produced by the skin, has been correlated with multiple metabolic and cardiovascular outcomes [58]. Since 2020, the possible relation between COVID-19 and Vitamin D (VD) levels has been widely studied. A meta-analysis from 2021 concluded that VD levels were significantly lower in patients infected with COVID-19 and in patients with severe disease [59].
Endothelial damage in COVID is a phenomenon that seems to be reversible, although in unpredictable times; the improvement of endothelial damage, accompanied in this case by the increase in the quality of the patient’s erections, may have been helped, as mentioned by the repairing effect on the endothelium of tadalafil, but we also hypothesized possible actions on the VD receptor as derived by a high-dose vitamin D supplementation. It has become clear that Vitamin D supplementation could improve some cardiovascular risk factors [60]. In fact, it is directly involved in endothelial function and integrity [61] through its anti-inflammatory and immunomodulatory effects [58]. It seems that vitamin D supplementation could also have a positive effect on inflammatory markers [62], reducing CRP levels [63]. Moreover, Vitamin D induces a shift from inflammatory Th1 to anti-inflammatory Th2 response [64].
The therapeutic strategy we have applied to this case, including LI-SWT, should not be considered a univocal approach to COVID-19-related ED, but has proved to be effective in our clinical context. In particular, since the remission of erectile dysfunction is not foreseeable, it is not yet possible to suggest a specific therapeutic strategy and the time to suspend therapy; however, we speculated that the clinical use of basic markers of inflammation and vascular damage could be helpful to determine the right time to attempt a dose reduction.
5. Conclusions
We reported a single case of ED with an abrupt onset of manifestations four months after poorly symptomatic COVID-19 with progressive responsiveness to LI-SWT and then to PDE-5is. In addition, we may speculate that the daily use of tadalafil and vitamin D supplementation contributed to the reduction in inflammatory markers observed throughout the follow-up period. However, due to the fact that our study reports on a single case, further research with a larger sample size is needed to support our hypothesis.
Even if COVID-19 has been deeply investigated, only a few studies have focused on ED as its consequence [65]. Our data suggest a good prognosis for COVID-19-related ED, although future studies are needed to better understand the physio-pathological effects of the SARS-CoV-2 infection and the underlying mechanisms by which erectile dysfunction may be determined.
Author Contributions
Conceptualization, M.C.Z., G.S. and S.I.; methodology A.A.; investigation, S.I. and G.S; data curation A.A.; writing—original draft preparation, G.S and S.I.; writing—review and editing, G.S. and S.I.; supervision, L.D.L. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, dealing with a case report conducted according to clinical practice guidelines.
Informed Consent Statement
Written informed consent for the publication of this case report was obtained from the patient. The study was performed in accordance with the Declaration of Helsinki.
Data Availability Statement
Data supporting the reported results are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Bernard, I.; Limonta, D.; Mahal, L.K.; Hobman, T.C. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- La Vignera, S.; Cannarella, R.; Condorelli, R.A.; Torre, F.; Aversa, A.; Calogero, A.E. Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated ACE2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. Int. J. Mol. Sci. 2020, 21, 2948. [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann. Intern. Med. 2020, 173, 362–367. [Google Scholar] [CrossRef]
- Crocerossa, F.; Visser, W.; Carbonara, U.; Falagario, U.G.; Pandolfo, S.D.; Loizzo, D.; Imbimbo, C.; Klausner, A.P.; Porpiglia, F.; Damiano, R.; et al. The impact the COVID-19 pandemic on urology literature: A bibliometric analysis. Cent. Eur. J. Urol. 2022, 75, 102–109. [Google Scholar] [CrossRef]
- Sansone, A.; Mollaioli, D.; Ciocca, G.; Limoncin, E.; Colonnello, E.; Vena, W.; Jannini, E.A. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J. Endocrinol. Invest. 2021, 44, 223–231. [Google Scholar] [CrossRef]
- Hu, B.; Ruan, Y.; Liu, K.; Wei, X.; Wu, Y.; Feng, H.; Deng, Z.; Liu, J.; Wang, T. A Mid-to-Long Term Comprehensive Evaluation of Psychological Distress and Erectile Function in COVID-19 Recovered Patients. J. Sex Med. 2021, 18, 1863–1871. [Google Scholar] [CrossRef]
- Vaccaro, M.G.; Izzo, G.; Sarica, A.; La Vignera, S.; Aversa, A. Cluster Analysis Method Reveals Gender Attitudes in Sociosexual Orientation of a Southern Italy Population During the COVID-19 Lockdown. Sex Res. Social Policy 2022, 24, 1–14. [Google Scholar] [CrossRef]
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction. Eur. Urol. 2021, 80, 333–357. [Google Scholar] [CrossRef]
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A.; et al. Erectile dysfunction. Nat. Rev. Dis. Prim. 2016, 2, 16003. [Google Scholar] [CrossRef]
- Sansone, A.; Mollaioli, D.; Ciocca, G.; Colonnello, E.; Limoncin, E.; Balercia, G.; Jannini, E.A. “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology 2021, 9, 1053–1059. [Google Scholar] [CrossRef]
- Kresch, E.; Achua, J.; Saltzman, R.; Khodamoradi, K.; Arora, H.; Ibrahim, E.; Kryvenko, O.N.; Almeida, V.W.; Firdaus, F.; Hare, J.M.; et al. COVID-19 Endothelial Dysfunction Can Cause Erectile Dysfunction: Histopathological, Immunohistochemical, and Ultrastructural Study of the Human Penis. World J. Mens Health 2021, 39, 466–469. [Google Scholar] [CrossRef]
- Kaynar, M.; Gomes, A.L.Q.; Sokolakis, I.; Gül, M. Tip of the iceberg: Erectile dysfunction and COVID-19. Int. J. Impot. Res. 2022, 34, 152–157. [Google Scholar] [CrossRef]
- Pizzol, D.; Shin, J.I.; Trott, M.; Ilie, P.C.; Ippoliti, S.; Carrie, A.M.; Ghayda, R.A.; Lozano, J.M.O.; Muyor, J.M.; Butler, L.; et al. Social environmental impact of COVID-19 and erectile dysfunction: An explorative review. J. Endocrinol. Invest. 2022, 45, 483–487. [Google Scholar] [CrossRef]
- Adeyemi, D.H.; Odetayo, A.F.; Hamed, M.A.; Akhigbe, R.E. Impact of COVID 19 on erectile function. Aging Male 2022, 25, 202–216. [Google Scholar] [CrossRef]
- Jannini, E.A.; McCabe, M.P.; Salonia, A.; Montorsi, F.; Sachs, B.D. Organic vs. psychogenic? The Manichean diagnosis in sexual medicine. J. Sex. Med. 2010, 7, 1726–1733. [Google Scholar] [CrossRef]
- De Leonardis, F.; Colalillo, G.; Finazzi Agrò, E.; Miano, R.; Fuschi, A.; Asimakopoulos, A.D. Endothelial Dysfunction, Erectile Deficit and Cardiovascular Disease: An Overview of the Pathogenetic Links. Biomedicines 2022, 10, 1848. [Google Scholar] [CrossRef]
- Barthelmes, J.; Nägele, M.P.; Ludovici, V.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Endothelial dysfunction in cardiovascular disease and Flammer syndrome—Similarities and differences. EPMA J. 2017, 8, 99–109. [Google Scholar] [CrossRef]
- Neumann, P.; Gertzberg, N.; Johnson, A. TNF-alpha induces a decrease in eNOS promoter activity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L452–L459. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xie, W.; Li, D.; Shi, L.; Mao, Y.; Xiong, Y.; Zhang, Y.; Zhang, M. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. medRxiv 2020. [Google Scholar] [CrossRef]
- Okçelik, S. COVID-19 pneumonia causes lower testosterone levels. Andrologia 2021, 53, e13909. [Google Scholar] [CrossRef] [PubMed]
- Magadum, A.; Kishore, R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020, 9, 2508. [Google Scholar] [CrossRef] [PubMed]
- Graney, B.A.; Wamboldt, F.S.; Baird, S.; Churney, T.; Fier, K.; Korn, M.; McCormick, M.; Vierzba, T.; Swigris, J.J. Looking ahead and behind at supplemental oxygen: A qualitative study of patients with pulmonary fibrosis. Heart Lung 2017, 46, 387–393. [Google Scholar] [CrossRef]
- Sansone, A.; Mollaioli, D.; Limoncin, E.; Ciocca, G.; Bắc, N.H.; Cao, T.N.; Hou, G.; Yuan, J.; Zitzmann, M.; Giraldi, A.; et al. The Sexual Long COVID (SLC): Erectile Dysfunction as a Biomarker of Systemic Complications for COVID-19 Long Haulers. Sex Med. Rev. 2022, 10, 271–285. [Google Scholar] [CrossRef]
- Romano, L.; Pellegrino, R.; Sciorio, C.; Barone, B.; Gravina, A.G.; Santonastaso, A.; Mucherino, C.; Astretto, S.; Napolitano, L.; Aveta, A.; et al. Erectile and sexual dysfunction in male and female patients with celiac disease: A cross-sectional observational study. Andrology 2022, 10, 910–918. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Cappelleri, J.C.; Rosen, R.C.; Smith, M.D.; Mishra, A.; Osterloh, I.H. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology 1999, 54, 346–351. [Google Scholar] [CrossRef]
- Rosen, R.C.; Cappelleri, J.C.; Smith, M.D.; Lipsky, J.; Peña, B.M. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int. J. Impot. Res. 1999, 11, 319–326. [Google Scholar] [CrossRef]
- Steidle, C.P.; McCullough, A.R.; Kaminetsky, J.C.; Crowley, A.R.; Siegel, R.L.; Deriesthal, H.; Tseng, L.J. Early sildenafil dose optimization and personalized instruction improves the frequency, flexibility, and success of sexual intercourse in men with erectile dysfunction. Int. J. Impot. Res. 2007, 19, 154–160. [Google Scholar] [CrossRef]
- Aversa, A.; Crafa, A.; Alessandra Greco, E.; Chiefari, E.; Brunetti, A.; La Vignera, S. The Penile Duplex Ultrasound: How and when to perform it? Andrology 2021, 9, 1457–1466. [Google Scholar] [CrossRef]
- Vena, W.; Vaccalluzzo, L.; Morenghi, E.; D’Agostino, C.; Perri, A.; Giammusso, B.; Lania, A.G.; Aversa, A.; Pizzocaro, A. Low-intensity shockwave treatment (liswt) improves penile rigidity in eugonadal subjects with erectile dysfunction: A pilot study. Minerva Endocrinol. 2021. [Google Scholar] [CrossRef]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef]
- Moncada, I.; Martinez-Salamanca, J.; Ruiz-Castañe, E.; Romero, J. Combination therapy for erectile dysfunction involving a PDE5 inhibitor and alprostadil. Int. J. Impot. Res. 2018, 30, 203–208. [Google Scholar] [CrossRef]
- McMahon, C.N.; Smith, C.J.; Shabsigh, R. Treating erectile dysfunction when PDE5 inhibitors fail. BMJ Clin. Res. Ed. 2006, 332, 589–592. [Google Scholar] [CrossRef]
- Herrero, A.; Marcos, M.; Galindo, P.; Miralles, J.M.; Corrales, J.J. Clinical and biochemical correlates of male hypogonadism in type 2 diabetes. Andrology 2018, 6, 58–63. [Google Scholar] [CrossRef]
- Aversa, A.; Basciani, S.; Visca, P.; Arizzi, M.; Gnessi, L.; Frajese, G.; Fabbri, A. Platelet-derived growth factor (PDGF) and PDGF receptors in rat corpus cavernosum: Changes in expression after transient in vivo hypoxia. J. Endocrinol. 2001, 170, 395–402. [Google Scholar] [CrossRef]
- Hellstrom, W.J.; Elhilali, M.; Homering, M.; Taylor, T.; Gittleman, M. Vardenafil in patients with erectile dysfunction: Achieving treatment optimization. J. Androl. 2005, 26, 604–609. [Google Scholar] [CrossRef]
- Albersen, M.; Mwamukonda, K.B.; Shindel, A.W.; Lue, T.F. Evaluation and treatment of erectile dysfunction. Med. Clin. North Am. 2011, 95, 201–212. [Google Scholar] [CrossRef]
- Hackett, G.; Kirby, M.; Wylie, K.; Heald, A.; Ossei-Gerning, N.; Edwards, D.; Muneer, A. British Society for Sexual Medicine Guidelines on the Management of Erectile Dysfunction in Men-2017. J. Sex Med. 2018, 15, 430–457. [Google Scholar] [CrossRef] [PubMed]
- Eardley, I.; Cartledge, J. Tadalafil (Cialis) for men with erectile dysfunction. Int. J. Clin. Pract. 2002, 56, 300–304. [Google Scholar] [PubMed]
- Porst, H.; Padma-Nathan, H.; Giuliano, F.; Anglin, G.; Varanese, L.; Rosen, R. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: A randomized controlled trial. Urology 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Bai, W.J.; Dai, Y.T.; Xu, W.P.; Wang, C.N.; Li, H.Z. An analysis of treatment preferences and sexual quality of life outcomes in female partners of Chinese men with erectile dysfunction. Asian J. Androl. 2016, 18, 773–779. [Google Scholar] [CrossRef]
- McMahon, C. Efficacy and safety of daily tadalafil in men with erectile dysfunction previously unresponsive to on-demand tadalafil. J. Sex. Med. 2004, 1, 292–300. [Google Scholar] [CrossRef]
- Albersen, M.; Joniau, S.; Claes, H.; Van Poppel, H. Preclinical Evidence for the Benefits of Penile Rehabilitation Therapy following Nerve-Sparing Radical Prostatectomy. Adv. Urol. 2008, 2008, 594868. [Google Scholar] [CrossRef]
- Aversa, A.; Greco, E.; Bruzziches, R.; Pili, M.; Rosano, G.; Spera, G. Relationship between chronic tadalafil administration and improvement of endothelial function in men with erectile dysfunction: A pilot study. Int. J. Impot. Res. 2007, 19, 200–207. [Google Scholar] [CrossRef]
- Frairia, R.; Berta, L. Biological effects of extracorporeal shock waves on fibroblasts. A review. Muscles Ligaments Tendons J. 2011, 1, 138–147. [Google Scholar]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Kunichoff, D.; Garshick, M.; Shah, B.; Pillinger, M.; Hochman, J.S.; Berger, J.S. C-reactive protein and clinical outcomes in patients with COVID-19. Eur. Heart J. 2021, 42, 2270–2279. [Google Scholar] [CrossRef]
- Willems, L.H.; Nagy, M.; Ten Cate, H.; Spronk, H.M.H.; Groh, L.A.; Leentjens, J.; Janssen, N.A.F.; Netea, M.G.; Thijssen, D.H.J.; Hannink, G.; et al. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb. Res. 2022, 209, 106–114. [Google Scholar] [CrossRef]
- Perri, A.; Bossio, S.; Rago, V.; Greco, E.A.; Lofaro, D.; Aversa, A. NLRP3-inflammasome activation in male reproductive system diseases. Minerva Endocrinol. 2022, 14, 5323. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Harirugsakul, K.; Wainipitapong, S.; Phannajit, J.; Paitoonpong, L.; Tantiwongse, K. Erectile dysfunction after COVID-19 recovery: A follow-up study. PLoS ONE 2022, 17, e0276429. [Google Scholar] [CrossRef]
- Ottaviano, G.; Zuccarello, D.; Frasson, G.; Scarpa, B.; Nardello, E.; Foresta, C.; Marioni, G.; Staffieri, A. Olfactory sensitivity and sexual desire in young adult and elderly men: An introductory investigation. Am. J. Rhinol. Allergy 2013, 27, 157–161. [Google Scholar] [CrossRef]
- Siegel, J.K.; Kung, S.Y.; Wroblewski, K.E.; Kern, D.W.; McClintock, M.K.; Pinto, J.M. Olfaction Is Associated With Sexual Motivation and Satisfaction in Older Men and Women. J. Sex. Med. 2021, 18, 295–302. [Google Scholar] [CrossRef]
- Bertolo, R.; Cipriani, C.; Bove, P. Anosmia and ageusia: A piece of the puzzle in the etiology of COVID-19-related transitory erectile dysfunction. J. Endocrinol. Invest. 2021, 44, 1123–1124. [Google Scholar] [CrossRef]
- Crafa, A.; Cannarella, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Is There an Association Between Vitamin D Deficiency and Erectile Dysfunction? A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1411. [Google Scholar] [CrossRef]
- Crafa, A.; Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; Barbagallo, F.; Aversa, A.; La Vignera, S.; Calogero, A.E. Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: A systematic review and meta-analysis. Eclinical Med. 2021, 37, 100967. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Rainsbury, J.; Kimball, S.M. Vitamin D Supplementation, Serum 25(OH)D Concentrations and Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2018, 5, 87. [Google Scholar] [CrossRef]
- Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin D and regulation of vascular cell function. Am. J. Physiol. -Heart Circ. Physiol. 2018, 314, H753–H765. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Krajewska, M.; Witkowska-Sędek, E.; Rumińska, M.; Stelmaszczyk-Emmel, A.; Sobol, M.; Majcher, A.; Pyrżak, B. Vitamin D Effects on Selected Anti-Inflammatory and Pro-Inflammatory Markers of Obesity-Related Chronic Inflammation. Front. Endocrinol. 2022, 13, 920340. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Leung, P.S.C.; Adamopoulos, I.E.; Gershwin, M.E. The Implication of Vitamin D and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2013, 45, 217–226. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, W.; Zou, M.; Zeng, Q.; Feng, Y.; Luo, Z.; Gan, H. Prevalence and risk factors of erectile dysfunction in COVID-19 patients: A systematic review and meta-analysis. J. Endocrinol. Investig. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).