A Thermal Sublimation Generator of 131mXe
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Parent Source
2.2. I Source Manufacturing
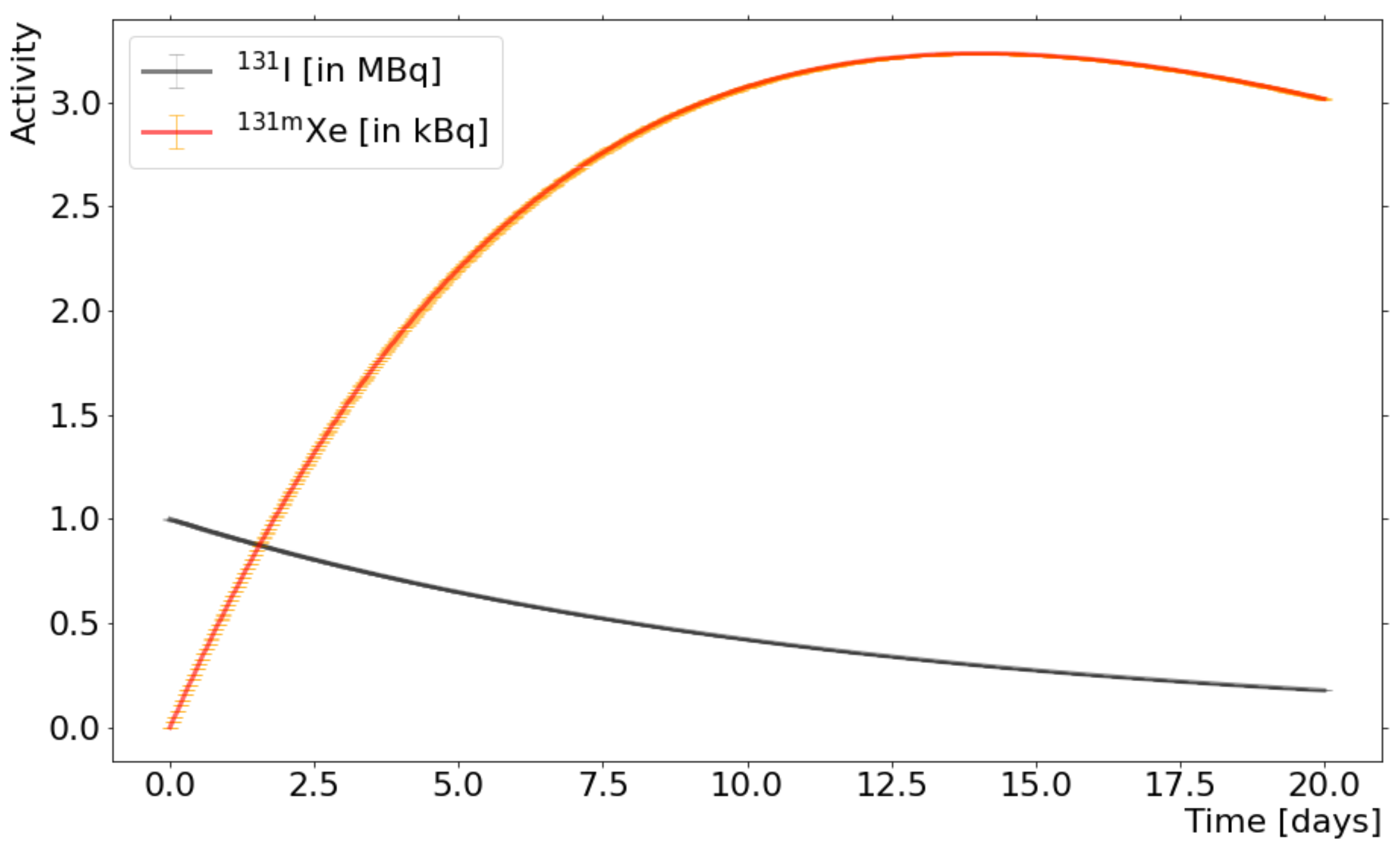
2.3. Purchase of 131I and Radioactive Decay
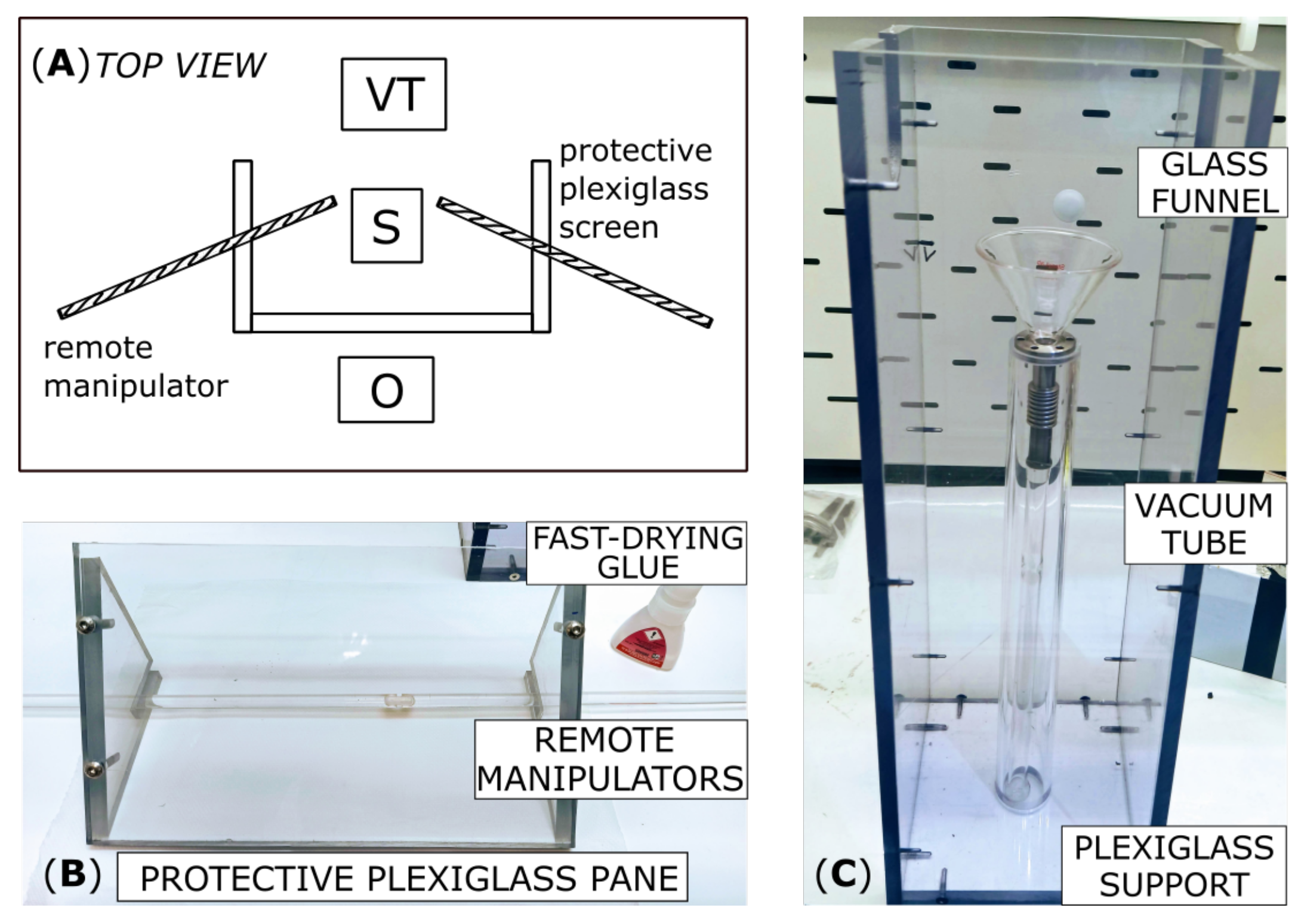
2.4. I Extraction and Xe Collection
3. Results
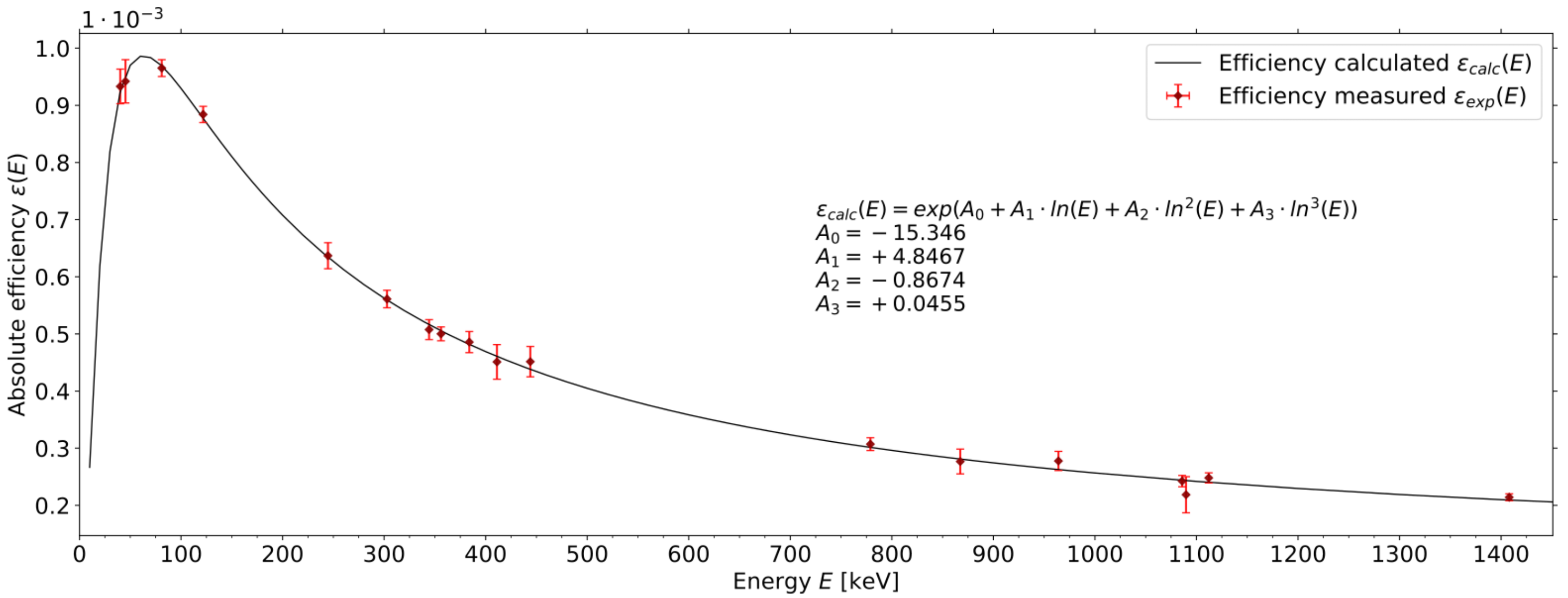
3.1. Efficiency of Xe Generator
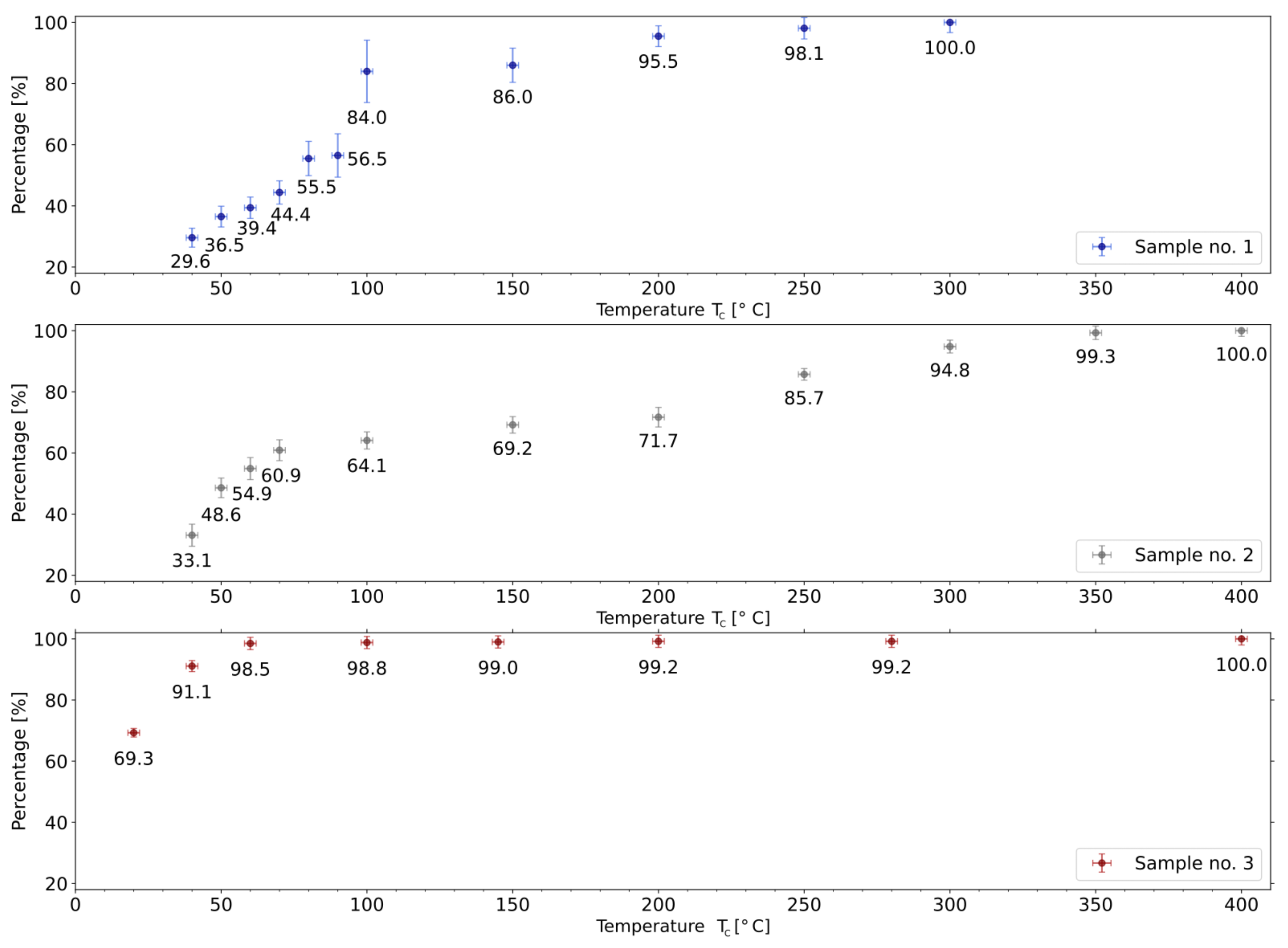
3.2. Collection Efficiency as a Function of Temperature
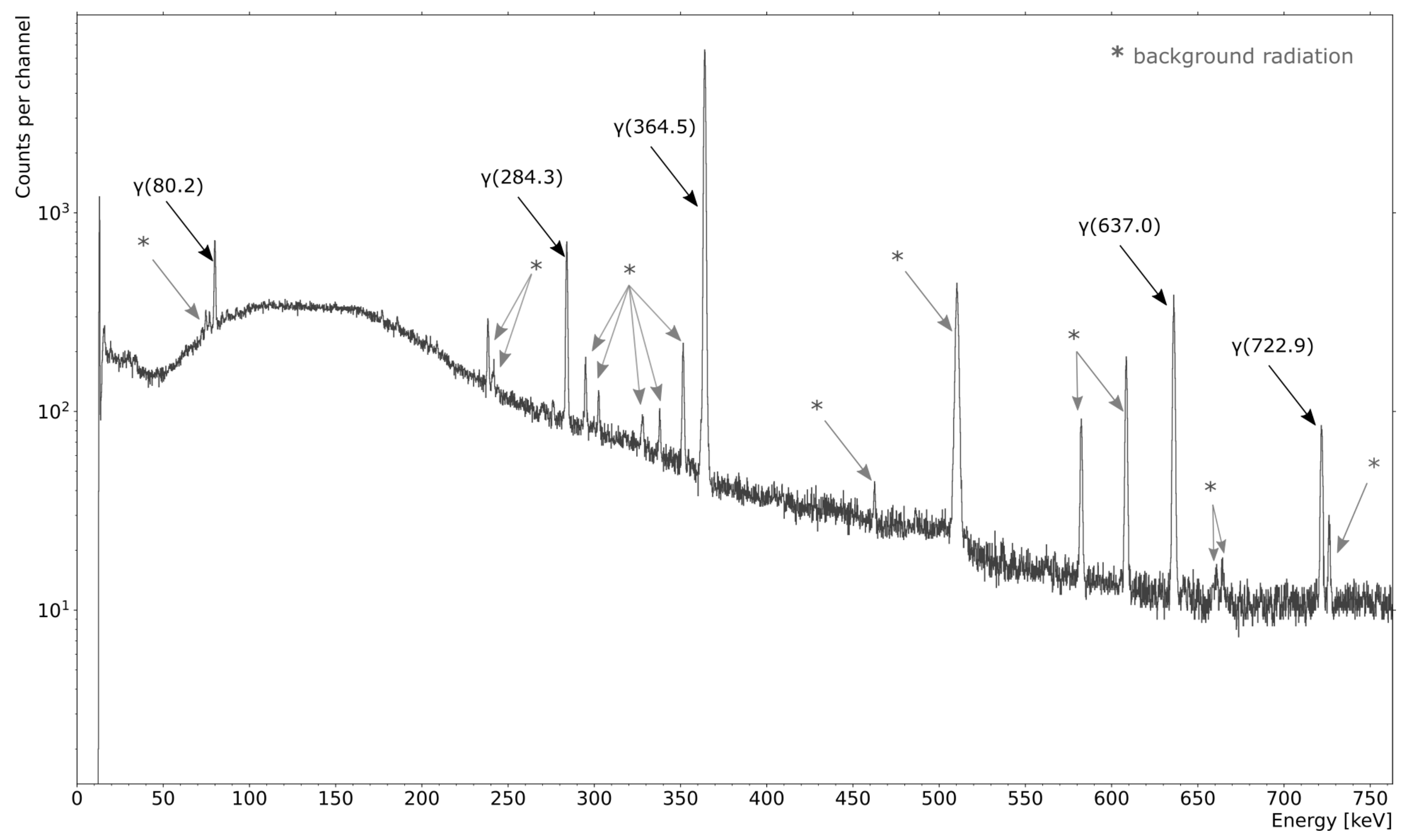
3.3. Radionuclidic Purity of Generated Xe
4. Discussion
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oros, A.M.; Shah, N.J. Hyperpolarized xenon in NMR and MRI. Phys. Med. Biol. 2004, 49, R105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, Z. SPECT and PET in Vascular Dementia. In PET and SPECT in Neurology; Springer International Publishing: New York, NY, USA, 2021; pp. 563–575. [Google Scholar]
- Wisser, D.; Hartmann, M. 129Xe NMR on Porous Materials: Basic Principles and Recent Applications. Adv. Mater. Interfaces 2021, 8, 2001266. [Google Scholar] [CrossRef]
- Boventi, M.; Mauri, M.; Simonutti, R. 129Xe Xe: A Wide-Ranging NMR Probe for Multiscale Structures. Appl. Sci. 2022, 12, 3152. [Google Scholar] [CrossRef]
- Walker, T.G.; Happer, W. Spin-exchange optical pumping of noble-gas nuclei. Rev. Mod. Phys. 1997, 69, 629–642. [Google Scholar] [CrossRef]
- Rao, M.R.; Norquay, G.; Stewart, N.J.; Wild, J.M. Measuring 129Xe transfer across the blood-brain barrier using MR spectroscopy. Magn. Reson. Med. 2021, 85, 2939. [Google Scholar] [CrossRef] [PubMed]
- Berthault, P.; Boutin, C. Biosensing and study of biological cells using hyperpolarized 129Xe. In Hyperpolarized Xenon-129 Magnetic Resonance: Concepts, Production, Techniques and Applications; The Royal Society of Chemistry: London, UK, 2015; pp. 261–271. [Google Scholar]
- Stupic, K.F.; Cleveland, Z.I.; Pavlovskaya, G.E.; Meersmann, T. Hyperpolarized 131Xe NMR spectroscopy. J. Magn. Reson. 2011, 208, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Imai, H.; Fujiwara, H. Continuous flow and dissolved phase 129Xe NMR/MRI for quantification in preclinical study as well as materials science. In Hyperpolarized Xenon-129 Magnetic Resonance: Concepts, Production, Techniques and Applications; The Royal Society of Chemistry: London, UK, 2015; pp. 301–316. [Google Scholar]
- Wang, L.Q. Hyperpolarized 129Xe NMR in materials sciences: Pore structure, interconnectivity and functionality. In Hyperpolarized Xenon-129 Magnetic Resonance: Concepts, Production, Techniques and Applications; The Royal Society of Chemistry: London, UK, 2015; pp. 142–163. [Google Scholar]
- Yokoe, K.; Satoh, K.; Yamamoto, Y.; Nishiyama, Y.; Asakura, H.; Haba, R.; Ohkawa, M. Usefulness of 99mTc-Technegas and 133Xe dynamic SPECT in ventilatory impairment. Nucl. Med. Commun. 2006, 27, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Ziessman, H.A.; O’Malley, J.P.; Thrall, J.H. (Eds.) Chapter 10—Pulmonary System. In Nuclear Medicine, 4th ed.; W.B. Saunders: Philadelphia, PA, USA, 2014; pp. 204–226. [Google Scholar]
- GAMMA-MRI Project Website. Available online: https://gamma-mri.eu (accessed on 30 August 2022).
- The Supply of Medical Radioisotopes: Interim Report of the OECD/NEA High-Level Group on Security of Supply of Medical Radioisotopes; Technical Report; Organisation for Economic Co-Operation and Development, Nuclear Energy Agency (NEA): Paris, France, 2010.
- Manual on Therapeutic Uses of Iodine-131, Practical Radiation Safety Manual No. 6; Technical Report; International Atomic Energy Agency (IAEA): Vienna, Austria, 1996.
- Molybdenum-99 for Medical Imaging; Technical Report; National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 2016.
- Salacz, J. Reprocessing of irradiated 235U for the production of 99Mo, 131I and 133Xe radioisotopes. Fission molybdenum for medical use. In Proceedings of the Technical Committee Meeting Organized by the IAEA, Vienna, Austria, 3–6 November 1987; International Atomic Energy Agency (IAEA): Vienna, Austria, 1989. [Google Scholar]
- Bedrossian, P.; Tóth, G.; Zsinka, L. Herstellung von tragerarmem 131mXe durch eine Adsorptionsmethode. Isot. Isot. Environ. Health Stud. 1968, 4, 117–118. [Google Scholar] [CrossRef]
- McFarland, R. An Improved Generator of 131mXe. Int. J. Appl. Radiat. Isot. 1974, 25, 567–568. [Google Scholar] [CrossRef]
- Zheng, Y.; Miller, G.W.; Tobias, W.A.; Cates, G.D. A method for imaging and spectroscopy using γ-rays and magnetic resonance. Nature 2016, 537, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Khazov, Y.; Mitropolsky, I.; Rodionov, A. Nuclear Data Sheets for A = 131. Nucl. Data Sheets 2006, 107, 2715–2930. [Google Scholar] [CrossRef]
- Bateman, H. The solution of a system of differential equations occurring in the theory of radioactive transformations. Proc. Camb. Philos. Soc. 1910, 15, 423–427. [Google Scholar]
- Nahler, G. European Pharmacopoeia (Eur Ph). In Dictionary of Pharmaceutical Medicine; Springer: Berlin/Heidelberg, Germany, 2009; p. 69. [Google Scholar]
- Kulesz, K.; (CERN, Geneva, Switzerland); Wuillemin, M.; (B.E. Imaging AG, Schwyz, Switzerland). Private communication, 2021.
- Specification Sheet of DT5730/DT5730S 8 Channel 14 bit 500 MS/s Digitizer from CAEN. Available online: https://www.caen.it/products/dt5730/ (accessed on 30 August 2022).
- Livi, R. MAESTRO Multi-Channel Analyzer Software User’s Manual; AMETEK ORTEC Inc.: Vienna, Austria, 2007. [Google Scholar]
- Raeside, D.E.; Brnetich, J. A High-Resolution γ-ray Spectrum of Background Radiation. Am. J. Phys. 2005, 39, 1396. [Google Scholar] [CrossRef]
- Committee on Thyroid Screening Related to I-131 Exposure, Institute of Medicine; Committee on Exposure of the American People to I-131 from the Nevada Atomic Bomb Tests, National Research Council. Health Risks of I-131 Exposure. In Exposure of the American People to Iodine-131 from Nevada Nuclear-Bomb Tests: Review of the National Cancer Institute Report and Public Health Implications; National Academies Press: Washington, DC, USA, 1999; Chapter 3; pp. 45–55. [Google Scholar]
- Currie, L.A. Limits for Qualitative Detection and Quantitative Determination: Application to Radiochemistry. Anal. Chem. 1968, 40, 586–593. [Google Scholar] [CrossRef]
- Mirion Technologies (Canberra). Spectrum Analysis Manual. 2017. Available online: https://www.canberra.com/literature/fundamental-principles/pdf/Spectrum-Analysis.pdf (accessed on 1 August 2022).
- Curium US LLC. Xenon Xe-133 Gas Factsheet; Curium US LLC: Saint Louis, MO, USA, 2018. [Google Scholar]
- Nordion Inc. Xenon Xe-133 Gas Factsheet; Nordion Inc.: Ottawa, ON, Canada, 2016. [Google Scholar]
- Lantheus Medical Imaging Inc. Xenon Xe-133 Gas Factsheet; Lantheus Medical Imaging Inc.: North Billerica, MA, USA, 2018. [Google Scholar]
- GAMMA-MRI Collaboration. Gamma-MRI: The Future of Molecular Imaging; Grant Agreement ID: 964644; European Commission: Brussels, Belgium, 2021.
- Le Conseil Fédéral Suisse. Ordonnance du 26 Avril 2017 sur la Radioprotection (ORaP). Etat le 1er Janvier 2022; The Swiss Federal Council: Bern, Switzerland, 2022.
- Kulesz, K.; Chojnacki, M.J.; Köster, U.; Crepieux, B.; Jolivet, R.B.; Korgul, A.; Lica, R.; Michelon, I.; Murawski, L.; Prokopowicz, R.; et al. CERN: Geneva, Switzerland, 2022; (manuscript in preparation; to be submitted Applied Radiation and Isotopes).


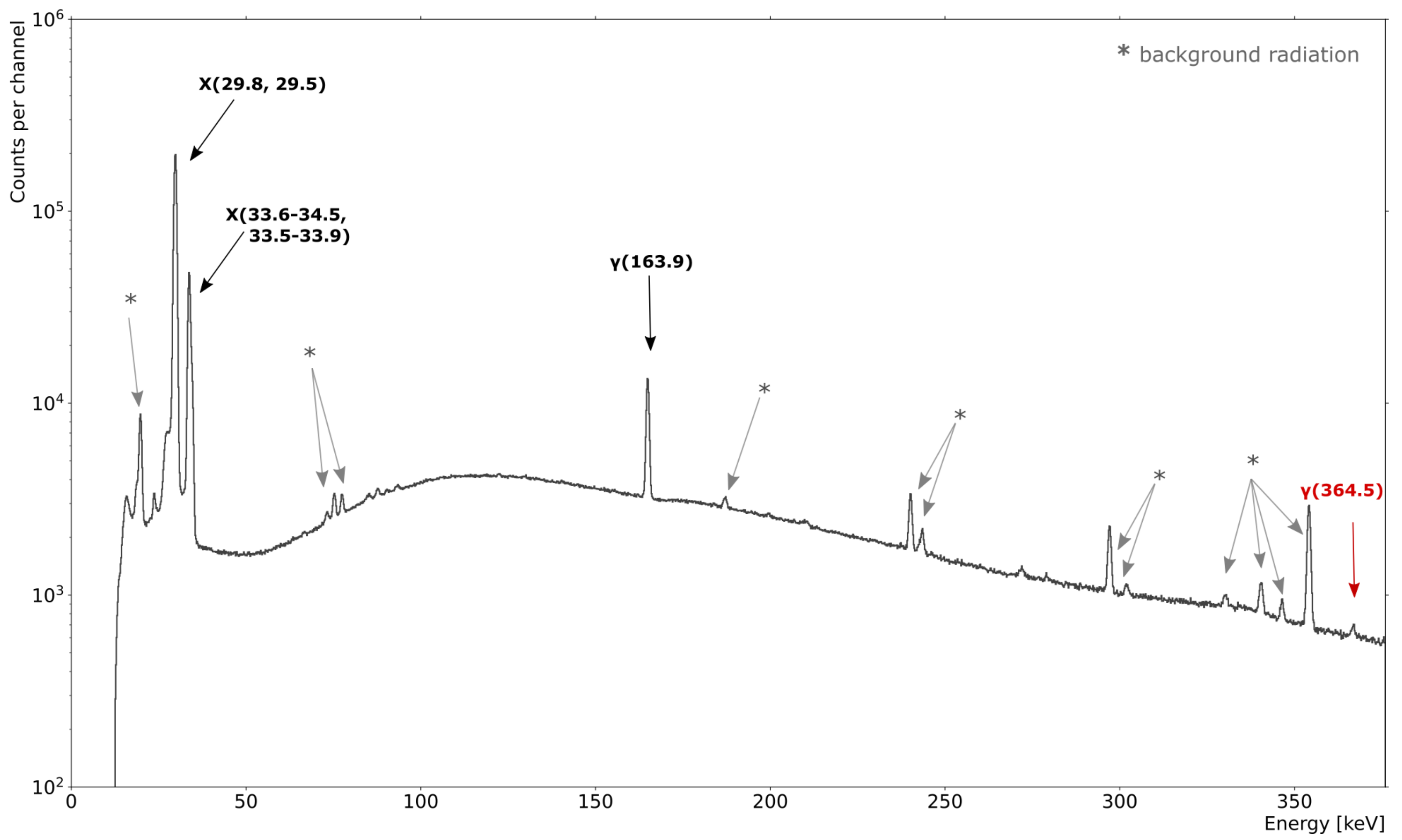
| Source | Distance d: Source–Detector [cm] | Energy [keV] | Detection Efficiency |
|---|---|---|---|
| Xe vial | 50 | 163.9 | 7.79(15) |
| 364.5 | 4.98(12) | ||
| NaI powder | 154 | 364.5 | 5.65(13) |
| ID | Measured I Activity at Delivery [MBq] * | Time T between EOM and Delivery [Days] | I Transfer Rate | Determined Xe Activity at EOC (Given and ) [kBq] | Measured Xe Activity at EOC [kBq] * | Efficiency of Xe Collection |
|---|---|---|---|---|---|---|
| 1 | 49.0(5) | 6 | 64(1)% | 119(4) | 99(2) | 83(3)% |
| 2 | 51.1(6) | 2 | 85(2)% | 149(5) | 131(4) | 88(3)% |
| 3 | 47.5(4) | 22 | 96(2)% | 240(7) | 204(1) | 85(3)% |
| ID | MDA [Bq] | Determined I Activity [Bq] | Radiation from I | Ratio I:Xe |
|---|---|---|---|---|
| 1 | 28.6 | 186(50) | Present | 0.2% |
| 2 | 4.1 | 37(13) | Present | 0.03% |
| 3 | 7.4 | 56(9) | Present | 0.03% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulesz, K.; Azaryan, N.; Baranowski, M.; Chojnacki, M.J.; Köster, U.; Lica, R.; Pascu, S.G.; Jolivet, R.B.; Kowalska, M. A Thermal Sublimation Generator of 131mXe. Instruments 2022, 6, 76. https://doi.org/10.3390/instruments6040076
Kulesz K, Azaryan N, Baranowski M, Chojnacki MJ, Köster U, Lica R, Pascu SG, Jolivet RB, Kowalska M. A Thermal Sublimation Generator of 131mXe. Instruments. 2022; 6(4):76. https://doi.org/10.3390/instruments6040076
Chicago/Turabian StyleKulesz, Karolina, Nikolay Azaryan, Mikołaj Baranowski, Mateusz Jerzy Chojnacki, Ulli Köster, Razvan Lica, Sorin Gabriel Pascu, Renaud Blaise Jolivet, and Magdalena Kowalska. 2022. "A Thermal Sublimation Generator of 131mXe" Instruments 6, no. 4: 76. https://doi.org/10.3390/instruments6040076
APA StyleKulesz, K., Azaryan, N., Baranowski, M., Chojnacki, M. J., Köster, U., Lica, R., Pascu, S. G., Jolivet, R. B., & Kowalska, M. (2022). A Thermal Sublimation Generator of 131mXe. Instruments, 6(4), 76. https://doi.org/10.3390/instruments6040076






