Electrode Materials for Supercapacitors in Hybrid Electric Vehicles: Challenges and Current Progress
Abstract
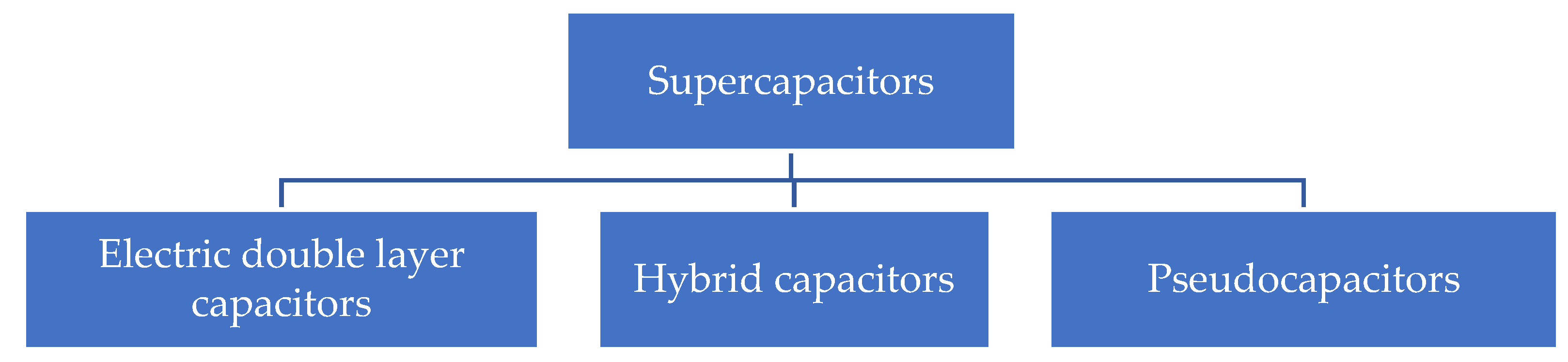
1. Introduction
2. Electrode Materials
2.1. Carbonaceous Materials
2.1.1. Graphene and Graphene Nanocomposites
2.1.2. Renewable Materials Based Activated Carbon
- Biomass-derived carbon
2.1.3. Carbide Derived Carbons (CDC)
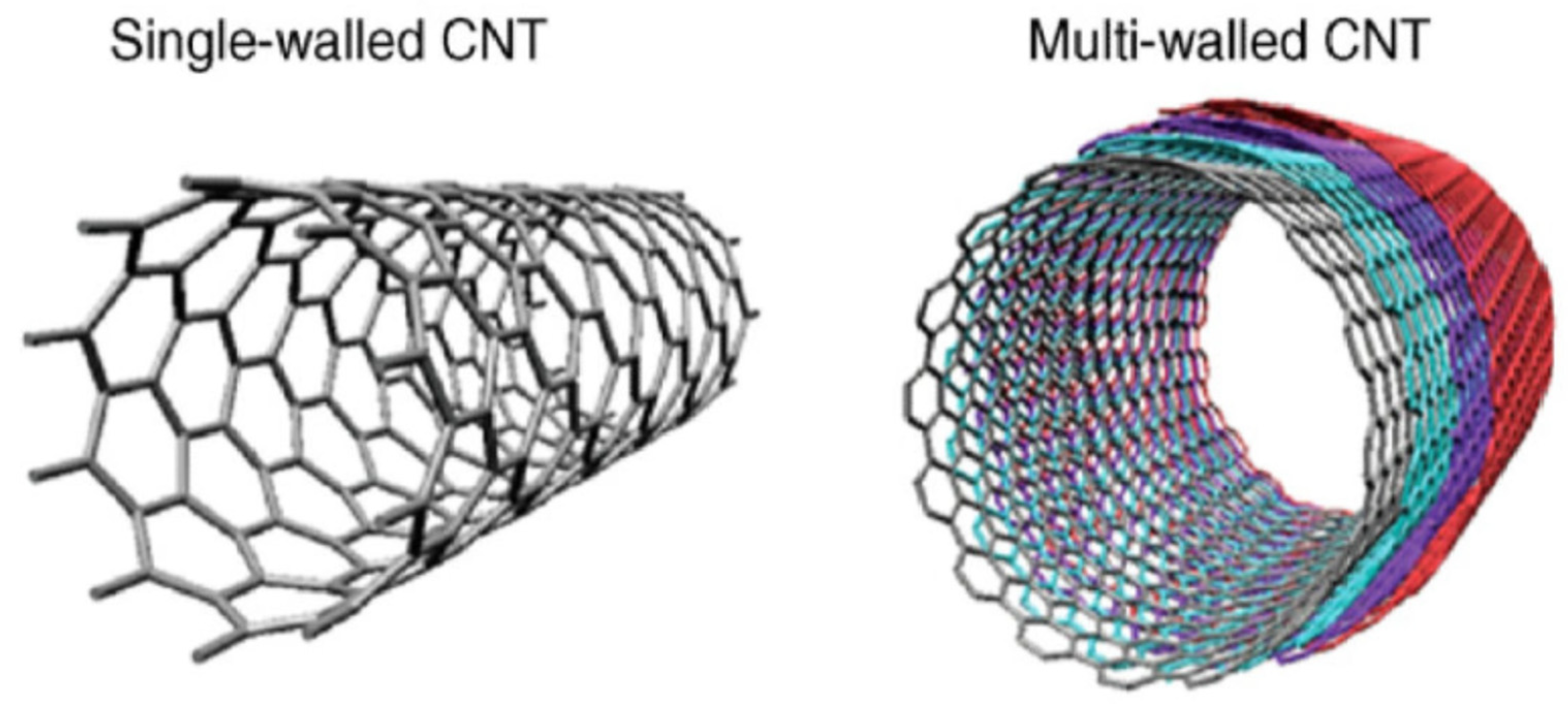
2.1.4. Carbon Nanotubes (CNT)
2.1.5. Carbon Aerogel
2.2. Metal-Organic Framework (MOF) Based Electrode Materials
2.2.1. Pristine MOFs
2.2.2. MOFs Composite Materials
2.2.3. MOF-Derived Materials
2.3. Bimetallic Metal-Organic Framework (BMOF)
2.3.1. Bimetallic-Organic Framework
2.3.2. BMOF-Derived Metal-Carbon Composite
2.3.3. Bimetallic Metal Organic Framework Derived Metal Oxides
2.3.4. BMOF-Derived Metal Sulphur Composite
2.3.5. BMOF-Derived Metal-Phosphorous Composite
2.3.6. Hybrid Materials from Pristine Bimetallic Metal Organic Frameworks and Their Derivatives as Electrodes of Supercapacitors
2.4. Conducting Polymers
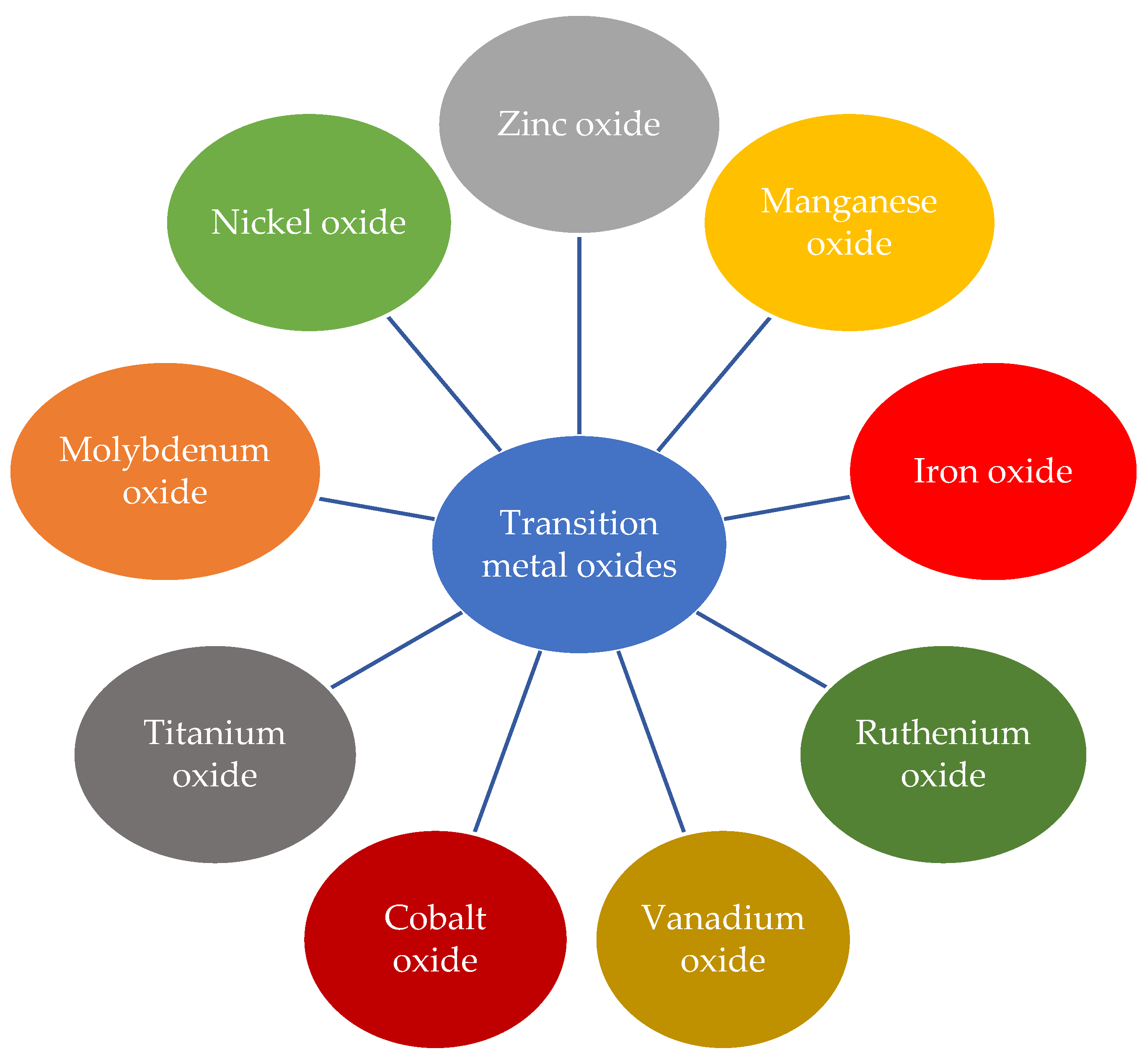
2.5. Transition Metal Oxides
2.5.1. Nickel-Based Supercapacitor Electrodes
- Nanoparticles based on nickel
- Nickel-based nanowires
- Nickel-based thin films
- Nickel-based nanofibers
- Spherical structured materials based on nickel
- Nickel-based nanosheets
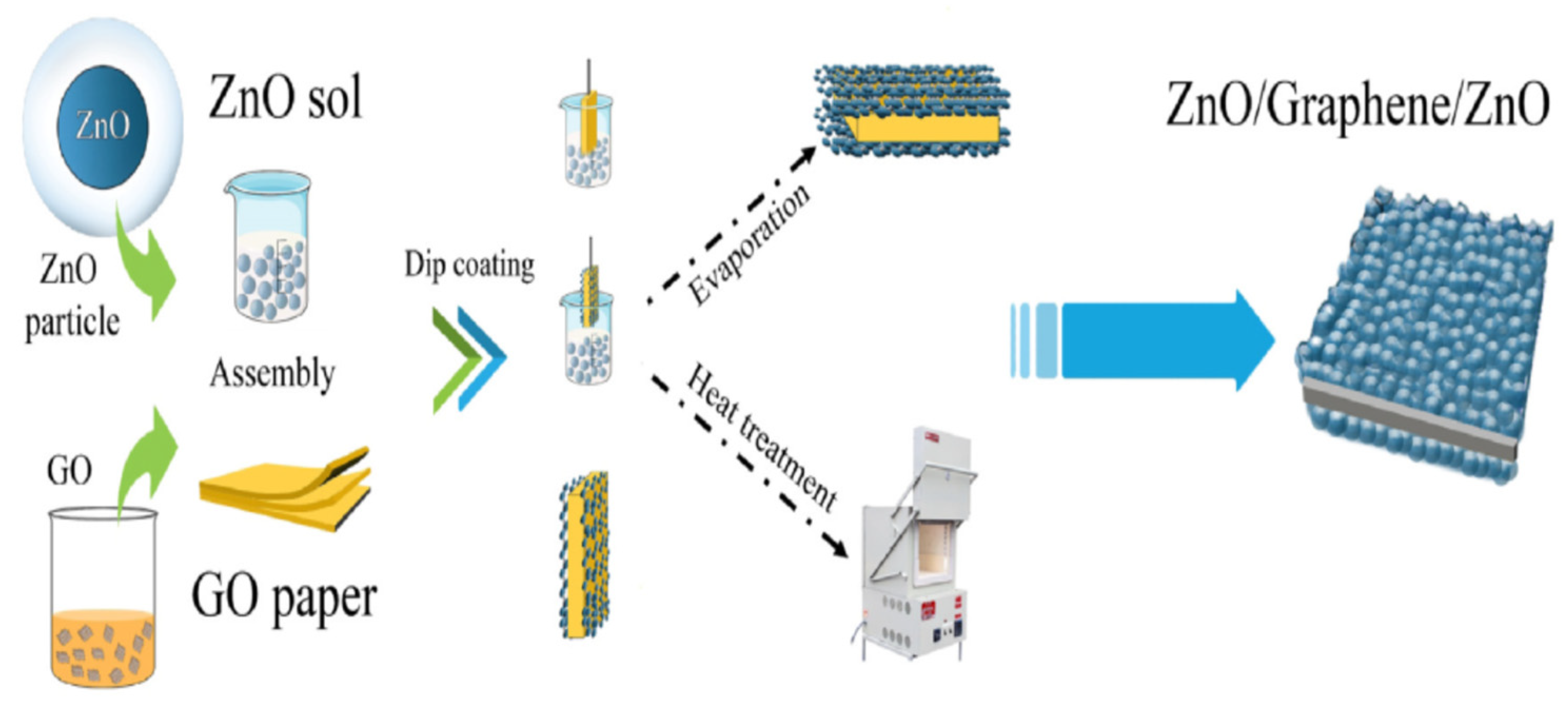
2.5.2. Zinc Oxide
2.5.3. Titanium Oxide
2.5.4. Cobalt Oxide
2.5.5. Iron Oxide
2.5.6. Molybdenum Oxide
2.5.7. Manganese Oxide
2.6. Transition Metal Nitrides
2.6.1. Vanadium Nitrides
2.6.2. Titanium Nitride
2.6.3. Molybdenum Nitride
2.6.4. Niobium Nitride
2.7. Mesoporous Cobalt Silicate Nanosheets (Co2SiO4 NSs)
2.8. CuCo2O4-Based Electrode Materials
2.9. Redox Polymers
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Shalan, A.E.; Abd Elkodous, M.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Awed, A.S.; Ashour, A.H.; et al. Advanced Materials and Technologies for Supercapacitors Used in Energy Conversion and Storage: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- Zhang, Y.; Mei, H.X.; Cao, Y.; Yan, X.H.; Yan, J.; Gao, H.L.; Luo, H.W.; Wang, S.W.; Jia, X.D.; Kachalova, L.; et al. Recent advances and challenges of electrode materials for flexible supercapacitors. Coord. Chem. Rev. 2021, 438, 213910. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Yaïci, W.; Entchev, E. Hybrid battery/supercapacitor energy storage system for the electric vehicles. J. Power Sources 2018, 374, 237–248. [Google Scholar] [CrossRef]
- Wang, C.; Song, Z.; Shi, P.; Lv, L.; Wan, H.; Tao, L.; Zhang, J.; Wang, H.; Wang, H. High-rate transition metal-based cathode materials for battery-supercapacitor hybrid devices. Nanoscale Adv. 2021, 3, 5222–5239. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islam, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Raj, B.; Padhy, A.K.; Basu, S.; Mohapatra, M. Review—Futuristic Direction for R&D Challenges to Develop 2D Advanced Materials Based Supercapacitors. J. Electrochem. Soc. 2020, 167, 136501. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, G.; Duan, X. Self-Assembled Three-Dimensional Graphene Macrostructures: Synthesis and Applications in Supercapacitors. Acc. Chem. Res. 2015, 48, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Brousse, T.; Bélanger, D.; Chiba, K.; Egashira, M.; Favier, F.; Long, J.; Miller, J.R.; Morita, M.; Naoi, K.; Simon, P.; et al. Materials for electrochemical capacitors. In Springer Handbook of Electrochemical Energy; Springer: Berlin/Heidelberg, Germany, 2017; pp. 495–561. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Mohanty, A.; Balasingam, S.K.; Kim, S.J.; Ramadoss, A. Comprehensive Insight into the Mechanism, Material Selection and Performance Evaluation of Supercapatteries. Nano-Micro Lett. 2020, 12, 85. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Xie, L.; Sun, G.; Su, F.; Guo, X.; Kong, Q.; Li, X.; Huang, X.; Wan, L.; Song, W.; Li, K.; et al. Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. J. Mater. Chem. A 2016, 4, 1637–1646. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon N. Y. 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Wu, Z.S.; Zhou, G.; Yin, L.C.; Ren, W.; Li, F.; Cheng, H.M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef]
- Kakaei, K.; Esrafili, M.D.; Ehsani, A. Graphene-Based Electrochemical Supercapacitors. Interface Sci. Technol. 2019, 27, 339–386. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murali, S.; Stoller, M.D.; Ruoff, R.S. Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials.pdf. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Xia, J.; Chen, F.; Li, J.; Tao, N. Measurement of the quantum capacitance of graphene. Nat. Nanotechnol. 2009, 4, 505–509. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.G.; Tan, J.; Wu, Z.S.; Gentle, I.; Lu, G.Q.; Cheng, H.M. Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.; Han, B.; Wei, Z. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef]
- He, Y.S.; Bai, D.W.; Yang, X.; Chen, J.; Liao, X.Z.; Ma, Z.F. A Co(OH)2-graphene nanosheets composite as a high performance anode material for rechargeable lithium batteries. Electrochem. Commun. 2010, 12, 570–573. [Google Scholar] [CrossRef]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H. Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 2010, 132, 7472–7477. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yan, J.; Zhi, L.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.; Qian, W.; Wei, F. A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.K.; Jin, M.; Ra, E.J.; Sheem, K.Y.; Han, G.H.; Arepalli, S.; Lee, Y.H. Enhanced electric double layer capacitance of graphite oxide intercalated by poly(sodium 4-styrensulfonate) with high cycle stability. ACS Nano 2010, 4, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Robinson, J.T.; Diankov, G.; Dai, H. Supporting Information Nanocrystal Growth on Graphene with Various Degrees of Oxidation. J. Am. Chem. Soc. 2010, 132, 3270–3271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Hu, Y.; Cheng, H.; Hu, C.; Jiang, C.; Jiang, L.; Cao, A.; Qu, L. Highly Compression-Tolerant Supercapacitor Based on Polypyrrole-mediated Graphene Foam Electrodes. Adv. Mater. 2013, 25, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Alabadi, A.; Razzaque, S.; Dong, Z.; Wang, W.; Tan, B. Graphene oxide-polythiophene derivative hybrid nanosheet for enhancing performance of supercapacitor. J. Power Sources 2016, 306, 241–247. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Huang, M.; Kang, L.; Lei, Z.; Xu, H.; Shi, F.; Liu, Z.-H. Reduced graphene oxide/Mn3O4 nanocrystals hybrid fiber for flexible all-solid-state supercapacitor with excellent volumetric energy density. Electrochim. Acta 2017, 242, 10–18. [Google Scholar] [CrossRef]
- Liao, Q.; Li, N.; Jin, S.; Yang, G.; Wang, C. All-Solid-State Symmetric Supercapacitor Based on Co3O4 Nanoparticles on Vertically Aligned Graphene. ACS Nano 2015, 9, 5310–5317. [Google Scholar] [CrossRef]
- Velmurugan, V.; Srinivasarao, U.; Ramachandran, R.; Saranya, M.; Grace, A.N. Synthesis of tin oxide/graphene (SnO2/G) nanocomposite and its electrochemical properties for supercapacitor applications. Mater. Res. Bull. 2016, 84, 145–151. [Google Scholar] [CrossRef]
- Balamurugan, J.; Karthikeyan, G.; Thanh, T.D.; Kim, N.H.; Lee, J.H. Facile synthesis of vanadium nitride/nitrogen-doped graphene composite as stable high performance anode materials for supercapacitors. J. Power Sources 2016, 308, 149–157. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, W.; Shen, Z.; Zheng, Q.; Wu, H.; Wang, X.; Song, W.; Ding, K. A novel Ni3N/graphene nanocomposite as supercapacitor electrode material with high capacitance and energy density. J. Mater. Chem. A 2015, 3, 16633–16641. [Google Scholar] [CrossRef]
- Venkateshalu, S.; Goban Kumar, P.; Kollu, P.; Jeong, S.K.; Grace, A.N. Solvothermal synthesis and electrochemical properties of phase pure pyrite FeS2 for supercapacitor applications. Electrochim. Acta 2018, 290, 378–389. [Google Scholar] [CrossRef]
- Zhou, R.; Han, C.; Wang, X. Hierarchical MoS2-coated three-dimensional graphene network for enhanced supercapacitor performances. J. Power Sources 2017, 352, 99–110. [Google Scholar] [CrossRef]
- Lee, I.; Jeong, G.H.; An, S.; Kim, S.-W.; Yoon, S. Facile synthesis of 3D MnNi-layered double hydroxides (LDH)/graphene composites from directly graphites for pseudocapacitor and their electrochemical analysis. Appl. Surf. Sci. 2018, 429, 196–202. [Google Scholar] [CrossRef]
- Qin, K.; Wang, L.; Wen, S.; Diao, L.; Liu, P.; Li, J.; Ma, L.; Shi, C.; Zhong, C.; Hu, W.; et al. Designed synthesis of NiCo-LDH and derived sulfide on heteroatom-doped edge-enriched 3D rivet graphene films for high-performance asymmetric supercapacitor and efficient OER. J. Mater. Chem. A 2018, 6, 8109–8119. [Google Scholar] [CrossRef]
- Couly, C.; Alhabeb, M.; Van Aken, K.L.; Kurra, N.; Gomes, L.; Navarro-Suárez, A.M.; Anasori, B.; Alshareef, H.N.; Gogotsi, Y. Asymmetric Flexible MXene-Reduced Graphene Oxide Micro-Supercapacitor. Adv. Electron. Mater. 2018, 4, 1700339. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xie, Z.; Wang, D.; Yuan, Y.; Kang, H.; Su, B.; Cheng, Z.; Liu, Y. Modified MXene/Holey Graphene Films for Advanced Supercapacitor Electrodes with Superior Energy Storage. Adv. Sci. 2018, 5, 1800750. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wei, T.; Shao, B.; Ma, F.; Fan, Z.; Zhang, M.; Zheng, C.; Shang, Y.; Qian, W.; Wei, F. Electrochemical properties of graphene nanosheet/carbon black composites as electrodes for supercapacitors. Carbon N. Y. 2010, 48, 1731–1737. [Google Scholar] [CrossRef]
- Lee, J.K.; Smith, K.B.; Hayner, C.M.; Kung, H.H. Silicon nanoparticles-graphene paper composites for Li ion battery anodes. Chem. Commun. 2010, 46, 2025–2027. [Google Scholar] [CrossRef]
- Chen, P.; Yang, J.J.; Li, S.S.; Wang, Z.; Xiao, T.Y.; Qian, Y.H.; Yu, S.H. Hydrothermal synthesis of macroscopic nitrogen-doped graphene hydrogels for ultrafast supercapacitor. Nano Energy 2013, 2, 249–256. [Google Scholar] [CrossRef]
- Śliwak, A.; Grzyb, B.; Díez, N.; Gryglewicz, G. Nitrogen-doped reduced graphene oxide as electrode material for high rate supercapacitors. Appl. Surf. Sci. 2017, 399, 265–271. [Google Scholar] [CrossRef]
- Wu, Z.S.; Winter, A.; Chen, L.; Sun, Y.; Turchanin, A.; Feng, X.; Müllen, K. Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 2012, 24, 5130–5135. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.B.; Hou, S.F.; Zhang, J.L.; Zhou, J.; Li, Z.; Si, W.; Zhuo, S. A facile preparation of three dimensional N, S co-doped graphene hydrogels with thiocarbohydrazide for electrode materials in supercapacitor. Mater. Lett. 2015, 147, 97–100. [Google Scholar] [CrossRef]
- An, H.; Li, Y.; Long, P.; Gao, Y.; Qin, C.; Cao, C.; Feng, Y.; Feng, W. Hydrothermal preparation of fluorinated graphene hydrogel for high-performance supercapacitors. J. Power Sources 2016, 312, 146–155. [Google Scholar] [CrossRef]
- Ates, M.; Cinar, D.; Caliskan, S.; Gecgel, U.; Uner, O.; Bayrak, Y.; Candan, I. Active carbon/graphene hydrogel nanocomposites as a symmetric device for supercapacitors. Fuller. Nanotub. Carbon Nanostructures 2016, 24, 427–434. [Google Scholar] [CrossRef]
- Manoharan, S.; Krishnamoorthy, K.; Sathyaseelan, A.; Kim, S.-J. High-power graphene supercapacitors for the effective storage of regenerative energy during the braking and deceleration process in electric vehicles. Mater. Chem. Front. 2021, 5, 6200–6211. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, G.; Gao, P.; Bi, S.; Tang, X.; Wang, D. High-performance supercapacitor of macroscopic graphene hydrogels by partial reduction and nitrogen doping of graphene oxide. Electrochim. Acta 2016, 221, 167–176. [Google Scholar] [CrossRef]
- Ni, L.; Zhang, W.; Wu, Z.; Sun, C.; Cai, Y.; Yang, G.; Chen, M.; Piao, Y.; Diao, G. Supramolecular assembled three-dimensional graphene hybrids: Synthesis and applications in supercapacitors. Appl. Surf. Sci. 2017, 396, 412–420. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Ismanto, A.E.; Wang, S.; Soetaredjo, F.E.; Ismadji, S. Preparation of capacitor’s electrode from cassava peel waste. Bioresour. Technol. 2010, 101, 3534–3540. [Google Scholar] [CrossRef] [PubMed]
- Rachiy, B.I.; Nykoliuk, M.O.; Budzulyak, I.M.; Kachmar, A.I. Ultrasonic modification of carbon materials for electrochemical capacitors. Nanoscale Res. Lett. 2017, 12, 1–5. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Li, L.; Jin, Y.; Wang, Y.; Yuan, C.; Wei, Y.; Chen, G.; Ge, J.; Lu, H. Porous carbon made from rice husk as electrode material for electrochemical double layer capacitor. Appl. Energy 2015, 153, 41–47. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Cheng, Y.; Zhong, H.; Tian, H.; Pan, J.; Pareek, V.K.; Jiang, S.P.; Lamonier, J.F.; Jaroniec, M.; Liu, J. From waste Coca Cola® to activated carbons with impressive capabilities for CO2 adsorption and supercapacitors. Carbon N. Y. 2017, 116, 490–499. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Simon, P.; Burke, A. Nanostructured carbons: Double-layer capacitance and more. Electrochem. Soc. Interface 2008, 17, 38–43. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.L.; Simon, P.; Fauvarque, J.F.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Qu, D. Studies of the activated mesocarbon microbeads used in double-layer supercapacitors. J. Power Sources 2002, 109, 403–411. [Google Scholar] [CrossRef]
- Shi, H. Activated carbons and double layer capacitance. Electrochim. Acta 1996, 41, 1633–1639. [Google Scholar] [CrossRef]
- Kim, Y.J.; Horie, Y.; Ozaki, S.; Matsuzawa, Y.; Suezaki, H.; Kim, C.; Miyashita, N.; Endo, M. Correlation between the pore and solvated ion size on capacitance uptake of PVDC-based carbons. Carbon N. Y. 2004, 42, 1491–1500. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Soavi, F. New trends in electrochemical supercapacitors. J. Power Sources 2001, 100, 164–170. [Google Scholar] [CrossRef]
- Jurewicz, K.; Vix-Guterl, C.; Frackowiak, E.; Saadallah, S.; Reda, M.; Parmentier, J.; Patarin, J.; Béguin, F. Capacitance properties of ordered porous carbon materials prepared by a templating procedure. J. Phys. Chem. Solids 2004, 65, 287–293. [Google Scholar] [CrossRef]
- Fernández, J.A.; Morishita, T.; Toyoda, M.; Inagaki, M.; Stoeckli, F.; Centeno, T.A. Performance of mesoporous carbons derived from poly(vinyl alcohol) in electrochemical capacitors. J. Power Sources 2008, 175, 675–679. [Google Scholar] [CrossRef]
- Salitra, G.; Soffer, A.; Eliad, L.; Cohen, Y.; Aurbach, D. Carbon Electrodes for Double-Layer Capacitors I. Relations Between Ion and Pore Dimensions. J. Electrochem. Soc. 2000, 147, 2486. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Edo, M.G.; Alemán, C. Powering the future: Application of cellulose-based materials for supercapacitors. Green Chem. 2016, 18, 5930–5956. [Google Scholar] [CrossRef]
- He, S.; Hu, C.; Hou, H.; Chen, W. Ultrathin MnO2 nanosheets supported on cellulose based carbon papers for high-power supercapacitors. J. Power Sources 2014, 246, 754–761. [Google Scholar] [CrossRef]
- Zheng, C.; Yue, Y.; Gan, L.; Xu, X.; Mei, C.; Han, J. Highly stretchable and self-healing strain sensors based on nanocellulose-supported graphene dispersed in electro-conductive hydrogels. Nanomaterials 2019, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, X.; Han, J.; Yu, L.; Chen, J.; Wu, Q.; Jiang, J. Effects of nanocellulose on sodium alginate/polyacrylamide hydrogel: Mechanical properties and adsorption-desorption capacities. Carbohydr. Polym. 2019, 206, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Fan, W.; Zhang, Y.; Huang, Y.; Gao, W.; Liu, T. Bacterial cellulose-based sheet-like carbon aerogels for the in situ growth of nickel sulfide as high performance electrode materials for asymmetric supercapacitors. Nanoscale 2017, 9, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Rana, H.H.; Lee, J.Y.; Park, H.S. Renewable flexible supercapacitors based on all-lignin-based hydrogel electrolytes and nanofiber electrodes. J. Mater. Chem. A 2019, 7, 16962–16968. [Google Scholar] [CrossRef]
- Herou, S.; Ribadeneyra, M.C.; Madhu, R.; Araullo-Peters, V.; Jensen, A.; Schlee, P.; Titirici, M. Ordered mesoporous carbons from lignin: A new class of biobased electrodes for supercapacitors. Green Chem. 2019, 21, 550–559. [Google Scholar] [CrossRef]
- Leguizamon, S.; Díaz-Orellana, K.P.; Velez, J.; Thies, M.C.; Roberts, M.E. High charge-capacity polymer electrodes comprising alkali lignin from the Kraft process. J. Mater. Chem. A 2015, 3, 11330–11339. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of electrochemical capacitor electrode materials: A review. Int. J. Hydrogen Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Fic, K.; Platek, A.; Piwek, J.; Frackowiak, E. Sustainable materials for electrochemical capacitors. Mater. Today 2018, 21, 437–454. [Google Scholar] [CrossRef]
- Rolison, D.R.; Long, J.W.; Lytle, J.C.; Fischer, A.E.; Rhodes, C.P.; Mc Evoy, T.M.; Bourg, M.E.; Lubers, A.M. Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem. Soc. Rev. 2009, 38, 226–252. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, M.; Jiang, Z.; Jiang, S.; Zhang, Q.; Xiong, R.; Huang, C. Green Electrospun Nanofibers and Their Application in Air Filtration. Macromol. Mater. Eng. 2018, 303, 1–18. [Google Scholar] [CrossRef]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; De Smedt, S.; Xiong, R.; Huang, C. Ecofriendly Electrospun Membranes Loaded with Visible-Light-Responding Nanoparticles for Multifunctional Usages: Highly Efficient Air Filtration, Dye Scavenging, and Bactericidal Activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef]
- Tang, G.; Xiong, R.; Lv, D.; Xu, R.X.; Braeckmans, K.; Huang, C.; De Smedt, S.C. Gas-Shearing Fabrication of Multicompartmental Microspheres: A One-Step and Oil-Free Approach. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Guo, Z.; Tang, G.; Zhou, Y.; Shuwu, L.; Hou, H.; Chen, Z.; Chen, J.; Hu, C.; Wang, F.; De Smedt, S.C.; et al. Fabrication of Sustained-release CA-PU Coaxial Electrospun Fiber Membranes for Plant Grafting Application. Carbohydr. Polym. 2017, 169, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xiong, R.; Huang, C. Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration. Carbohydr. Polym. 2019, 205, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon N. Y. 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Ersoy, D.A.; McNallan, M.J.; Gogotsi, Y. Carbon coatings produced by high temperature chlorination of silicon carbide ceramics. Mater. Res. Innov. 2001, 5, 55–62. [Google Scholar] [CrossRef]
- Gogotsi, Y.G.; Jeon, I.D.; McNallan, M.J. Carbon coatings on silicon carbide by reaction with chlorine-containing gases. J. Mater. Chem. 1997, 7, 1841–1848. [Google Scholar] [CrossRef]
- Cambaz, Z.G.; Yushin, G.N.; Gogotsi, Y.; Vyshnyakova, K.L.; Pereselentseva, L.N. Formation of carbide-derived carbon on β-silicon carbide whiskers. J. Am. Ceram. Soc. 2006, 89, 509–514. [Google Scholar] [CrossRef]
- Chmiola, J. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2014, 313, 1760–1763. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Nikitin, A.; Ye, H.; Zhou, W.; Fischer, J.E.; Yi, B.; Foley, H.C.; Barsoum, M.W. Nanoporous carbide-derived carbon with tunable pore size. Nat. Mater. 2003, 2, 591–594. [Google Scholar] [CrossRef]
- Dash, R.; Chmiola, J.; Yushin, G.; Gogotsi, Y.; Laudisio, G.; Singer, J.; Fischer, J.; Kucheyev, S. Titanium carbide derived nanoporous carbon for energy-related applications. Carbon N. Y. 2006, 44, 2489–2497. [Google Scholar] [CrossRef]
- Erdemir, A.; Kovalchenko, A.; McNallan, M.J.; Welz, S.; Lee, A.; Gogotsi, Y.; Carroll, B. Effects of high-temperature hydrogenation treatment on sliding friction and wear behavior of carbide-derived carbon films. Surf. Coatings Technol. 2004, 188–189, 588–593. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Dash, R.; Gogotsi, Y. Effect of pore size and surface area of carbide derived carbons on specific capacitance. J. Power Sources 2006, 158, 765–772. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Frackowiak, E.; Metenier, K.; Bertagna, V.; Beguin, F. Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 2421–2423. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S.; Moon, J.M.; Bae, D.J.; Lim, S.C.; Lee, Y.S.; Lee, Y.H. Electrochemical properties of high-power supercapacitors using single-walled carbon nanotube electrodes. Adv. Funtional Mater. 2001, 11, 387–392. [Google Scholar] [CrossRef]
- Emmenegger, C.; Mauron, P.; Züttel, A.; Nützenadel, C.; Schneuwly, A.; Gallay, R.; Schlapbach, L. Carbon nanotube synthesized on metallic substrates. Appl. Surf. Sci. 2000, 162, 452–456. [Google Scholar] [CrossRef]
- Chen, J.H.; Li, W.Z.; Wang, D.Z.; Yang, S.X.; Wen, J.G.; Ren, Z.F. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double-layer capacitors. Carbon N. Y. 2002, 40, 1193–1197. [Google Scholar] [CrossRef]
- Talapatra, S.; Kar, S.; Pal, S.K.; Vajti, R.; Ci, L.; Victor, P.; Shaijumon, M.M.; Kaur, S.; Nalamasu, O.; Ajayan, P.M. Direct growth of aligned carbon nanotubes on bulk metals. Nat. Nanotechnol. 2006, 1, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Delpeux, S.; Jurewicz, K.; Szostak, K.; Cazorla-Amoros, D.; Béguin, F. Enhanced capacitance of carbon nanotubes through chemical activation. Chem. Phys. Lett. 2002, 361, 35–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Yan, B. Functionalized carbon nanotubes for potential medicinal applications. Drug Discov. Today 2010, 15, 428–435. [Google Scholar] [CrossRef]
- Peng, C.; Jin, J.; Chen, G.Z. A comparative study on electrochemical co-deposition and capacitance of composite films of conducting polymers and carbon nanotubes. Electrochim. Acta 2007, 53, 525–537. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Chien, H.C.; Cheng, W.Y.; Wang, Y.H.; Lu, S.Y. Ultrahigh specific capacitances for supercapacitors achieved by nickel cobaltite/carbon aerogel composites. Adv. Funct. Mater. 2012, 22, 5038–5043. [Google Scholar] [CrossRef]
- Wang, R.; Jin, D.; Zhang, Y.; Wang, S.; Lang, J.; Yan, X.; Zhang, L. Engineering metal organic framework derived 3D nanostructures for high performance hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 292–302. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal-organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal organic frameworks for energy storage and conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Li, D.; Lawes, S.; Sun, X. Significant impact of 2D graphene nanosheets on large volume change tin-based anodes in lithium-ion batteries: A review. J. Power Sources 2015, 274, 869–884. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically Conductive Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal-Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef]
- Kaur, R.; Rana, A.; Singh, R.K.; Chhabra, V.A.; Kim, K.H.; Deep, A. Efficient photocatalytic and photovoltaic applications with nanocomposites between CdTe QDs and an NTU-9 MOF. RSC Adv. 2017, 7, 29015–29024. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, B.; Qu, C.; Chen, D.; Dang, D.; Song, B.; deGlee, B.M.; Fu, J.; Hu, C.; Wong, C.P.; et al. Controlled synthesis of three-phase NixSy/rGO nanoflake electrodes for hybrid supercapacitors with high energy and power density. Nano Energy 2017, 33, 522–531. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J.; Yamauchi, Y. New Strategies for Novel MOF-Derived Carbon Materials Based on Nanoarchitectures. Chem 2020, 6, 19–40. [Google Scholar] [CrossRef]
- Meng, F.; Fang, Z.; Li, Z.; Xu, W.; Wang, M.; Liu, Y.; Zhang, J.; Wang, W.; Zhao, D.; Guo, X. Porous Co3O4 materials prepared by solid-state thermolysis of a novel Co-MOF crystal and their superior energy storage performances for supercapacitors. J. Mater. Chem. A 2013, 1, 7235–7241. [Google Scholar] [CrossRef]
- Meng, W.; Chen, W.; Zhao, L.; Huang, Y.; Zhu, M.; Huang, Y.; Fu, Y.; Geng, F.; Yu, J.; Chen, X.; et al. Porous Fe3O4/carbon composite electrode material prepared from metal-organic framework template and effect of temperature on its capacitance. Nano Energy 2014, 8, 133–140. [Google Scholar] [CrossRef]
- Du, W.; Bai, Y.L.; Xu, J.; Zhao, H.; Zhang, L.; Li, X.; Zhang, J. Advanced metal-organic frameworks (MOFs) and their derived electrode materials for supercapacitors. J. Power Sources 2018, 402, 281–295. [Google Scholar] [CrossRef]
- Choi, K.M.; Jeong, H.M.; Park, J.H.; Zhang, Y.B.; Kang, J.K.; Yaghi, O.M. Supercapacitors of nanocrystalline metal-organic frameworks. ACS Nano 2014, 8, 7451–7457. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Jiao, Y.; Zhao, B.; Chen, D.; Zou, R.; Walton, K.S.; Liu, M. Nickel-based pillared MOFs for high-performance supercapacitors: Design, synthesis and stability study. Nano Energy 2016, 26, 66–73. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.L.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Energy Applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Ren, L.; Piao, Q.; Zhong, J.; Wang, Y.; Li, H.; Chen, Y.; Wang, B. Flexible solid-state supercapacitor based on a metal-organic framework interwoven by electrochemically-deposited PANI. J. Am. Chem. Soc. 2015, 137, 4920–4923. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, N.; Chen, W.; Wang, X.; Sun, C.; Su, Z. Supercapacitor with high cycling stability through electrochemical deposition of metal-organic frameworks/polypyrrole positive electrode. Dalt. Trans. 2018, 47, 13472–13478. [Google Scholar] [CrossRef]
- Dang, S.; Zhu, Q.L.; Xu, Q. Nanomaterials derived from metal-organic frameworks. Nat. Rev. Mater. 2017, 3. [Google Scholar] [CrossRef]
- Nai, J.; Lou, X.W. Hollow Structures Based on Prussian Blue and Its Analogs for Electrochemical Energy Storage and Conversion. Adv. Mater. 2019, 31, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Guan, B.; Xia, B.; Lou, X.W. (David) Designed Formation of Co3O4/NiCo2O4 Double-Shelled Nanocages with Enhanced Pseudocapacitive and Electrocatalytic Properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Guo, Y.; Ying, Y.; Peng, X. Flexible and Binder-Free Hierarchical Porous Carbon Film for Supercapacitor Electrodes Derived from MOFs/CNT. ACS Appl. Mater. Interfaces 2017, 9, 14043–14050. [Google Scholar] [CrossRef]
- Qu, C.; Zhao, B.; Jiao, Y.; Chen, D.; Dai, S.; deglee, B.M.; Chen, Y.; Walton, K.S.; Zou, R.; Liu, M. Functionalized Bimetallic Hydroxides Derived from Metal–Organic Frameworks for High-Performance Hybrid Supercapacitor with Exceptional Cycling Stability. ACS Energy Lett. 2017, 2, 1263–1269. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Bhardwaj, N.; Kaur, R.; Mehta, J.; Sharma, A.L.; Kim, K.H.; Deep, A. An overview of different strategies to introduce conductivity in metal-organic frameworks and miscellaneous applications thereof. J. Mater. Chem. A 2018, 6, 14992–15009. [Google Scholar] [CrossRef]
- Young, C.; Kim, J.; Kaneti, Y.V.; Yamauchi, Y. One-Step Synthetic Strategy of Hybrid Materials from Bimetallic Metal-Organic Frameworks for Supercapacitor Applications. ACS Appl. Energy Mater. 2018, 1, 2007–2015. [Google Scholar] [CrossRef]
- Chen, C.; Wu, M.-K.; Tao, K.; Zhou, J.-J.; Li, Y.-L.; Han, X.; Han, L. Formation of bimetallic metal–organic framework nanosheets and their derived porous nickel–cobalt sulfides for supercapacitors. Dalt. Trans. 2018, 47, 5639–5645. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Chen, Z.; Yu, C.; Song, X.; Zhong, W. Bimetallic-organic coordination polymers to prepare N-doped hierarchical porous carbon for high performance supercapacitors. Prog. Nat. Sci. Mater. Int. 2019, 29, 495–503. [Google Scholar] [CrossRef]
- Shinde, P.A.; Khan, M.F.; Rehman, M.A.; Jung, E.; Pham, Q.N.; Won, Y.; Jun, S.C. Nitrogen-doped carbon integrated nickel–cobalt metal phosphide marigold flowers as a high capacity electrode for hybrid supercapacitors. CrystEngComm 2020, 22, 6360–6370. [Google Scholar] [CrossRef]
- Huang, S.; Shi, X.R.; Sun, C.; Duan, Z.; Ma, P.; Xu, S. The application of metal–organic frameworks and their derivatives for supercapacitors. Nanomaterials 2020, 10, 2268. [Google Scholar] [CrossRef]
- Tan, L.; Guo, D.; Chu, D.; Yu, J.; Zhang, L.; Yu, J.; Wang, J. Metal organic frameworks template-directed fabrication of hollow nickel cobalt selenides with pentagonal structure for high-performance supercapacitors. J. Electroanal. Chem. 2019, 851, 113469. [Google Scholar] [CrossRef]
- Wang, X.; Yang, N.; Li, Q.; He, F.; Yang, Y.; Cong, S.; Li, K.; Xiong, S.; Zhou, A. Hydrothermal Synthesis of Humate-Layer-Based Bimetal Organic Framework Composites as High Rate-Capability and Enery-Density Electrode Materials for Supercapacitors. ChemistrySelect 2020, 5, 2794–2804. [Google Scholar] [CrossRef]
- Rajak, R.; Saraf, M.; Mobin, S.M. Robust heterostructures of a bimetallic sodium-zinc metal-organic framework and reduced graphene oxide for high-performance supercapacitors. J. Mater. Chem. A 2019, 7, 1725–1736. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, C.; Xiong, P.; Li, Y.; Wei, M. Zn-doped Ni-MOF material with a high supercapacitive performance. J. Mater. Chem. A 2014, 2, 19005–19010. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Shao, Z.; Jiang, S.P. Metal-organic frameworks derived porous carbon, metal oxides and metal sulfides-based compounds for supercapacitors application. Energy Storage Mater. 2020, 26, 1–22. [Google Scholar] [CrossRef]
- Gholipour-Ranjbar, H.; Soleimani, M.; Naderi, H.R. Application of Ni/Co-based metal-organic frameworks (MOFs) as an advanced electrode material for supercapacitors. New J. Chem. 2016, 40, 9187–9193. [Google Scholar] [CrossRef]
- Liu, X.; Zang, W.; Guan, C.; Zhang, L.; Qian, Y.; Elshahawy, A.M.; Zhao, D.; Pennycook, S.J.; Wang, J. Ni-Doped Cobalt-Cobalt Nitride Heterostructure Arrays for High-Power Supercapacitors. ACS Energy Lett. 2018, 3, 2462–2469. [Google Scholar] [CrossRef]
- Tao, K.; Han, X.; Cheng, Q.; Yang, Y.; Yang, Z.; Ma, Q.; Han, L. A Zinc Cobalt Sulfide Nanosheet Array Derived from a 2D Bimetallic Metal–Organic Frameworks for High-Performance Supercapacitors. Chem.-A Eur. J. 2018, 24, 12584–12591. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Li, F.; Song, X.; Ma, H.; Tan, L.; Pang, H.; Wang, X.; Guo, D.; Xiao, B. A novel dual-tasking hollow cube NiFe2O4-NiCo-LDH@rGO hierarchical material for high preformance supercapacitor and glucose sensor. J. Colloid Interface Sci. 2020, 568, 130–138. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.; Yu, L.; Zhang, Y.; Hou, D.; Li, T. Facile synthesis of NiMn2O4 nanosheet arrays grown on nickel foam as novel electrode materials for high-performance supercapacitors. Ceram. Int. 2016, 42, 14963–14969. [Google Scholar] [CrossRef]
- Gao, Y.; Mi, L.; Wei, W.; Cui, S.; Zheng, Z.; Hou, H.; Chen, W. Double metal ions synergistic effect in hierarchical multiple sulfide microflowers for enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 2015, 7, 4311–4319. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Hua, H.; Bao, R.; Chen, Z.; Yang, C.; Zhu, S.; Pang, G.; Tong, L.; Yuan, C.; Zhang, X. Anion-Exchange Formation of Hollow NiCo2S4 Nanoboxes from Mesocrystalline Nickel Cobalt Carbonate Nanocubes towards Enhanced Pseudocapacitive Properties. Chempluschem 2016, 81, 557–563. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, X.; Yang, X.; Duan, L.; Zhao, L. Synthesis of a MnS/NixSy composite with nanoparticles coated on hexagonal sheet structures as an advanced electrode material for asymmetric supercapacitors. RSC Adv. 2018, 8, 17754–17763. [Google Scholar] [CrossRef]
- Hou, S.; Xu, X.; Wang, M.; Xu, Y.; Lu, T.; Yao, Y.; Pan, L. Carbon-incorporated Janus-type Ni2P/Ni hollow spheres for high performance hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 19054–19061. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, H.; Zhao, P.; Hou, H.; Guo, L. Acetylene black enhancing the electrochemical performance of NiCo-MOF nanosheets for supercapacitor electrodes. Appl. Surf. Sci. 2019, 492, 455–463. [Google Scholar] [CrossRef]
- Ates, M. Graphene and its nanocomposites used as an active materials for supercapacitors. J. Solid State Electrochem. 2016, 20, 1509–1526. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Fan, Z.; Qian, W.; Zhang, M.; Shen, X.; Wei, F. Preparation of graphene nanosheet/carbon nanotube/polyaniline composite as electrode material for supercapacitors. J. Power Sources 2010, 195, 3041–3045. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, D.; Hu, N.; Yang, C.; Li, M.; Wei, H.; Yang, Z.; Su, Y.; Zhang, Y. Three-dimensional structures of graphene/polyaniline hybrid films constructed by steamed water for high-performance supercapacitors. J. Power Sources 2017, 342, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, X.; Jiang, J.; Yan, M.; Shi, W. Nitrogen and carbon co-doped Ni-TiO2 spindles for high performance electrochemical capacitor electrodes. Appl. Surf. Sci. 2017, 396, 774–779. [Google Scholar] [CrossRef]
- Jung, M.-J.; Kim, Y.; Lee, Y.-S. Enhancement of the electrochemical capacitance of TiOF2 obtained via control of the crystal structure. J. Ind. Eng. Chem. 2016, 47. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, L.B.; Liu, M.C.; Dai, Y.H.; Yan, K.; Hu, B.; Luo, Y.C.; Kang, L. Design and preparation of MoO2/MoS2 as negative electrode materials for supercapacitors. Mater. Des. 2016, 112, 88–96. [Google Scholar] [CrossRef]
- Gigot, A.; Fontana, M.; Serrapede, M.; Castellino, M.; Bianco, S.; Armandi, M.; Bonelli, B.; Pirri, C.F.; Tresso, E.; Rivolo, P. Mixed 1T-2H Phase MoS2/Reduced Graphene Oxide as Active Electrode for Enhanced Supercapacitive Performance. ACS Appl. Mater. Interfaces 2016, 8, 32842–32852. [Google Scholar] [CrossRef]
- Tu, C.C.; Lin, L.Y.; Xiao, B.C.; Chen, Y.S. Highly efficient supercapacitor electrode with two-dimensional tungsten disulfide and reduced graphene oxide hybrid nanosheets. J. Power Sources 2016, 320, 78–85. [Google Scholar] [CrossRef]
- Ratha, S.; Rout, C.S. Supercapacitor electrodes based on layered tungsten disulfide-reduced graphene oxide hybrids synthesized by a facile hydrothermal method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef]
- Hu, B.; Qin, X.; Asiri, A.M.; Alamry, K.A.; Al-Youbi, A.O.; Sun, X. WS2 nanoparticles-encapsulated amorphous carbon tubes: A novel electrode material for supercapacitors with a high rate capability. Electrochem. Commun. 2013, 28, 75–78. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Moo, J.G.S.; Khezri, B.; Song, P.; Fisher, A.C.; Sofer, Z.; Pumera, M. Self-Propelled Supercapacitors for On-Demand Circuit Configuration Based on WS2 Nanoparticles Micromachines. Adv. Funct. Mater. 2016, 26, 6662–6667. [Google Scholar] [CrossRef]
- Jing, C.; Song, X.; Li, K.; Zhang, Y.; Liu, X.; Dong, B.; Dong, F.; Zhao, S.; Yao, H.; Zhang, Y. Optimizing the rate capability of nickel cobalt phosphide nanowires on graphene oxide by the outer/inter-component synergistic effects. J. Mater. Chem. A 2020, 8, 1697–1708. [Google Scholar] [CrossRef]
- Jing, C.; Liu, X.D.; Li, K.; Liu, X.; Dong, B.; Dong, F.; Zhang, Y. The pseudocapacitance mechanism of graphene/CoAl LDH and its derivatives: Are all the modifications beneficial? J. Energy Chem. 2021, 52, 218–227. [Google Scholar] [CrossRef]
- Li, X.; Du, D.; Zhang, Y.; Xing, W.; Xue, Q.; Yan, Z. Layered double hydroxides toward high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 15460–15485. [Google Scholar] [CrossRef]
- Jing, C.; Dong, B.; Zhang, Y. Chemical Modifications of Layered Double Hydroxides in the Supercapacitor. Energy Environ. Mater. 2020, 3, 346–379. [Google Scholar] [CrossRef]
- Verma, S.; Khosla, A.; Arya, S. Performance of Electrochemically Synthesized Nickel-Zinc and Nickel-Iron (Ni–Zn//Ni–Fe) Nanowires as Battery Type Supercapacitor. J. Electrochem. Soc. 2020, 167, 120527. [Google Scholar] [CrossRef]
- Feng, M.; Gu, J.; Zhang, G.C.; Xu, M.; Yu, Y.; Liu, X. Homogeneous nickel bicarbonate nanocrystals as electrode materials for high-performance asymmetric supercapacitors. J. Solid State Chem. 2019, 282, 121084. [Google Scholar] [CrossRef]
- Bivo, T.; Visible, E.; Photocatalytic, L.; Zhang, G.C.; Zhong, J.; Xu, M.; Yang, Y.; Li, Y.; Fang, Z.; Tang, S.; et al. Ternary BiVO4/NiS/Au Nanocomposites with Efficient Charge Separations for Enhanced Visible Light Photocatalytic Performance. Chem. Eng. J. 2019, 375, 122093. [Google Scholar] [CrossRef]
- Natarajan, S.; Ulaganathan, M.; Aravindan, V. Building next-generation supercapacitors with battery type Ni(OH)2. J. Mater. Chem. A 2021, 9, 15542–15585. [Google Scholar] [CrossRef]
- Cai, G.; Wang, X.; Cui, M.; Darmawan, P.; Wang, J.; Eh, A.L.; See, P. Electrochromo-supercapacitor based on direct growth of NiO nanoparticles. Nano Energy 2015, 12, 258–267. [Google Scholar] [CrossRef]
- Trung, N.B.; Van Tam, T.; Dang, D.K.; Babu, K.F.; Kim, E.J.; Kim, J.; Choi, W.M. Facile synthesis of three-dimensional graphene/nickel oxide nanoparticles composites for high performance supercapacitor electrodes. Chem. Eng. J. 2015, 264, 603–609. [Google Scholar] [CrossRef]
- Su, Y.; Xiao, K.; Li, N.; Liu, Z.; Qiao, S. Amorphous Ni(OH)2@ three-dimensional Ni core-shell nanostructures for high capacitance pseudocapacitors and asymmetric supercapacitors. J. Mater. Chem. A Mater. energy Sustain. 2014, 2, 13845–13853. [Google Scholar] [CrossRef]
- Vidhyadharan, B.; Khayyriah, N.; Zain, M.; Misnon, I.I.; Aziz, R.A.; Ismail, J.; Yusoff, M.M.; Jose, R. High performance supercapacitor electrodes from electrospun nickel oxide nanowires. J. Alloys Compd. 2014, 610, 143–150. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, P.; Zhang, H.; Yu, X.; Zhu, J.; Li, Q.; Wang, T. NiMoO4 nanowires supported on Ni foam as novel advanced electrodes for supercapacitors. J. Mater. Chem. A 2013, 1, 9024–9027. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Saeed, G.; Kim, N.H.; Hee, J. Zinc-nickel cobalt oxide@ NiMoO4 core-shell nanowire/nanosheet arrays for solid state asymmetric supercapacitors. Chem. Eng. J. 2019, 384, 123357. [Google Scholar] [CrossRef]
- Patil, U.M.; Salunkhe, R.R.; Gurav, K.V.; Lokhande, C.D. Chemically deposited nanocrystalline NiO thin films for supercapacitor application. Appl. Surf. Sci. 2008, 255, 2603–2607. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Bavani, T.; Arunachalam, P.; Lee, S.J.; Theerthagiri, J.; Madhavan, J.; Pollet, B.G.; Choi, M.Y. Nanofiber NiMoO 4/g-C3N4 Composite Electrode Materials for Redox Supercapacitor Applications. Nanomaterials 2020, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Cheng, B.; You, W.; Yu, J. Fabrication of a hierarchical NiO/C hollow sphere composite and its enhanced supercapacitor performance. Chem. Commun. 2018, 54, 3731–3734. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, J.; Zhang, L.; Cheng, B.; Yu, J. Construction of nickel cobalt sulfide nanosheet arrays on carbon cloth for performance-enhanced supercapacitor. J. Mater. Sci. Technol. 2020, 47, 113–121. [Google Scholar] [CrossRef]
- Wan, L.; He, C.; Chen, D.; Liu, J.; Zhang, Y.; Du, C.; Wan, L.; He, C.; Chen, D.; Liu, J.; et al. In situ grown NiFeP@NiCo2S4 nanosheet arrays on carbon cloth for asymmetric supercapacitors. Chem. Eng. J. 2020, 399, 125778. [Google Scholar] [CrossRef]
- Sagadevan, S.; Pal, K.; Chowdhury, Z.Z.; Foley, M. Controllable synthesis of Graphene/ZnO-nanocomposite for novel switching. J. Alloys Compd. 2017, 728, 645–654. [Google Scholar] [CrossRef]
- Fahimi, Z.; Moradlou, O. Fabrication of ZnO@C foam: A fl exible free-standing electrode for energy storage devices. Mater. Des. 2020, 189, 108525. [Google Scholar] [CrossRef]
- Rong, P.; Ren, S.; Yu, Q. Fabrications and Applications of ZnO Nanomaterials in Flexible Functional Devices-A Review. Crit. Rev. Anal. Chem. 2019, 49, 336–349. [Google Scholar] [CrossRef]
- Guo, G.; Huang, L.; Chang, Q.; Ji, L.; Liu, Y.; Xie, Y.; Shi, W.; Jia, N. Sandwiched nanoarchitecture of reduced graphene oxide/ZnO nanorods/reduced graphene oxide on flexible PET substrate for supercapacitor. Appl. Phys. Lett. 2011, 99, 2012–2015. [Google Scholar] [CrossRef]
- Delbari, S.A.; Ghadimi, L.S.; Hadi, R.; Farhoudian, S.; Nedaei, M.; Babapoor, A.; Namini, A.S.; Van Le, Q.; Shokouhimehr, M.; Asl, M.S.; et al. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloys Compd. 2021, 857, 158281. [Google Scholar] [CrossRef]
- Xian, K.; Nie, B.; Li, Z.; Gao, M.; Li, Z.; Shang, C.; Liu, Y.; Guo, Z.; Pan, H. TiO2 decorated porous carbonaceous network structures offer confinement, catalysis and thermal conductivity for effective hydrogen storage of LiBH4. Chem. Eng. J. 2021, 407, 127156. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, E.; Ma, R.; Sun, Z.; Yang, Y.; Sevilla, M.; Fuertes, A.B.; Magasinski, A.; Yushin, G. Anatase TiO2 Confined in Carbon Nanopores for High-Energy Li-Ion Hybrid Supercapacitors Operating at High Rates and Subzero Temperatures. Adv. Energy Mater. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Subramani, K.; Kowsik, S.; Sathish, M. Facile and Scalable Ultra–fine Cobalt Oxide/Reduced Graphene Oxide Nanocomposites for High Energy Asymmetric Supercapacitors†. ChemistrySelect 2016, 1, 3455–3467. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.S.; Wu, H.B.; Wang, Z.; Lou, X.W. Quasiemulsion-templated formation of α-Fe2O3 hollow spheres with enhanced lithium storage properties. J. Am. Chem. Soc. 2011, 133, 17146–17148. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-J.; Dai, Y.; Chen, W. Electrochemical Deposited Nanoflakes Co(OH)2 Porous Films for Electrochemical Capacitors. J. Chinese Chem. Soc. 2010, 57, 423–428. [Google Scholar] [CrossRef]
- Yao, B.; Huang, L.; Zhang, J.; Gao, X.; Wu, J.; Cheng, Y.; Xiao, X.; Wang, B.; Li, Y.; Zhou, J. Flexible Transparent Molybdenum Trioxide Nanopaper for Energy Storage. Adv. Mater. 2016, 28, 6353–6358. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Influence of Microstucture on the Charge Storage Properties of Chemically Synthesized Manganese Dioxide. Chem. Mater. 2002, 14, 3946–3952. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge Storage Mechanism of MnO2 Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Chang, J.-K.; Lee, M.-T.; Tsai, W.-T. In situ Mn K-edge X-ray absorption spectroscopic studies of anodically deposited manganese oxide with relevance to supercapacitor applications. J. Power Sources 2007, 166, 590–594. [Google Scholar] [CrossRef]
- Pang, S.-C.; Anderson, M.A.; Chapman, T.W. Novel Electrode Materials for Thin-Film Ultracapacitors: Comparison of Electrochemical Properties of Sol-Gel-Derived and Electrodeposited Manganese Dioxide. J. Electrochem. Soc. 2000, 147, 444. [Google Scholar] [CrossRef]
- Wang, H.W.; Hu, Z.A.; Chang, Y.Q.; Chen, Y.L.; Wu, H.Y.; Zhang, Z.Y.; Yang, Y.Y. Design and synthesis of NiCo2O4-reduced graphene oxide composites for high performance supercapacitors. J. Mater. Chem. 2011, 21, 10504–10511. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, J.; Li, C. Hierarchical porous NiCo2O4 nanowires for high-rate supercapacitors. Chem. Commun. 2012, 48, 4465–4467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, Z.; Jian, N.; Gao, M.; Hu, J.; Du, F.; Pan, H.; Liu, Y. Vanadium oxide nanoparticles supported on cubic carbon nanoboxes as highly active catalyst precursors for hydrogen storage in MgH2. J. Mater. Chem. A 2018, 6, 16177–16185. [Google Scholar] [CrossRef]
- Kate, R.S.; Khalate, S.A.; Deokate, R.J. Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: A review. J. Alloys Compd. 2018, 734, 89–111. [Google Scholar] [CrossRef]
- Yan, Y.; Li, B.; Guo, W.; Pang, H.; Xue, H. Vanadium based materials as electrode materials for high performance supercapacitors. J. Power Sources 2016, 329, 148–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kim, B.; Chung, H.; Kim, W. Fabrication and characterization of flexible and high capacitance supercapacitors based on MnO2/CNT/papers. Synth. Met. 2010, 160, 2510–2514. [Google Scholar] [CrossRef]
- Li, Z.; Mi, Y.; Liu, X.; Liu, S.; Yang, S.; Wang, J. Flexible graphene/MnO2 composite papers for supercapacitor electrodes. J. Mater. Chem. 2011, 21, 14706–14711. [Google Scholar] [CrossRef]
- Alpen, U.V.; Rabenau, A.; Talat, G.H. Ionic conductivity in Li3N single crystals. Appl. Phys. Lett. 1977, 30, 621–623. [Google Scholar] [CrossRef]
- Chen, J.G. Carbide and nitride overlayers on early transition metal surfaces: Preparation, characterization, and reactivities. Chem. Rev. 1996, 96, 1477–1498. [Google Scholar] [CrossRef]
- Balogun, M.S.; Yu, M.; Li, C.; Zhai, T.; Liu, Y.; Lu, X.; Tong, Y. Facile synthesis of titanium nitride nanowires on carbon fabric for flexible and high-rate lithium ion batteries. J. Mater. Chem. A 2014, 2, 10825–10829. [Google Scholar] [CrossRef]
- Gage, S.H.; Trewyn, B.G.; Ciobanu, C.V.; Pylypenko, S.; Richards, R.M. Synthetic advancements and catalytic applications of nickel nitride. Catal. Sci. Technol. 2016, 6, 4059–4076. [Google Scholar] [CrossRef]
- Alexander, A.M.; Hargreaves, J.S.J. Alternative catalytic materials: Carbides, nitrides, phosphides and amorphous boron alloys. Chem. Soc. Rev. 2010, 39, 4388–4401. [Google Scholar] [CrossRef]
- Liu, T.-C.; Pell, W.G.; Conway, B.E.; Roberson, S.L. Behavior of Molybdenum Nitrides as Materials for Electrochemical Capacitors: Comparison with Ruthenium Oxide. J. Electrochem. Soc. 1998, 145, 1882–1888. [Google Scholar] [CrossRef]
- Choi, D.; Blomgren, G.E.; Kumta, P.N. Fast and reversible surface redox reaction in nanocrystalline vanadium nitride supercapacitors. Adv. Mater. 2006, 18, 1178–1182. [Google Scholar] [CrossRef]
- Cheng, F.; He, C.; Shua, D.; Chen, H.; Zhang, J.; Tang, S.; Finlow, D.E. Preparation of nanocrystalline VN by the melamine reduction of V2O5 xerogel and its supercapacitive behavior. Mater. Chem. Phys. 2011, 131, 268–273. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Li, G.R.; Gao, X.P. Highly ordered TiN nanotube arrays as counter electrodes for dye-sensitized solar cells. Chem. Commun. 2009, 6720–6722. [Google Scholar] [CrossRef]
- Sun, P.; Lin, R.; Wang, Z.; Qiu, M.; Chai, Z.; Zhang, B.; Meng, H.; Tan, S.; Zhao, C.; Mai, W. Rational design of carbon shell endows TiN@C nanotube based fiber supercapacitors with significantly enhanced mechanical stability and electrochemical performance. Nano Energy 2017, 31, 432–440. [Google Scholar] [CrossRef]
- Nagae, M.; Yoshio, T.; Takemoto, Y.; Takada, J. Microstructure of a Molybdenum Nitride Layer Formed by Nitriding Molybdenum Metal. J. Am. Ceram. Soc. 2001, 84, 1175–1177. [Google Scholar] [CrossRef]
- Inumaru, K.; Nishikawa, T.; Nakamura, K.; Yamanaka, S. High-Pressure Synthesis of Superconducting Molybdenum Nitride δ-MoN by in Situ Nitridation. Chem. Mater. 2008, 20, 4756–4761. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Ma, X.-J.; Kong, L.-B.; Liu, M.-C.; Luo, Y.-C.; Kang, L. Electrochemical Performance of Pseudo-Capacitive Intermetallic Molybdenum Nitride in Acid. J. Electrochem. Soc. 2016, 163, A1300–A1305. [Google Scholar] [CrossRef]
- Liu, J.; Huang, K.; Tang, H.L.; Lei, M. Porous and single-crystalline-like molybdenum nitride nanobelts as a non-noble electrocatalyst for alkaline fuel cells and electrode materials for supercapacitors. Int. J. Hydrogen Energy 2016, 41, 996–1001. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, Z. Novel [111] oriented γ-Mo2N thin films deposited by magnetron sputtering as an anode for aqueous micro-supercapacitors. Electrochim. Acta 2017, 245. [Google Scholar] [CrossRef]
- Shah, S.I.U.; Hector, A.L.; Owen, J.R. Redox supercapacitor performance of nanocrystalline molybdenum nitrides obtained by ammonolysis of chloride- and amide-derived precursors. J. Power Sources 2014, 266, 456–463. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, F. Capacitive performance of molybdenum nitride/titanium nitride nanotube array for supercapacitor. Mater. Sci. Eng. B 2017, 215, 64–70. [Google Scholar] [CrossRef]
- Deng, C.Z.; Pynenburg, R.A.J.; Tsai, K.C. Improved Porous Mixture of Molybdenum Nitride and Tantalum Oxide as a Charge Storage Material. J. Electrochem. Soc. 1998, 145. [Google Scholar] [CrossRef]
- Hardy, G.F.; Hulm, J.K. The Superconductivity of Some Transition Metal Compounds. Phys. Rev. 1954, 93, 1004–1016. [Google Scholar] [CrossRef]
- Meyer, O.; Friedland, E.; Scheerer, B. Superconductivity of niobium nitride single crystals after implantation of carbon and nitrogen. Solid State Commun. 1981, 39, 1217–1221. [Google Scholar] [CrossRef]
- Radparvar, M. Superconducting niobium and niobium nitride processes for medium-scale integration applications. Cryogenics (Guildf.) 1995, 35, 535–540. [Google Scholar] [CrossRef]
- Gao, B.; Xiao, X.; Su, J.; Zhang, X.; Peng, X.; Fu, J.; Chu, P.K. Synthesis of mesoporous niobium nitride nanobelt arrays and their capacitive properties. Appl. Surf. Sci. 2016, 383, 57–63. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, G.; Liu, X.; Liu, F.; Xie, Y.; Yang, C.; Lin, T.; Gu, H.; Huang, F. Niobium Nitride Nb4N5 as a New High-Performance Electrode Material for Supercapacitors. Adv. Sci. 2015, 2, 1500126. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Ma, X.-J.; Kong, L.-B.; Luo, Y.-C.; Kang, L. Capacitive Intermetallic Manganese Nitride with High Volumetric Energy Densities. J. Electrochem. Soc. 2016, 163, A2830–A2834. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Ma, X.-J.; Loh, A.; Li, X.; Walsh, F.C.; Kong, L.-B. High Volumetric Energy Density Capacitors Based on New Electrode Material Lanthanum Nitride. ACS Energy Lett. 2017, 2, 336–341. [Google Scholar] [CrossRef]
- Śliwak, A.; Moyseowicz, A.; Gryglewicz, G. Hydrothermal-assisted synthesis of an iron nitride–carbon composite as a novel electrode material for supercapacitors. J. Mater. Chem. A 2017, 5, 5680–5684. [Google Scholar] [CrossRef]
- Bouhtiyya, S.; Lucio Porto, R.; Laïk, B.; Boulet, P.; Capon, F.; Pereira-Ramos, J.P.; Brousse, T.; Pierson, J.F. Application of sputtered ruthenium nitride thin films as electrode material for energy-storage devices. Scr. Mater. 2013, 68, 659–662. [Google Scholar] [CrossRef]
- Arif, M.; Sanger, A.; Singh, A. Sputter deposited chromium nitride thin electrodes for supercapacitor applications. Mater. Lett. 2018, 220, 213–217. [Google Scholar] [CrossRef]
- Choi, D.; Kumta, P.N. Synthesis, Structure, and Electrochemical Characterization of Nanocrystalline Tantalum and Tungsten Nitrides. J. Am. Ceram. Soc. 2007, 90, 3113–3120. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, Z.; Zhao, S.; Zhang, T.F.; Wang, Q.M. Enhanced capacitive property of HfN film electrode by plasma etching for supercapacitors. Mater. Lett. 2019, 235, 148–152. [Google Scholar] [CrossRef]
- Keraudy, J.; Athouël, L.; Hamon, J.; Girault, B.; Gloaguen, D.; Richard-Plouet, M.; Jouan, P.-Y. Electrochemical characteristics of NixN thin films deposited by DC and HiPIMS reactive magnetron sputtering. Thin Solid Films 2019, 669, 659–664. [Google Scholar] [CrossRef]
- Dong, X.; Yu, Y.; Zhang, Y.; Xu, Z.; Jiang, H.; Meng, C.; Huang, C. Synthesis of cobalt silicate nanosheets with mesoporous structure and high surface area as the promising electrode for high-performing hybrid supercapacitor. Electrochim. Acta 2021, 380, 138225. [Google Scholar] [CrossRef]
- Qiu, C.; Jiang, J.; Ai, L. When Layered Nickel—Cobalt Silicate Hydroxide Nanosheets Meet Carbon Nanotubes: A Synergetic Coaxial Nanocable Structure for Enhanced Electrocatalytic Water Oxidation. ACS Appl Mater Interfaces 2016, 8, 945–951. [Google Scholar] [CrossRef]
- Mueller, F.; Bresser, D.; Minderjahn, N.; Menne, S.; Winter, M.; Passerini, S. Cobalt orthosilicate as a new electrode material for secondary lithium-ion batteries. Dalton Trans. 2014, 43, 15013–15021. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Zhang, Y.; Sun, C. Cobalt silicate hierarchical hollow spheres for lithium-ion batteries. Nanotechnology 2016, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Rechberger, F.; Ilari, G.; Ma, H.; Lin, W.-I.; Niederberger, M. Amorphous cobalt silicate nanobelts@carbon composites as a stable anode material for lithium ion batteries. Chem. Sci. 2015, 6, 6908–6915. [Google Scholar] [CrossRef]
- Treacher, J.C.; Wood, S.M.; Islam, M.S.; Kendrick, E. Na2CoSiO4 as a cathode material for sodium-ion batteries: Structure, electrochemistry and diffusion pathways. Phys. Chem. Chem. Phys. 2016, 18, 32744–32752. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, Y.; Jiang, H.; Dong, X.; Meng, C. Coupled cobalt silicate nanobelt-on-nanobelt hierarchy structure with reduced graphene oxide for enhanced supercapacitive performance. J. Power Sources 2020, 448, 227407. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Jiang, H.; Li, X.; Cheng, Y.; Meng, C. Designed Mesoporous Hollow Sphere Architecture Metal (Mn, Co, Ni) Silicate: A Potential Electrode Material for Flexible All Solid-State Asymmetric Supercapacitor. Chem. Eng. J. 2021, 403, 126285. [Google Scholar] [CrossRef]
- Rong, Q.; Long, L.; Zhang, X.; Huang, Y.; Yu, H. Layered cobalt nickel silicate hollow spheres as a highly-stable supercapacitor material q. Appl. Energy 2015, 153, 63–69. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Jiang, H.; Dong, X.; Zheng, J.; Meng, C. Synthesis of Amorphous Cobalt Silicate Nanobelts@Manganese Silicate Core-shell Structures as Enhanced Electrode for High-Performance Hybrid Supercapacitors. J. Colloid Interface Sci. 2019, 561, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Angelov, S.; Zhecheva, E.; Petrov, K.; Menandjiev, D. The properties of a spinel copper cobaltite prepared at low temperatures and normal pressure. Mater. Res. Bull. 1982, 17, 235–240. [Google Scholar] [CrossRef]
- Sun, J.; Xu, C.; Chen, H. A review on the synthesis of CuCo2O4-based electrode materials and their applications in supercapacitors. J. Mater. 2020, 7, 98–126. [Google Scholar] [CrossRef]
- Boopathiraja, R.; Parthibavarman, M.; Begum, A.N. Hydrothermal induced novel CuCo2O4 electrode for high performance supercapacitor applications. Vacuum 2019, 165, 96–104. [Google Scholar] [CrossRef]
- Jin, C.; Cui, Y.; Zhang, G.; Luo, W.; Liu, Y.; Sun, Y.; Tian, Z.; Zheng, W. Synthesis of copper-cobalt hybrid oxide microflowers as electrode material for supercapacitors. Chem. Eng. J. 2018, 343, 331–339. [Google Scholar] [CrossRef]
- Pendashteh, A.; Moosavifard, S.E.; Rahmanifar, M.S.; Wang, Y.; El-kady, M.F.; Kaner, R.B.; Mousavi, M.F. Highly Ordered Mesoporous CuCo2O4 Nanowires, a Promising Solution for High-Performance Supercapacitors Highly Ordered Mesoporous CuCo2O4 Nanowires, a Promising So- lution for High-Performance Supercapacitors. Chem. Mater. 2015, 27, 3919–3926. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J. Towards greener and more sustainable batteries for electrical energy storage. Nat. Publ. Gr. 2014, 7, 19–29. [Google Scholar] [CrossRef]
- Esser, B.; Dolhem, F.; Becuwe, M.; Poizot, P.; Vlad, A.; Brandell, D. A perspective on organic electrode materials and technologies for next generation batteries. J. Power Sources 2021, 482, 228814. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, T.; Wang, Y.; Lai, W.; Pang, H.; Huang, W. A Simple Approach to Boost Capacitance: Flexible Supercapacitors Based on Manganese Oxides@MOFs via Chemically Induced In Situ Self-Transformation. Adv. Mater. 2016, 28, 5242–5258. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tang, J.; Qian, H.; Hou, S.; Bando, Y.; Pan, L.; Yamauchi, Y. Three-Dimensional Networked Metal—Organic Frameworks with Conductive Polypyrrole Tubes for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 38737–38744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Lai, W.-Y.; Pang, H.; Huang, W. Flexible supercapacitors based on paper substrates: A new paradigm for low-cost energy storage. Chem. Soc. Rev. 2015, 44, 5181–5199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, Z.; Liu, X.; Mei, P.; Yang, Y. Redox-active polymers as organic electrode materials for sustainable supercapacitors. Renew. Sustain. Energy Rev. 2021, 147, 111247. [Google Scholar] [CrossRef]
| Comparison Parameter | Battery | Supercapacitor |
|---|---|---|
| Storage mechanism | Chemical | Physical |
| Power limitations | Reaction kinetics, mass transport | Electrolyte conductivity |
| Charge rate | Kinetically limited | High |
| Energy storage | High | Limited |
| Cycle life limitations | Mechanical stability, chemical reversibility | Side reactions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajagopal, S.; Pulapparambil Vallikkattil, R.; Mohamed Ibrahim, M.; Velev, D.G. Electrode Materials for Supercapacitors in Hybrid Electric Vehicles: Challenges and Current Progress. Condens. Matter 2022, 7, 6. https://doi.org/10.3390/condmat7010006
Rajagopal S, Pulapparambil Vallikkattil R, Mohamed Ibrahim M, Velev DG. Electrode Materials for Supercapacitors in Hybrid Electric Vehicles: Challenges and Current Progress. Condensed Matter. 2022; 7(1):6. https://doi.org/10.3390/condmat7010006
Chicago/Turabian StyleRajagopal, Sivakumar, Rameez Pulapparambil Vallikkattil, M. Mohamed Ibrahim, and Dimiter Georgiev Velev. 2022. "Electrode Materials for Supercapacitors in Hybrid Electric Vehicles: Challenges and Current Progress" Condensed Matter 7, no. 1: 6. https://doi.org/10.3390/condmat7010006
APA StyleRajagopal, S., Pulapparambil Vallikkattil, R., Mohamed Ibrahim, M., & Velev, D. G. (2022). Electrode Materials for Supercapacitors in Hybrid Electric Vehicles: Challenges and Current Progress. Condensed Matter, 7(1), 6. https://doi.org/10.3390/condmat7010006







