Socially Acceptable Feed Formulations May Impact the Voluntary Feed Intake and Growth, but Not Robustness of Nile Tilapia (Oreochromis niloticus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish Husbandry
2.2.1. Growth Trial
2.2.2. Digestibility Trial
2.3. Sample Collection
2.4. Key Performance Indicators
whole-body protein, lipid or energy content) × (crude protein, crude lipid or gross energy intake−1 × ADC% of
protein, lipid, or energy)
2.5. Analytical Procedures
2.6. Reverse Transcription–Quantitative Real-Time PCR (qPCR)
2.7. Data Analysis and Statistics
3. Results
3.1. Apparent Digestibility Coefficients of Diets
3.2. Growth Performance, Feed Utilization and Somatic Indices
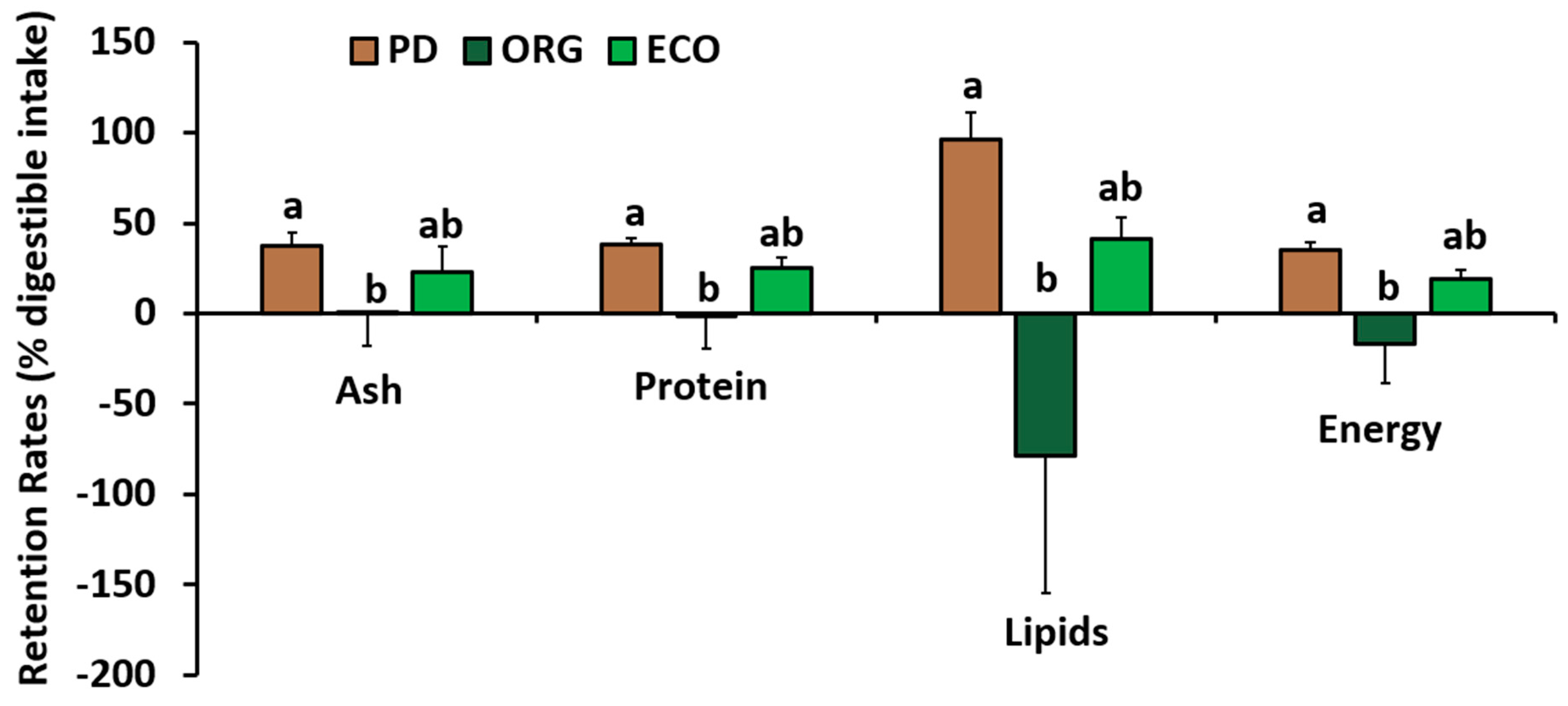
3.3. Whole Body Composition and Retention
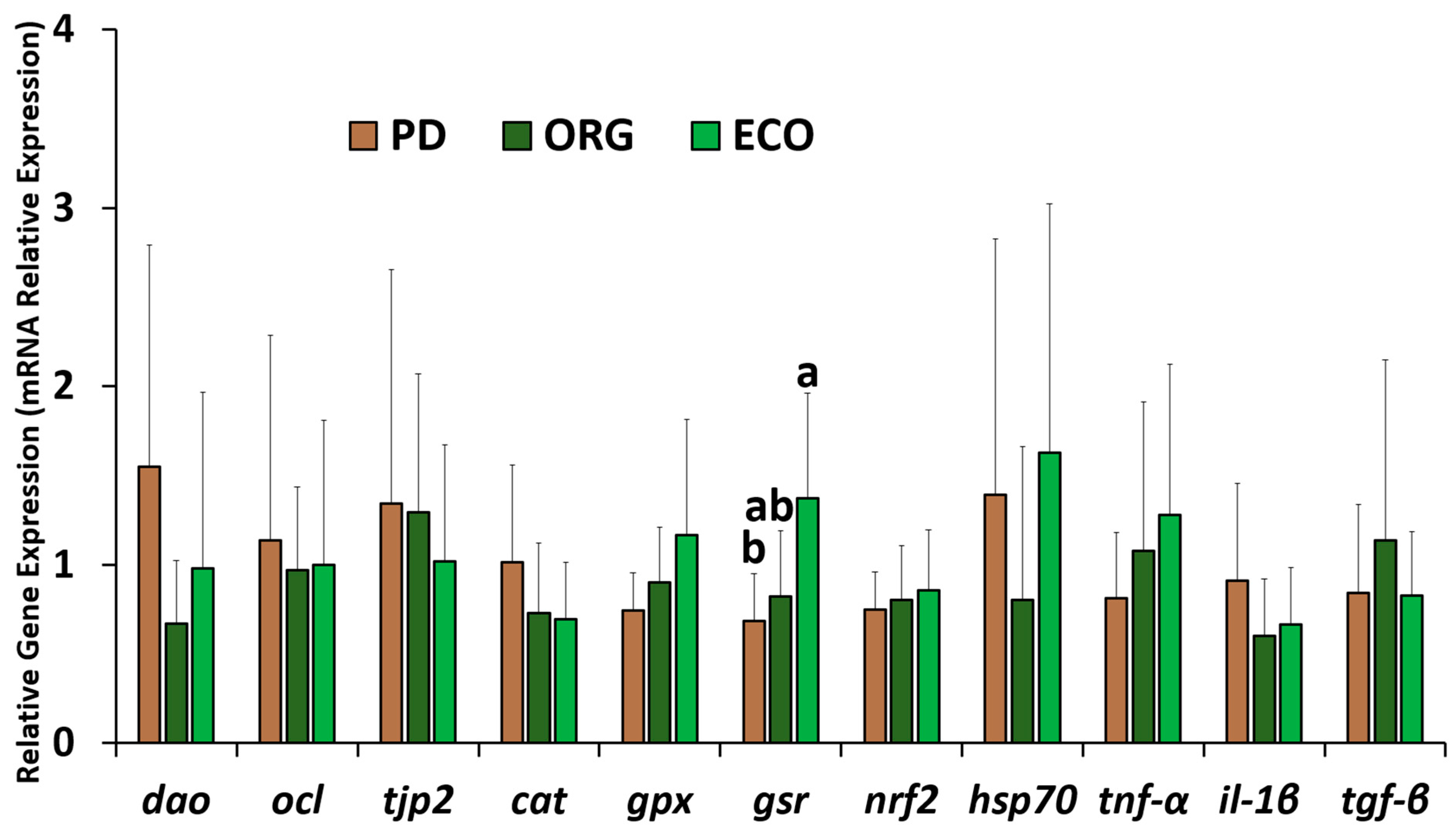
3.4. Relative Gene Expression
4. Discussion
4.1. Diet Formulation and Fish Performance
4.2. Diet Palatability
4.3. Diet Digestibility
4.4. Whole-Body Composition and Retentions
4.5. Fish Health and Somatic Indices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Roma, Italy, 2022; ISBN 978-92-5-136364-5. [Google Scholar]
- El-Sayed, A.-F.M. Tilapia Culture; Academic Press: Cambridge, MA, USA, 2019; ISBN 0-12-816541-3. [Google Scholar]
- Feucht, Y.; Zander, K. Of Earth Ponds, Flow-through and Closed Recirculation Systems—German Consumers’ Understanding of Sustainable Aquaculture and Its Communication. Aquaculture 2015, 438, 151–158. [Google Scholar] [CrossRef]
- López-Mas, L.; Claret, A.; Reinders, M.J.; Banovic, M.; Krystallis, A.; Guerrero, L. Farmed or Wild Fish? Segmenting European Consumers Based on Their Beliefs. Aquaculture 2021, 532, 735992. [Google Scholar] [CrossRef]
- Zander, K.; Risius, A.; Feucht, Y.; Janssen, M.; Hamm, U. Sustainable Aquaculture Products: Implications of Consumer Awareness and of Consumer Preferences for Promising Market Communication in Germany. J. Aquat. Food Prod. Technol. 2018, 27, 5–20. [Google Scholar] [CrossRef]
- Ellingsen, K.; Grimsrud, K.; Nielsen, H.M.; Mejdell, C.; Olesen, I.; Honkanen, P.; Navrud, S.; Gamborg, C.; Sandøe, P. Who Cares about Fish Welfare? A Norwegian Study. Br. Food J. 2015, 117, 257–273. [Google Scholar] [CrossRef]
- Reig, L.; Escobar, C.; Carrassón, M.; Constenla, M.; Gil, J.M.; Padrós, F.; Piferrer, F.; Flos, R. Aquaculture Perceptions in the Barcelona Metropolitan Area from Fish and Seafood Wholesalers, Fishmongers, and Consumers. Aquaculture 2019, 510, 256–266. [Google Scholar] [CrossRef]
- Bjørhusdal, V.; Haugen, S. Circular Economy Implications for Sustainable Supply Chain Practices: Comparative Case Study within the Norwegian Fish Farming Industry. Master’s Thesis, Høgskolen i Molde-Vitenskapelig Høgskole i Logistikk, Molde, Norway, 2023. [Google Scholar]
- Regueiro, L.; Newton, R.; Soula, M.; Méndez, D.; Kok, B.; Little, D.C.; Pastres, R.; Johansen, J.; Ferreira, M. Opportunities and Limitations for the Introduction of Circular Economy Principles in EU Aquaculture Based on the Regulatory Framework. J. Ind. Ecol. 2022, 26, 2033–2044. [Google Scholar] [CrossRef]
- Stentiford, G.; Bateman, I.; Hinchliffe, S.; Bass, D.; Hartnell, R.; Santos, E.; Devlin, M.; Feist, S.; Taylor, N.; Verner-Jeffreys, D. Sustainable Aquaculture through the One Health Lens. Nat. Food 2020, 1, 468–474. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; McKuin, B.; Fitzgerald, D.S.; Nash, H.M.; Greenwood, C. Microalgae-Blend Tilapia Feed Eliminates Fishmeal and Fish Oil, Improves Growth, and Is Cost Viable. Sci. Rep. 2020, 10, 19328. [Google Scholar] [CrossRef]
- Malcorps, W.; Kok, B.; van ‘t Land, M.; Fritz, M.; van Doren, D.; Servin, K.; van der Heijden, P.; Palmer, R.; Auchterlonie, N.A.; Rietkerk, M. The Sustainability Conundrum of Fishmeal Substitution by Plant Ingredients in Shrimp Feeds. Sustainability 2019, 11, 1212. [Google Scholar] [CrossRef]
- Newton, R.W.; Maiolo, S.; Malcorps, W.; Little, D.C. Life Cycle Inventories of Marine Ingredients. Aquaculture 2023, 565, 739096. [Google Scholar] [CrossRef]
- Chary, K.; Van Riel, A.; Muscat, A.; Wilfart, A.; Harchaoui, S.; Verdegem, M.; Filgueira, R.; Troell, M.; Henriksson, P.J.G.; De Boer, I.J.M.; et al. Transforming Sustainable Aquaculture by Applying Circularity Principles. Rev. Aquac. 2023, 16, 656–673. [Google Scholar] [CrossRef]
- Do Vale Pereira, G.; Teixeira, C.; Couto, J.; Dias, J.; Rema, P.; Gonçalves, A.T. Dietary Protein Source Affects the Interplay between Gut Microbiota and Host Performance in Nile Tilapia. In Biology and Life Sciences; MDPI: Basel, Switzerland, 2023. [Google Scholar]
- Hoerterer, C.; Petereit, J.; Lannig, G.; Johansen, J.; Conceição, L.E.C.; Buck, B.H. Effects of Dietary Plant and Animal Protein Sources and Replacement Levels on Growth and Feed Performance and Nutritional Status of Market-Sized Turbot (Scophthalmus Maximus) in RAS. Front. Mar. Sci. 2022, 9, 1023001. [Google Scholar] [CrossRef]
- Petereit, J.; Hoerterer, C.; Bischoff-Lang, A.A.; Conceição, L.E.C.; Pereira, G.; Johansen, J.; Pastres, R.; Buck, B.H. Adult European Seabass (Dicentrarchus Labrax) Perform Well on Alternative Circular-Economy-Driven Feed Formulations. Sustainability 2022, 14, 7279. [Google Scholar] [CrossRef]
- Ahmed, N.; Thompson, S.; Turchini, G.M. Organic Aquaculture Productivity, Environmental Sustainability, and Food Security: Insights from Organic Agriculture. Food Sec. 2020, 12, 1253–1267. [Google Scholar] [CrossRef]
- Cao, L.; Diana, J.S.; Keoleian, G.A. Role of Life Cycle Assessment in Sustainable Aquaculture. Rev. Aquac. 2013, 5, 61–71. [Google Scholar] [CrossRef]
- Aragão, C.; Gonçalves, A.T.; Costas, B.; Azeredo, R.; Xavier, M.J.; Engrola, S. Alternative Proteins for Fish Diets: Implications beyond Growth. Animals 2022, 12, 1211. [Google Scholar] [CrossRef]
- Vale Pereira, G.; Conceição, L.E.C.; Soares, F.; Petereit, J.; Buck, B.H.; Johansen, J.; Dias, J.; Faccenda, F. Alternative Feed Formulations Impact Growth Performance, Flesh Quality and Consumer Acceptance of Rainbow Trout (Oncorhynchus Mykiss). J. Mar. Sci. Eng. 2023, 11, 1135. [Google Scholar] [CrossRef]
- Little, D.C.; Newton, R.W.; Beveridge, M.C.M. Aquaculture: A Rapidly Growing and Significant Source of Sustainable Food? Status, Transitions and Potential. Proc. Nutr. Soc. 2016, 75, 274–286. [Google Scholar] [CrossRef]
- Newton, R.W.; Little, D.C. Mapping the Impacts of Farmed Scottish Salmon from a Life Cycle Perspective. Int. J. Life Cycle Assess 2018, 23, 1018–1029. [Google Scholar] [CrossRef]
- Han, P.; Li, J.; Zhong, H.; Xie, J.; Zhang, P.; Lu, Q.; Li, J.; Xu, P.; Chen, P.; Leng, L.; et al. Anti-Oxidation Properties and Therapeutic Potentials of Spirulina. Algal Res. 2021, 55, 102240. [Google Scholar] [CrossRef]
- Hossain, M.d.S.; Small, B.C.; Kumar, V.; Hardy, R. Utilization of Functional Feed Additives to Produce Cost-Effective, Ecofriendly Aquafeeds High in Plant-Based Ingredients. Rev. Aquac. 2024, 16, 121–153. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Schneider, R.G. The Role of Amaranth, Quinoa, and Millets for the Development of Healthy, Sustainable Food Products—A Concise Review. Foods 2022, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the Nutritional Composition of Quinoa (Chenopodium Quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.A.; Abd El-Rahman, G.I.; Behairy, A.; Beheiry, R.R.; Hendam, B.M.; Alsubaie, F.M.; Khalil, S.R. Influence of Feeding Quinoa (Chenopodium Quinoa) Seeds and Prickly Pear Fruit (Opuntia Ficus Indica) Peel on the Immune Response and Resistance to Aeromonas Sobria Infection in Nile Tilapia (Oreochromis niloticus). Animals 2020, 10, 2266. [Google Scholar] [CrossRef]
- Aragão, C.; Cabano, M.; Colen, R.; Fuentes, J.; Dias, J. Alternative Formulations for Gilthead Seabream Diets: Towards a More Sustainable Production. Aquacult. Nutr. 2020, 26, 444–455. [Google Scholar] [CrossRef]
- Tippayadara, N.; Dawood, M.A.O.; Krutmuang, P.; Hoseinifar, S.H.; Doan, H.V.; Paolucci, M. Replacement of Fish Meal by Black Soldier Fly (Hermetia Illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef]
- Velasquez, S.F.; Chan, M.A.; Abisado, R.G.; Traifalgar, R.F.M.; Tayamen, M.M.; Maliwat, G.C.F.; Ragaza, J.A. Dietary Spirulina (Arthrospira platensis) Replacement Enhances Performance of Juvenile Nile Tilapia (Oreochromis niloticus). J. Appl. Phycol. 2016, 28, 1023–1030. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, L.; Liu, W.; Yang, Y.; Du, Z.; Zhou, Z. Effects of Partially Replacing Dietary Soybean Meal or Cottonseed Meal with Completely Hydrolyzed Feather Meal (Defatted Rice Bran as the Carrier) on Production, Cytokines, Adhesive Gut Bacteria, and Disease Resistance in Hybrid Tilapia (Oreochromis niloticus ♀ × Oreochromis Aureus ♂). Fish Shellfish. Immunol. 2014, 41, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Fortes-Silva, R.; Kitagawa, A.; Sanchez Vazquez, F.J. Dietary Self-Selection in Fish: A New Approach to Studying Fish Nutrition and Feeding Behavior. Rev. Fish Biol. Fish. 2016, 26, 39–51. [Google Scholar] [CrossRef]
- Conceição, L.E.C.; Aragão, C.; Dias, J.; Costas, B.; Terova, G.; Martins, C.; Tort, L. Dietary Nitrogen and Fish Welfare. Fish Physiol. Biochem. 2012, 38, 119–141. [Google Scholar] [CrossRef]
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal Availability in the Scenarios of Climate Change: Inevitability of Fishmeal Replacement in Aquafeeds and Approaches for the Utilization of Plant Protein Sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important Antinutrients in Plant Feedstuffs for Aquaculture: An Update on Recent Findings Regarding Responses in Salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Magbanua, T.O.; Ragaza, J.A. Selected Dietary Plant-Based Proteins for Growth and Health Response of Nile Tilapia Oreochromis niloticus. Aquac. Fish. 2024, 9, 3–19. [Google Scholar] [CrossRef]
- Sallam, E.A.; Matter, A.F.; Mohammed, L.S.; Azam, A.E.; Shehab, A.; Mohamed Soliman, M. Replacing Fish Meal with Rapeseed Meal: Potential Impact on the Growth Performance, Profitability Measures, Serum Biomarkers, Antioxidant Status, Intestinal Morphometric Analysis, and Water Quality of Oreochromis niloticus and Sarotherodon Galilaeus Fingerlings. Vet. Res. Commun. 2021, 45, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, K.S.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of Farmed Fish: Its Relation to Fish Welfare and Its Utility as Welfare Indicator. Fish Physiol. Biochem. 2012, 38, 85–105. [Google Scholar] [CrossRef]
- Assan, D.; Huang, Y.; Mustapha, U.F.; Addah, M.N.; Li, G.; Chen, H. Fish Feed Intake, Feeding Behavior, and the Physiological Response of Apelin to Fasting and Refeeding. Front. Endocrinol. 2021, 12, 798903. [Google Scholar] [CrossRef]
- EC. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance; European Commission: Brussels, Belgium, 2010; Volume 276. [Google Scholar]
- Maynard, L.; Loosli, J.; Hintz, H.; Warner, R. Digestive Processes in Different Species. Anim. Nutr. 1979, 7, 21–46. [Google Scholar]
- Ricker, W.E. Handbook of Computations for Biological Statistics of Fish Populations; Fisheries Research Board of Canada: Vancouver, BC, Canada, 1958; Volume Bulletin 119. [Google Scholar]
- Association of Official Analytical Chemists Official. Methods of Analysis of AOAC International; Association of Official Analysis Chemists International: Rockville, MD, USA, 2007; Volume 1. [Google Scholar]
- ISO 27085: 2009; Animal Feeding Stuffs—Determination of Calcium, Sodium, Phosphorus, Magnesium, Potassium, Iron, Zinc, Copper, Manganese, Cobalt, Molybdenum, Arsenic, Lead and Cadmium by ICP-AES. ISO: Geneva, Switzerland, 2009.
- Brooks, S.P.J.; Oberleas, D.; Dawson, B.A.; Belonje, B.; Lampi, B.J. Proposed Phytic Acid Standard Including a Method for Its Analysis. J. AOAC Int. 2001, 84, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.A.; Valente, L.M.P.; Almeida, C.M.R. A Fast and Simple Methodology for Determination of Yttrium as an Inert Marker in Digestibility Studies. Food Chem. 2008, 108, 1094–1098. [Google Scholar] [CrossRef]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef]
- Pfaffl, M.W. Quantification Strategies in Real-Time PCR. In A–Z of Quantitative PCR; International University Line: La Jolla, CA, USA, 2004; Volume 5. [Google Scholar]
- Ennos, R. Statistical and Data Handling Skills in Biology; Pearson Education: London, UK, 2007; ISBN 0-13-195584-5. [Google Scholar]
- Zhou, Q.-C.; Yue, Y.-R. Effect of Replacing Soybean Meal with Canola Meal on Growth, Feed Utilization and Haematological Indices of Juvenile Hybrid Tilapia, Oreochromis niloticus×Oreochromis Aureus. Aquac. Res. 2010, 41, 982–990. [Google Scholar] [CrossRef]
- El-Saidy, D.M.S.D.; Gaber, M.M.A. Replacement of Fish Meal with a Mixture of Different Plant Protein Sources in Juvenile Nile Tilapia, Oreochromis niloticus (L.) Diets: Plant Protein Mixture in Nile Tilapia Diets. Aquac. Res. 2003, 34, 1119–1127. [Google Scholar] [CrossRef]
- Teodósio, R.; Engrola, S.; Colen, R.; Masagounder, K.; Aragão, C. Optimizing Diets to Decrease Environmental Impact of Nile Tilapia (Oreochromis niloticus) Production. Aquacult. Nutr. 2020, 26, 422–431. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing Fish. In Feed and Feeding Practices in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2015; pp. 203–233. ISBN 978-0-08-100506-4. [Google Scholar]
- Soltan, N.M.; Soaudy, M.R.; Abdella, M.M.; Hassaan, M.S. Partial Dietary Fishmeal Replacement with Mixture of Plant Protein Sources Supplemented with Exogenous Enzymes Modify Growth Performance, Digestibility, Intestinal Morphology, Haemato-Biochemical and Immune Responses for Nile Tilapia, Oreochromis niloticus. Anim. Feed Sci. Technol. 2023, 299, 115642. [Google Scholar] [CrossRef]
- Agbo, N.W.; Madalla, N.; Jauncey, K. Mixtures of Oilseed Meals as Dietary Protein Sources in Diets of Juvenile Nile Tilapia (Oreochromis niloticus L.). J. Sci. Technol. 2015, 35, 11–24. [Google Scholar] [CrossRef][Green Version]
- González-Félix, M.L.; Perez-Velazquez, M.; Villalba-Villalba, A.G.; Civera-Cerecedo, R.; Ezquerra, J.M.; Goytortúa-Bores, E. Tailoring a Diet for Nile Tilapia (Oreochromis niloticus) Culture in Northwest Mexico. J. Mar. Sci. Technol. 2010, 18, 674–681. [Google Scholar]
- National Research Council. Nutrient Requirements of Fish; National Academies Press: Washington, DC, USA, 1993; p. 2115. ISBN 978-0-309-04891-0. [Google Scholar]
- Christopher, R.B.; Ahilan, B.; Cheryl, A.; Samuel, M. Sunflower Meal as an Alternative Protein Source to Replace Soybean Meal in the Diet of GIFT Strain of Nile Tilapia Oreochromis niloticus. Indian J. Fish. 2020, 67, 82–88. [Google Scholar] [CrossRef]
- Desouky, A.; Hwihy, H.; Shaban, W.; Azab, A. Evaluating of Pea Peels Meal as a Fishmeal Alternative in Formulated Diet Ingredients of Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2023, 27, 725–737. [Google Scholar] [CrossRef]
- Madalla An, N.J. Effects of Soaked Pigeon Peas on the Growth of Nile Tilapia (Oreochromis niloticus L.) Fingerlings. J. Fish. Livest. Prod. 2014, 3, 1000125. [Google Scholar] [CrossRef]
- Nogales Mérida, S.; Tomás-Vidal, A.; Martínez-Llorens, S.; Jover Cerdá, M. Sunflower Meal as a Partial Substitute in Juvenile Sharpsnout Sea Bream (Diplodus Puntazzo) Diets: Amino Acid Retention, Gut and Liver Histology. Aquaculture 2010, 298, 275–281. [Google Scholar] [CrossRef]
- Ozório, R.O.A.; Portz, L.; Borghesi, R.; Cyrino, J.E.P. Effects of Dietary Yeast (Saccharomyces Cerevisia) Supplementation in Practical Diets of Tilapia (Oreochromis niloticus). Animals 2012, 2, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Pereira-da-Silva, E.; Pezzato, L. Response of Nile Tilapia (Oreochromis niloticus) to the Attraction and Palatability of the Used Ingredients in the Feeding of Fishes. Rev. Bras. Zootec. 2000, 29, 1273–1280. [Google Scholar]
- Schulz, C.; Wickert, M.; Kijora, C.; Ogunji, J.; Rennert, B. Evaluation of Pea Protein Isolate as Alternative Protein Source in Diets for Juvenile Tilapia (Oreochromis niloticus). Aquac. Res. 2007, 38, 537–545. [Google Scholar] [CrossRef]
- Alves, D.R.S.; de Oliveira, S.R.; Luczinski, T.G.; Paulo, I.G.P.; Boscolo, W.R.; Bittencourt, F.; Signor, A. Palatability of Protein Hydrolysates from Industrial Byproducts for Nile Tilapia Juveniles. Animals 2019, 9, 311. [Google Scholar] [CrossRef]
- Bertini, A.; Natale, S.; Gisbert, E.; Andrée, K.B.; Concu, D.; Dondi, F.; De Cesare, A.; Indio, V.; Gatta, P.P.; Bonaldo, A.; et al. Exploring the Application of Corynebacterium Glutamicum Single Cell Protein in the Diet of Flathead Grey Mullet (Mugil Cephalus): Effects on Growth Performance, Digestive Enzymes Activity and Gut Microbiota. Front. Mar. Sci. 2023, 10, 1172505. [Google Scholar] [CrossRef]
- Bureau, D. Feather Meal. Improving its nutrient value characterization for fish. Render 2010, 39, 14–16. [Google Scholar]
- Colombo, S.M.; Roy, K.; Mraz, J.; Wan, A.H.L.; Davies, S.J.; Tibbetts, S.M.; Øverland, M.; Francis, D.S.; Rocker, M.M.; Gasco, L.; et al. Towards Achieving Circularity and Sustainability in Feeds for Farmed Blue Foods. Rev. Aquac. 2022, 15, 1115–1141. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M.; Hasan, M.R. Feed Ingredients and Fertilizers for Farmed Aquatic Animals: Sources and Composition; Food and Agriculture Organization of the United Nations (FAO): Roma, Italy, 2009; ISBN 92-5-106421-0. [Google Scholar]
- Mendes, R.; Conceição, L.E.C.; Dias, J.; Engrola, S.; Sánchez-Vázquez, F.J. Nile Tilapia and Gilthead Seabream Dietary Self-Selection of Alternative Feeds. Fish Physiol. Biochem. 2024, 50, 1849–1860. [Google Scholar] [CrossRef]
- Abdel-Warith, A.-A.A.; Elsayed, E.A. Use of Arthrospira platensis as a Feed Additive to Improve Growth Performance, Feed Utilization, Body Composition, and Immune Response of Nile Tilapia. J. Sci. Ind. Res. 2019, 78, 681–686. [Google Scholar]
- AlMulhim, N.M.; Virk, P.; Abdelwarith, A.A.; AlKhulaifi, F.M. Effect of Incorporation of Spirulina Platensis into Fish Diets, on Growth Performance and Biochemical Composition of Nile Tilapia, Oreochromis niloticus. Egypt. J. Aquat. Res. 2023, 49, 537–541. [Google Scholar] [CrossRef]
- Al-Zayat, A.M. Effect of Various Levels of Spirulina (Arthrospira platensis) as Feed Supplement on Growth Performance, Feed Utilization, Immune Response and Hematology of the Nile Tilapia (Oreochromis niloticus) Fingerlings. Egypt. J. Aquat. Biol. Fish. 2019, 23, 361–370. [Google Scholar] [CrossRef]
- Youssef, I.M.I.; Saleh, E.S.E.; Tawfeek, S.S.; Abdel-Fadeel, A.A.A.; Abdel-Razik, A.-R.H.; Abdel-Daim, A.S.A. Effect of Spirulina Platensis on Growth, Hematological, Biochemical, and Immunological Parameters of Nile Tilapia (Oreochromis niloticus). Trop. Anim. Health Prod. 2023, 55, 275. [Google Scholar] [CrossRef]
- Clandinin, D.R. Rapeseed Oil Meal Studies. Poult. Sci. 1961, 40, 484–487. [Google Scholar] [CrossRef]
- Enami, H.R. A Review of Using Canola/Rapeseed Meal in Aquaculture Feeding. J. Fish. Aquat. Sci. 2010, 6, 22–36. [Google Scholar] [CrossRef]
- McCurdy, S.M.; March, B.E. Processing of Canola Meal for Incorporation in Trout and Salmon Diets. J. Am. Oil. Chem. Soc. 1992, 69, 213–220. [Google Scholar] [CrossRef]
- Montoya-Camacho, N.; Marquez-Ríos, E.; Castillo-Yáñez, F.J.; Cárdenas López, J.L.; López-Elías, J.A.; Ruíz-Cruz, S.; Jiménez-Ruíz, E.I.; Rivas-Vega, M.E.; Ocaño-Higuera, V.M. Advances in the Use of Alternative Protein Sources for Tilapia Feeding. Rev. Aquac. 2019, 11, 515–526. [Google Scholar] [CrossRef]
- Morais, S.; Angotzi, R.; Cerdá-Reverter, J.M.; Rosel, J.; Puchol, S. Evaluating Feed Discrimination in Seabream Sparus Aurata Using a Dualchoice Self-Feeding System. In Proceedings of the Aquaculture Europe 2019, Berlin, Germany, 7–10 October 2019. [Google Scholar]
- Puchol, S.; Leal, E.; Angotzi, R.; Rosel, J.; Morais, S.; Cerdá-Reverter, J.M. Dietary Discrimination Using a Dual-Choice Self-Feeding System in Seabream (Sparus Aurata). Aquaculture 2022, 559, 738449. [Google Scholar] [CrossRef]
- Nemati Shizari, F. Rapeseed Cake as a Feed Ingredient for Nile Tilapia: Responses to Replacing Protein from Soybean Meal with Rapeseed Cake, and Fine Milling and Autoclaving of the Rapeseed Cake. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2014. [Google Scholar]
- Piedad-Pascual, F. Handbook on Ingredients for Aquaculture Feeds; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; ISBN 0-412-62760-4. [Google Scholar]
- Trigo, M.; Rodríguez, A.; Dovale, G.; Pastén, A.; Vega-Gálvez, A.; Aubourg, S.P. The Effect of Glazing Based on Saponin-Free Quinoa (Chenopodium Quinoa) Extract on the Lipid Quality of Frozen Fatty Fish. LWT 2018, 98, 231–236. [Google Scholar] [CrossRef]
- Molina-Poveda, C.; Cárdenas, R.; Jover, M. Evaluation of Amaranth (Amaranthus Caudatus L.) and Quinoa (Chenopodium Quinoa) Protein Sources as Partial Substitutes for Fish Meal in Litopenaeus Vannamei Grow-out Diets. Aquac. Res. 2017, 48, 822–835. [Google Scholar] [CrossRef]
- Rigos, G.; Karagouni, E.; Kyriazis, I.; Athanasiou, E.; Grigorakis, K.; Kotou, E.; Katharios, P. In Vitro and in Vivo Evaluation of Quinine in Gilthead Sea Bream, Sparus Aurata Naturally Infected with the Ciliate Cryptocaryon Irritans. Aquaculture 2013, 416–417, 185–191. [Google Scholar] [CrossRef]
- Song, L.M.; Yu, Y.; Du, L.D.; Ji, X.Y.; Gao, H.; Cai, Y.Q.; Li, C.J.; Xue, P. Does Saponin in Quinoa Really Embody the Source of Its Bitterness? Food Chem. 2024, 437, 137872. [Google Scholar] [CrossRef] [PubMed]
- Timaná Morales, M.F.; Naspiran-Jojoa, D.C.; Mafla-Mejía, S.M.; Imúes-Figueroa, M.A.; Guerrero-Romero, C.L. Efecto de la inclusión de diferentes niveles de quinua (Chenopodium quinoa) sobre el crecimiento de tilapia nilotica (Oreochromis niloticus). REVIP 2022, 8, 7. [Google Scholar] [CrossRef]
- Değirmencioğlu, T. Possibility of Using Quinoa (Chenopodium Quinoa) as an Alternative Energy Source in the Goldfish (Carassius Auratus Auratus) Diet. J. Adv. Res. Nat. Appl. Sci. 2023, 9, 457–464. [Google Scholar] [CrossRef]
- Adeniji, C.A.; Fakoya, K.A.; Omamohwo, V.R. Partial Replacement of Soybean Cake with Amaranth Us Spinosus Leaf Meal in the Diet of Nile Tilapia (Oreochromis niloticus). Biol. Sci.—PJSIR 2007, 50, 335–338. [Google Scholar]
- Mhada, M.; Metougui, M.L.; El Hazzam, K.; El Kacimi, K.; Yasri, A. Variations of Saponins, Minerals and Total Phenolic Compounds Due to Processing and Cooking of Quinoa (Chenopodium Quinoa Willd.) Seeds. Foods 2020, 9, 660. [Google Scholar] [CrossRef]
- Sharma, S.; Kataria, A.; Singh, B. Effect of Thermal Processing on the Bioactive Compounds, Antioxidative, Antinutritional and Functional Characteristics of Quinoa (Chenopodium quinoa). LWT 2022, 160, 113256. [Google Scholar] [CrossRef]
- Fetahi, T.; Getahun, A. Food and Feeding Habits of Juvenile and Adult Nile Tilapia, Oreochromis niloticus (L.) (Pisces: Cichlidae) in Lake Ziway, Ethiopia. SINET Ethiop. J. Sci. 2020, 43, 25–33. [Google Scholar]
- Tefal, E.; Jauralde, I.; Tomás-Vidal, A.; Martínez-Llorens, S.; Peñaranda, D.S.; Jover-Cerdá, M. New Organic Raw Materials for Gilthead Seabream (Sparus Aurata) Feeding and the Effects on Growth, Nutritive Parameters, Digestibility, and Histology. Fishes 2023, 8, 330. [Google Scholar] [CrossRef]
- Telles, M. Efeito da Fortificação de Spirulina Maxima em Rações para Tilápia do Nilo (Oreochromis niloticus): Análise Bromatológica e Avaliação da Digestibilidade; Universidade Federal do Rio de Janeiro: Rio de Janeiro, Brazil, 2021. [Google Scholar]
- Ahmed, I. Effect of Ration Size on Growth, Body Composition, and Energy and Protein Maintenance Requirement of Fingerling Indian Major Carp, Labeo Rohita (Hamilton). Fish Physiol. Biochem. 2007, 33, 203–212. [Google Scholar] [CrossRef]
- Adebayo, O.; Balogun, A.; Fagbenro, O. Effects of Feeding Rates on Growth, Body Composition and Economic Performance of Juvenile Clariid Catfish Hybrid (Female Clarias Gariepinus× Male Heterobranchus Bidorsalis). J. Aquac. Trop. 2000, 15, 109–117. [Google Scholar]
- Gallardo-Collí, A.; Pérez-Fuentes, M.; Pérez-Rostro, C.I.; Hernández-Vergara, M.P. Compensatory Growth of Nile Tilapia Oreochromis niloticus, L. Subjected to Cyclic Periods of Feed Restriction and Feeding in a Biofloc System. Aquac. Res. 2020, 51, 1813–1823. [Google Scholar] [CrossRef]
- Marais, J.F.K.; Kissil, G.W. The Influence of Energy Level on the Feed Intake, Growth, Food Conversion and Body Composition of Sparus Aurata. Aquaculture 1979, 17, 203–219. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Yang, Y.; Cai, F. Compensatory Growth in Hybrid Tilapia, Oreochromis Mossambicus× O. Niloticus, Reared in Seawater. Aquaculture 2000, 189, 101–108. [Google Scholar] [CrossRef]
- Dong, G.; Yang, Y.; Yao, F.; Chen, L.; Yue, D.; Yu, D.; Huang, F.; Liu, J.; Liu, L. Growth Performance and Whole-body Composition of Yellow Catfish (Pelteobagrus Fulvidraco Richardson) under Feeding Restriction. Aquac. Nutr. 2017, 23, 101–110. [Google Scholar] [CrossRef]
- Lui, T.A.; Da Silva, W.P.; Nervis, J.A.L.; Brum, J.M.D.; Bittencourt, F.; Neu, D.H.; Boscolo, W.R. Food Restriction in Nile Tilapia Juveniles (Oreochromis niloticus). Span. J. Agric. Res. 2020, 18, e0607. [Google Scholar] [CrossRef]
- Rodde, C.; Vandeputte, M.; Trinh, T.Q.; Douchet, V.; Canonne, M.; Benzie, J.A.H.; de Verdal, H. The Effects of Feed Restriction and Isolated or Group Rearing on the Measurement of Individual Feed Intake and Estimation of Feed Conversion Ratio in Juvenile Nile Tilapia (Oreochromis niloticus) for Selective Breeding Purposes. Front. Genet. 2021, 11, 596521. [Google Scholar] [CrossRef]
- Sarsangi Aliabad, H.; Naji, A.; Mortezaei, S.R.S.; Sourinejad, I.; Akbarzadeh, A. Effects of Restricted Feeding Levels and Stocking Densities on Water Quality, Growth Performance, Body Composition and Mucosal Innate Immunity of Nile Tilapia (Oreochromis niloticus) Fry in a Biofloc System. Aquaculture 2022, 546, 737320. [Google Scholar] [CrossRef]
- Xiao, J.-X.; Zhou, F.; Yin, N.; Zhou, J.; Gao, S.; Li, H.; Shao, Q.-J.; Xu, J. Compensatory Growth of Juvenile Black Sea Bream, Acanthopagrus Schlegelii with Cyclical Feed Deprivation and Refeeding. Aquac. Res. 2013, 44, 1045–1057. [Google Scholar] [CrossRef]
- Dong, C.; He, G.; Mai, K.; Zhou, H.; Xu, W. Palatability of Water-Soluble Extracts of Protein Sources and Replacement of Fishmeal by a Selected Mixture of Protein Sources for Juvenile Turbot (Scophthalmus Maximus). J. Ocean Univ. China 2016, 15, 561–567. [Google Scholar] [CrossRef]
- Salze, G.; Alami-Durante, H.; Barbut, S.; Marcone, M.; Bureau, D.P. Nutrient Deposition Partitioning and Priorities between Body Compartments in Two Size Classes of Rainbow Trout in Response to Feed Restriction. Br. J. Nutr. 2014, 111, 1361–1372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Couto, N.; Wood, J.; Barber, J. The Role of Glutathione Reductase and Related Enzymes on Cellular Redox Homoeostasis Network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, N.K.M.; Eissa, I.A.M.; Ahmed, E.; Kilany, O.E.; El-Adl, M.; Dawood, M.A.O.; Hassan, A.M.; Abdel-Daim, M.M. Protective Role of Dietary Spirulina Platensis against Diazinon-Induced Oxidative Damage in Nile Tilapia; Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2017, 54, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M.; Valdivia, W.; Bustamante, L.; Graf, F.; Obal, I. Protein and Antioxidant Composition of Quinoa (Chenopodium Quinoa Willd.) Sprout from Seeds Submitted to Water Stress, Salinity and Light Conditions. Ind. Crops Prod. 2017, 107, 558–564. [Google Scholar] [CrossRef]
- Kumar, A.; Ramamoorthy, D.; Verma, D.K.; Kumar, A.; Kumar, N.; Kanak, K.R.; Marwein, B.M.; Mohan, K. Antioxidant and Phytonutrient Activities of Spirulina Platensis. Energy Nexus 2022, 6, 100070. [Google Scholar] [CrossRef]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Izewska, A.; Krosniak, M.; Gawlik, M.; Gawlik, M.; Gorinstein, S. Effect of Diet Supplemented with Quinoa Seeds on Oxidative Status in Plasma and Selected Tissues of High Fructose-Fed Rats. Plant Foods Hum. Nutr. 2010, 65, 146–151. [Google Scholar] [CrossRef]
- Kim, S.-S.; Rahimnejad, S.; Kim, K.-W.; Lee, K.-J. Partial Replacement of Fish Meal with Spirulina Pacifica in Diets for Parrot Fish (Oplegnathus Fasciatus). Turk. J. Fish. Aquat. Sci. 2013, 13, 197–204. [Google Scholar] [CrossRef]
- Rosas, V.T.; Monserrat, J.M.; Bessonart, M.; Magnone, L.; Romano, L.A.; Tesser, M.B. Fish Oil and Meal Replacement in Mullet (Mugil Liza) Diet with Spirulina (Arthrospira platensis) and Linseed Oil. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 218, 46–54. [Google Scholar] [CrossRef]
- Paredes-Trujillo, A.; Velázquez-Abunader, I.; Papiol, V.; del Rio-Rodríguez, R.E.; Vidal-Martínez, V.M. Negative Effect of Ectoparasite Burdens on the Condition Factor from Farmed Tilapia Oreochromis niloticus in the Yucatan, Mexico. Vet. Parasitol. 2021, 292, 109393. [Google Scholar] [CrossRef]
- Asmamaw, B.; Beyene, B.; Tessema, M.; Assefa, A. Length-Weight Relationships and Condition Factor of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) (Cichlidae) in Koka Reservoir, Ethiopia. Int. J. Fish. Aquat. Res. 2019, 4, 47–51. [Google Scholar]
- Ighwela, K.A.; Ahmed, A.; Abol-Munafi, A. Condition Factor as an Indicator of Growth and Feeding Intensity of Nile Tilapia Fingerlings (Oreochromis niloticus) Feed on Different Levels of Maltose. Am.-Eurasian J. Agric. Environ. Sci. 2011, 11, 559–563. [Google Scholar]
- Keyombe, J.L.; Malala, J.O.; Waithaka, E.; Lewo, R.M.; Obwanga, B.O. Seasonal Changes in Length-Weight Relationship and Condition Factor of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) (Cichlidae) in Lake Naivasha, Kenya. Int. J. Aquat. Biol. 2017, 5, 231. [Google Scholar] [CrossRef]
| Ingredients (% Inclusion Levels) | PD | ORG | ECO |
|---|---|---|---|
| 1 Poultry meal | 5.00 | 2.50 | |
| 2 Porcine blood meal | 5.00 | ||
| 3 Feathermeal hydrolysate | 5.00 | ||
| 4 Insect meal | 7.50 | ||
| 5 Microbial biomass | 5.50 | ||
| 6 Brewer’s yeast | 10.00 | 5.00 | |
| 7Spirulina | 10.00 | 2.50 | |
| 8 Soy protein concentrate | 5.00 | ||
| 9 Pea protein concentrate | 5.00 | ||
| 10 Corn gluten meal | 12.00 | ||
| 11 Soybean meal | 25.00 | 12.50 | |
| 12 Rapeseed meal | 13.00 | 26.00 | 13.00 |
| 13 Sunflower meal | 7.50 | 15.00 | 15.00 |
| 14 Wheat (whole) | 13.90 | 15.61 | |
| 15 Rice bran | 9.78 | 9.78 | |
| 16 Quinoa | 5.00 | 2.50 | |
| 17 Whole peas | 11.00 | ||
| 18 Vitamin and mineral premix | 1.00 | 1.00 | 1.00 |
| 19 Choline chloride | 0.20 | 0.20 | 0.20 |
| 20 Antioxidant powder | 0.20 | 0.20 | 0.20 |
| 21 Mono-calcium phosphate | 2.55 | 2.00 | 2.75 |
| 22 L-Lysine | 0.30 | 0.30 | |
| 23 DL-Methionine | 0.15 | 0.22 | |
| 24 Yttrium oxide | 0.02 | 0.02 | 0.02 |
| 25 Salmon oil | 2.00 | 2.00 | 2.00 |
| 26 Rapeseed oil | 2.40 | 1.30 | 3.20 |
| Proximate Composition (% as fed) | PD | ORG | ECO |
| Dry matter (DM) | 94.77 | 93.49 | 93.93 |
| Ash | 7.07 | 7.32 | 6.86 |
| Crude protein | 38.63 | 39.65 | 40.02 |
| Crude fat | 8.60 | 8.58 | 8.95 |
| Total phosphorus | 1.41 | 1.54 | 1.48 |
| Gross energy (kJ/g−1) | 19.24 | 19.14 | 19.32 |
| Amino Acids (g/100 g Fed Basis) | PD | ORG | ECO |
|---|---|---|---|
| Arginine | 2.24 | 2.57 | 2.33 |
| Histidine | 0.97 | 0.95 | 0.89 |
| Lysine | 2.03 | 2.00 | 2.12 |
| Threonine | 1.43 | 1.60 | 2.16 |
| Tryptophan | 0.42 | 0.51 | 0.46 |
| Isoleucine | 1.60 | 1.62 | 1.62 |
| Leucine | 3.54 | 2.89 | 2.93 |
| Valine | 1.88 | 1.98 | 2.13 |
| Methionine | 0.80 | 0.70 | 0.87 |
| Phenylalanine | 1.94 | 1.75 | 1.74 |
| Cysteine + Cystine | 0.66 | 0.65 | 0.70 |
| Tyrosine | 1.37 | 1.30 | 1.21 |
| Aspartic Acid | 3.36 | 3.48 | 3.08 |
| Glutamic Acid | 7.38 | 6.77 | 5.93 |
| Alanine | 2.10 | 2.02 | 2.11 |
| Glycine | 1.82 | 1.92 | 2.12 |
| Proline | 2.35 | 1.92 | 2.20 |
| Serine | 1.86 | 1.82 | 1.99 |
| Taurine | 0.02 | <0.002 | 0.01 |
| Gene | Forward Primer Sequence (5′ → 3′) | Reverse Primer Sequence (5′ → 3′) | NCBI GenBank Accession Number |
|---|---|---|---|
| dao | CAACCTTTGCAGTGAACCCG | TCACTCCCCTCTTTCGCAAC | XM_005473333 |
| ocl | TCAGATGAGCAGCGCAGAAA | TTCCAGTGCGTCCAACTCTC | XM_005476075 |
| tjp2 | GCTACATGGACTCCGGCTAC | GCGATCTGGGCTGTACTCTC | XM_025908597 |
| cat | TCCATTCCCAGAAGCGCAAT | ATTCATGTGACGGTGGCCAT | XM_019361816 |
| gpx | ACTTCCATTCCCCTGCGATG | GCTTGTAAGGTTCCCCGTCA | NM_001279711 |
| gsr | CAGCAGGAAGAGTCAGTGCA | ACCCATCTTGATGGCCACAG | XM_013271309 |
| nrf2 | TCTCAGCCCGATGACAGAGA | GTGCTGACCACTGCTCTCTT | XM_003447296 |
| hsp70 | CCAAAAGGTGTCCAACGCTG | CCCCACCCAGGTCAAAGATC | NM_001279671 |
| tnf-α | ATGGCAGAAGGATGTGGACC | GACCATGGGATGCGAAGACA | XM_013266976 |
| il-1β | CATGTCTTGCCGCATGGAAG | GTTCAACGGGCTGGTTTTCC | XM_005457887 |
| tgf-β | CACGCTGAAGGACAAATGGC | TCACAGTACCGCCGAAGTTC | NM_001311325 |
| ef1-α | TTGAGAAGGAAGCCGCTGAG | GCTGGTCTCGAACTTCCACA | AB075952 |
| Diets | ||||
|---|---|---|---|---|
| PD | ORG | ECO | p Value | |
| Dry matter (DM; %) | 66.0 ± 0.8 a | 64.0 ± 2.3 ab | 59.0 ± 4.7 b | 0.040 |
| Protein (%) | 85.3 ± 0.6 a | 81.0 ± 0.4 ab | 75.4 ± 4.5 b | 0.018 |
| Lipids (%) | 95.6 ± 0.2 a | 92.8 ± 0.8 b | 93.8 ± 1.2 b | 0.005 |
| Phosphorus (%) | 67.8 ± 2.0 | 68.8 ± 2.2 | 70.8 ± 2.6 | 0.247 |
| Energy (%) | 74.8 ± 0.9 a | 73.4 ± 1.5 ab | 67.1 ± 4.0 b | 0.043 |
| Diets | ||||
|---|---|---|---|---|
| PD | ORG | ECO | p Value | |
| FBW (g) | 107.8 ± 6.1 a | 32.7 ± 1.3 b | 62.7 ± 5.4 ab | 0.007 |
| RGR (%.day−1) | 2.3 ± 0.1 a | 0.1 ± 0.0 c | 1.3 ± 0.2 b | <0.001 |
| VFI (%ABW.day−1) | 2.1 ± 0.2 a | 0.02 ± 0.01 b | 0.9 ± 0.3 ab | 0.010 |
| FCR | 1.1 ± 0.1 b | 7.1 ± 2.3 a | 1.5 ± 0.4 ab | 0.012 |
| PER | 2.3 ± 0.2 a | 0.3 ± 0.3 c | 1.7 ± 0.4 b | <0.001 |
| VSI (%) | 7.8 ± 0.7 | 7.2 ± 0.6 | 8.1 ± 0.7 | 0.273 |
| HSI (%) | 1.8 ± 0.1 | 1.0 ± 0.2 | 1.2 ± 0.5 | 0.092 |
| K | 1.9 ± 0.1 a | 1.7 ± 0.2 b | 1.8 ± 0.0 ab | 0.014 |
| Diets | |||||
|---|---|---|---|---|---|
| (% WW) | Initial | PD | ORG | ECO | p Value |
| Dry matter (DM; %) | 26.0 ± 0.8 | 29.8 ± 1.6 a | 22.0 ± 1.6 b | 25.3 ± 1.9 b | <0.001 |
| Protein (%) | 14.8 ± 0.3 | 16.7 ± 1.1 a | 13.8 ± 0.5 b | 15.0 ± 0.8 b | 0.003 |
| Lipid (%) | 6.3 ± 0.2 | 9.2 ± 1.4 a | 3.1 ± 1.0 c | 5.9 ± 0.6 b | <0.001 |
| Ash (%) | 4.1 ± 0.3 | 3.4 ± 0.3 | 3.9 ± 0.3 | 3.2 ± 0.7 | 0.174 |
| Energy (kJ/g) | 6.1 ± 0.1 | 7.3 ± 0.3 a | 4.4 ± 0.4 c | 5.8 ± 0.4 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, R.; Rema, P.; Dias, J.; Gonçalves, A.T.; Teodósio, R.; Engrola, S.; Sánchez-Vázquez, F.J.; Conceição, L.E.C. Socially Acceptable Feed Formulations May Impact the Voluntary Feed Intake and Growth, but Not Robustness of Nile Tilapia (Oreochromis niloticus). Fishes 2024, 9, 361. https://doi.org/10.3390/fishes9090361
Mendes R, Rema P, Dias J, Gonçalves AT, Teodósio R, Engrola S, Sánchez-Vázquez FJ, Conceição LEC. Socially Acceptable Feed Formulations May Impact the Voluntary Feed Intake and Growth, but Not Robustness of Nile Tilapia (Oreochromis niloticus). Fishes. 2024; 9(9):361. https://doi.org/10.3390/fishes9090361
Chicago/Turabian StyleMendes, Rodrigo, Paulo Rema, Jorge Dias, Ana Teresa Gonçalves, Rita Teodósio, Sofia Engrola, Francisco J. Sánchez-Vázquez, and Luís E. C. Conceição. 2024. "Socially Acceptable Feed Formulations May Impact the Voluntary Feed Intake and Growth, but Not Robustness of Nile Tilapia (Oreochromis niloticus)" Fishes 9, no. 9: 361. https://doi.org/10.3390/fishes9090361
APA StyleMendes, R., Rema, P., Dias, J., Gonçalves, A. T., Teodósio, R., Engrola, S., Sánchez-Vázquez, F. J., & Conceição, L. E. C. (2024). Socially Acceptable Feed Formulations May Impact the Voluntary Feed Intake and Growth, but Not Robustness of Nile Tilapia (Oreochromis niloticus). Fishes, 9(9), 361. https://doi.org/10.3390/fishes9090361








