1. Introduction
In 2022, the total world aquaculture production has reached approximately 130.9 million tons, a historically high production, which accounts for 59% of the global fishery and aquaculture production. Even for aquatic animals alone, the aquaculture production surpassed the capture fishery for the first time [
1]. However, with rapid aquaculture growth, the gap in aquafeed ingredients, especially fishmeal and fish oil, is widening. Meeting the demand for aquafeed ingredients is becoming a big challenge, threatening the sustainability of aquaculture development. Therefore, exploring new ingredients is an urgent and important task.
Plant-based protein sources like soybean meal, rapeseed meal, peanut meal, cottonseed meal, corn gluten meal, and wheat flour are commonly used substitutes for fishmeal in the aquafeeds due to their wide availability, reasonable price, and stable supply [
2,
3,
4]. However, these ingredients often have big defects such as amino acid imbalance and anti-nutritional factors, hindering the nutrient digestion or absorption in aquatic animals and thus resulting in low utilization for many farmed species, especially carnivorous ones [
5,
6,
7,
8,
9,
10,
11,
12]. Animal processing by-products, like poultry by-product meal and meat and bone meal, have also been used in fish feeds, but they also have imbalanced amino acids and unstable nutrition compositions, which are easily affected by factors such as raw material type, processing method, and freshness [
13,
14,
15,
16]. Some other more novel feed raw materials, such as insect, algae, and microbial protein, although having been demonstrated to be highly effective, are still limited by small-scale production and high costs [
17,
18,
19,
20].
Marine polychaetes, represented by nereis, are natural feeds for aquatic animals. They possess excellent palatability and balanced nutritional compositions [
21,
22]. Some species contain up to 55–60% protein (on a dry matter basis) and 12–20% lipids (on a dry matter basis), along with high levels of polyunsaturated fatty acids (PUFA) [
23,
24,
25]. Polychaetes can serve as superior sources of protein and lipids in the feeds of fish and crustaceans [
26,
27], particularly at certain life stages such as broodstock and larval stages [
28,
29]. Polychaetes can be produced as by-products of multi-level integrated aquaculture. Typically, in pond aquaculture, the excessive feeds and feces of farmed aquatic animals (such as fish or shrimp) deposit at the bottom of the pond, which nourishes the polychaetes [
30]. In this model, along with the production of polychaetes, the excess organic matter in the entire aquaculture water environment can be removed and thus the influence of aquaculture activities on the environment can be reduced [
31,
32,
33,
34,
35,
36]. Therefore, developing polychaetes as substitutes for fishmeal in aqua-feeds also has considerable environmental benefits. However, to date, very few studies have investigated the comprehensive use of polychaetes in aquaculture ponds.
Tiger puffer
Takifugu rubripes is an important marine carnivorous fish species in Asia. The consumption of tiger puffer had been restricted due to the existence of tetrodotoxin. However, nowadays, the tetrodotoxin is no longer detectable in most farmed tiger puffer, accelerating the farming of this species [
37,
38,
39,
40]. Despite the fact that
Neanthes japonica is a by-product of pond aquaculture, there is currently no mechanized and low-cost harvesting technique available. The costs, mainly labor, during the harvesting process remain relatively high. This experiment aimed to investigate the potential functional effects of
N. japonica as a minor ingredient in juvenile tiger puffer; for instance, the feeding attracting function. Different drying methods were also compared, in order to evaluate the potential effects of drying temperature on biological efficacy of
N. japonica. Results of this study may help motivate the exploration of
N. japonica resources in aquaculture ponds.
2. Materials and Methods
2.1. Study Site
The rearing experiment was carried out in an indoor facility in at Huanghai Aquatic Products Co., Ltd. (Yantai, Shandong Province, China; 36°41′11.6″ N and 121°7′5.8″ E). The experimental duration was 56 days. The analysis experiment was carried out at the Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences.
2.2. Formulation and Preparation of Experimental Diets
The
N. japonica used in this experiment were collected from white shrimp
Litopenaeus vannamei farming ponds in Jimo (Qingdao, China). After the harvest of shrimp, the experimental
N. japonica was collected (on 4 December 2022) by draining water during the night and capturing them using nets at the drainage outlet. The collected
N. japonica were maintained in water, sealed, and frozen at −20 °C in a refrigerator. In May 2023, the samples were thawed, rinsed with freshwater, and subsequently placed in a vacuum freeze dryer (FDU-1100, EYELA, Tokyo, Japan) for freeze-drying. After being ground with a small grinder, they were vacuum-sealed as freeze-dried
N. japonica meal (FNM). Another part of the samples was dried in an electrothermal blast drying oven (Shanghai Yiheng Scientific Instrument Co., Ltd., Shanghai, China) at 105 °C until a constant weight was achieved, in order to prepare the oven-dried
N. japonica meal (ONM). The proximate compositions of the experimental
N. japonica, fishmeal, FNM, and ONM are presented in
Table 1.
The control experimental diet (CON) contained 40% fish meal (of dry matter). The treatment diets were obtained by adding different levels (3%, 6%, and 9%) of FNM or 6% ONM to CON. The treatment diets were named FNM3, FNM6, FNM9, and ONM6, respectively. Feed ingredients were sieved through 80 mesh, and then evenly mixed. Water was then added at 30%. Pellets with a diameter of 2.5 mm were prepared using a laboratory-level pelleter, and then dried in a 55 °C oven to a moisture content of about 6%. After drying and cooling, the pellets were packaged and sealed with double-layer plastic bags. The prepared diets were stored in a cold storage room at −20 °C until they were used. The formulation and proximate composition of the experimental feeds are presented in
Table 2, and the fatty acid, total amino acid, and free amino acid compositions of the experimental feeds are depicted in
Table 3,
Table 4 and
Table 5, respectively.
2.3. Experiment Fish and Feeding Management
The juvenile tiger puffer used in this experiment were all procured from Tangshan Haidu Seafood Food Co., Ltd. (Tangshan, China). The juvenile fish were transported to Haiyang Huanghai Aquatic Products Co., Ltd. (Haiyang, China) at 8:00 a.m. on 8 June 2023. Prior to the initiation of the rearing experiment, the fish were subjected to temporary rearing and acclimation with a commercial feed for a period of 30 days. During this interval, the fish teeth were cut to avoid cannibalism. A total of 450 juvenile fish (with an average initial body weight of 15.49 ± 0.02 g), which were free of external injuries on the body surface and of uniform specifications, were selected and randomly assigned to 15 fish tanks (72 × 72 × 50 cm, 200 L). Each experimental group was set up with 3 replicates, with 30 fish per replicate tank. The experimental fish were reared in an indoor flow-through seawater system for 56 days. They were hand-fed to satiation twice a day (07:30 and 18:30). The uneaten feed was removed using a siphoning method, and the quantity of uneaten feed in each fish tank following each feeding was recorded to adjust the data of feed consumption (based on the average weight of each pellet feed). During the experiment, a handheld multiparameter meter (ProQuatro, YSI, Yellow Springs, OH, USA) was used to measure the water quality. The water temperature was maintained within the range of 22–28 °C; the salinity was maintained within 28–32; the pH value was maintained within 7.4–7.8; the dissolved oxygen was greater than 5 mg/L; and the ammonia nitrogen and nitrite were less than 0.5 mg/L and 0.2 mg/L, respectively. The fish rearing handlings, as well as all the sampling handlings in this study, have been reviewed and approved by the Animal Care and Use Committee of the Yellow Sea Fisheries Research Institute (protocol code ACUC202303202514; date of approval, 20 March 2023).
2.4. Measurement of Fish Growth Performance
At the end of the experiment, the fish were first fasted for 24 h, and then the number of surviving fish in each aquaculture tank was counted and the fish weight in each tank was measured. The growth parameters can be calculated through the following equations:
where: Nt is the final fish number; No is the initial fish number; Wt is the final body weight, in grams; Wo is the initial body weight, in grams; D is the experimental duration, in days; Fd is the feed ingestion, in grams; Fp is the ingested protein mass; Dp is the body protein deposition mass, in grams; Wb is the fish body weight, in grams; Lb is the body length, in centimeters; Wl is the liver mass, in grams; Wv is the viscera mass.
2.5. Sample Collection
Seven fish were randomly selected from each tank, and they were anesthetized with eugenol (eugenol: water = 1/10,000) before dissection. Two fish were used for testing the proximate composition of the whole fish. A total of five fish were dissected for tissue collection. Blood was collected from the caudal vein and allowed to clot at 4 °C for 3–4 h. Subsequently, the blood was centrifuged (at 3500 r/min for 10 min at 4 °C) to obtain the serum samples. Tissue samples from the liver, muscle, and midgut were collected after dissection. The collected samples were immediately placed in liquid nitrogen on-site and subsequently transferred to a −80 °C freezer for storage until further use. Following the sampling process, two fish were randomly selected from each tank and stored in a −20 °C freezer as backups.
2.6. Chemical Analysis of Diets and Fish
The crude protein, crude lipid, ash, and moisture content of both the feed and fish body were determined using the AOAC (2005) method [
41]. FOSS Soxtec 2050 (Hillerod, Denmark) was used in the determination of crude protein. The gross energy of each of the diets was measured using a Compensated Calorimeter (6100, PARR, Moline, IL, USA).
The fatty acid composition of fishmeal,
N. japonica meals, feeds, and muscle was analyzed using a gas chromatograph (GC-2010 Pro, Shimadzu, Kyoto, Japan). Fatty acids were extracted from the samples using a chloroform–methanol method. The fatty acids were saponified and methylated using BF3–methanol and KOH–methanol, respectively. GC conditions: a quartz capillary column (SH-RT-2560, 100 m × 0.25 mm × 0.20 μm, Shimadzu, Kyoto, Japan) and a flame ionization detector. The heating program was as follows: an increase of 15 °C/min from 150 °C to 200 °C, then an increase of 2 °C/min from 200 °C to 250 °C. Results were expressed as % total fatty acids [
42].
Amino acid content determination: According to the protein content of fishmeal,
N. japonica meals, feeds, and muscle, appropriate amounts of samples were weighed and hydrolyzed with 6 mol/L HCl at 110 °C for 22–24 h. The amino acid content was determined using an amino acid analyzer (L-8900, Hitachi, Kyoto, Japan) [
43].
Determination of Free Amino Acids: The fish meal,
N. japonica meal, feed, and muscle samples were subjected to deproteinization using trichloroacetic acid (6%), then centrifuged at 10,000×
g for 10 min at 4 °C to obtain the supernatant. The content of free amino acids was determined using an amino acid analyzer (L-8900, Hitachi, Kyoto, Japan) [
44].
2.7. Muscle Texture Analysis
The texture properties of fish muscle were determined using a texture analyzer (TMS-Pro, FTC, Sterling, VA, USA) equipped with a 25 N gravity sensor. The texture properties measured included hardness, adhesiveness, cohesiveness, springiness, gumminess and chewiness. The test conditions were: ambient temperature of 23 °C; 8 mm round probe; compression rate of 30 mm/min; and a deformation ratio of 30%.
2.8. Analysis of Serum Biochemical Parameters
The serum biochemical indicators were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China): total protein (TP, A045-2), triglycerides (TG, A110-2-1), total cholesterol (TC, A111-2-1), malondialdehyde (MDA, A003-1), lysozyme (LZM, A050-1-1) and albumin (ALB, A028-1-1). The absorbance was measured using a microplate reader (Infinite M200, TECAN, Zurich, Switzerland). The operation was carried out in strict accordance with the instructions during the determination process.
2.9. Digestive Enzyme Activity
The digestive enzyme activities were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China): chymotrypsin (A080-3-1), trypsin (A080-2-2), lipase (A054-1-1) and β-amylase (C016-2-1). The absorbance was measured using a microplate reader (Infinite M200, TECAN, Zurich, Switzerland). The operation was carried out in strict accordance with the instructions during the determination process. The β-amylase activity was calculated by subtracting α-amylase activity from the total amylase activity.
2.10. Analysis of Relative mRNA Expression
RNAiso Plus (TaKaRa, Dalian, China) was used to extract the total RNA from liver and intestine samples. Agarose gel electrophoresis (2%) was used to detect the integrity of the purified RNA (1 μL), and the OD260/280 ratio was determined using a Colibri ultra-micro spectrophotometer (Titerek Berthold, Bad Wildbad, Germany) to assess the purity and concentration of the purified RNA. Evo M-MLV RT and gDNA Clean for qPCR Reverse Transcription Pre-Mix Kit (Acreo Biotech Co., Ltd., Hunan, China) were used to reverse transcribe the RNA into cDNA. Primers for target genes and reference genes were designed based on the sequence in GenBank sequences (
Table 6) and synthesized by Qingke Biotechnology Co., Ltd. (Qingdao, China).
β-actin and
ef1α were used as reference genes. The amplification efficiency of all specific primers was 95–105%. The R
2 value of the primer gradient dilution standard curve was all greater than 0.99. The Roche LightCycler 96 (Roche, Basel, Switzerland) was used as the fluorescence quantitative PCR instrument; the SYBR Green Pro Taq HS Pre-Mix Type II qPCR Reagent Kit II (Acreo Biotech Co., Ltd., Hunan, China) was used as the reagent kit. The reaction mixture was prepared as follows: 5 μL SYBR Green Pro Taq HS Premix II, 1 μL cDNA, 0.3 μL upstream primer and downstream primer, and 3.4 μL pure water. The fluorescence quantitative PCR program was set as follows: 95 °C denaturation for 30 s; 40 cycles as follows: 95 °C denaturation for 5 s, 57 °C annealing for 30 s, 72 °C extension for 30 s. Finally, the melting curve (6.4 °C/min from 65 °C to 95 °C) was drawn to determine that the system has a unique PCR specific product. The relative expression level of the gene was calculated using the 2
−ΔΔCt method.
2.11. Statistical Methods
All experimental data were analyzed using SPSS 26.0 for one-way ANOVA and Tukey’s test. When p < 0.05, there was a significant difference. The results are expressed as the mean ± standard error.
4. Discussion
To date, there have been few studies about the application of polychaete meal in aquafeeds. Limited studies in this area have shown that the sandworm
Alitta virens can replace 40% fishmeal in the feed of European seabass
Dicentrarchus labrax without affecting the growth and nutrient utilization [
41].
Alitta virens can also serve as the main dietary protein source for rainbow trout
Oncorhynchus mykiss. The growth performance of rainbow trout fed the diet with 35%
A. virens meal was similar to that of fish fed commercial feeds [
42]. In Nile tilapia
Oreochromis niloticus, supplementation of 51.4%
Nereis sp. meal into the feed (containing 40% crude protein) can promote the fish growth and feed efficiency [
43]. These studies suggested that polychaetes can be a valuable alternative protein source in aquatic feeds.
In contrast to the aforementioned studies, the dietary proportion of
N. japonica meal in this experiment was relatively low, ranging from 3% to 9%. This experimental design primarily took into account the low production of
N. japonica at present. The results of this experiment demonstrated that partial replacement of fishmeal by
N. japonica meal did not exert a significant influence on the growth performance of juvenile tiger puffer. Notably, the fish weight gain in the group with 3%
N. japonica meal was elevated by 9.43% compared to the control group. This indicates that the low-proportion substitution
N. japonica meal for fishmeal may have potential fish growth-promoting effects. A longer feeding duration may enlarge this growth-promoting effect. Similar results were observed in studies on European seabass, rainbow trout, and Nile tilapia [
45,
46,
47].
Besides, the supplementation of
N. japonica meal enhanced the feed intake of tiger puffer, which validated the experimental hypothesis. The feeding attracting effect of
N. japonica meal might be associated with the relatively high content of free amino acids. Free amino acids such as arginine, aspartic acid, glutamic acid, glycine, and alanine are flavor substances [
48], and an appropriate amount of these free amino acids could increase the palatability of the feed [
49].
Considering that the active substances which determine the function of N. japonica could be susceptible to high drying temperature, in this study different drying methods, namely, freeze-drying and oven-drying, were compared (at the 6% supplementation level). However, the results showed that different drying methods led to no significant difference in terms of the fish growth and physiological parameters. Irrespective of the drying method, dried N. japonica meal had higher contents of nearly all types of free amino acid, except for histidine and taurine. The oven-drying resulted in even higher free amino acids than freeze-drying (increased by 83.24%). This could be attributed to the fact that high temperatures during the oven-drying process facilitated the cleavage of peptide bonds into low-molecular peptides and free amino acids.
Regarding the fish body composition, the addition of
N. japonica meal did not exert a significant influence on the proximate composition of whole fish and fish tissues, nor did it have a notable effect on the fatty acid and amino acid profiles of the muscle. Currently, there have been very few studies on the impacts of replacing fishmeal with polychaetes on fish body composition. In the feed of European seabass, the addition of 2.5%, 5%, and 10% of polychaete
Alitta virens meal replacing fishmeal also had no significant effects on the proximate composition of fish body [
45]. The lack of changes in fish body composition could be due to the fact that the
N. japonica meal had very similar compositions to fishmeal, in terms of proximate composition, amino acid composition and fatty acid profiles. In contrast, the use of terrestrially-sourced insects, like black soldier fly
Hermetia illucens larvae meal, commonly resulted in changes in fish body composition [
50,
51]. Marine polychaetes seemed superior to terrestrially-sourced insects in maintaining fish body composition. Consequently, the use of
N. japonica meal in fish feeds could exert very minor effects on muscle texture, which was demonstrated in this study.
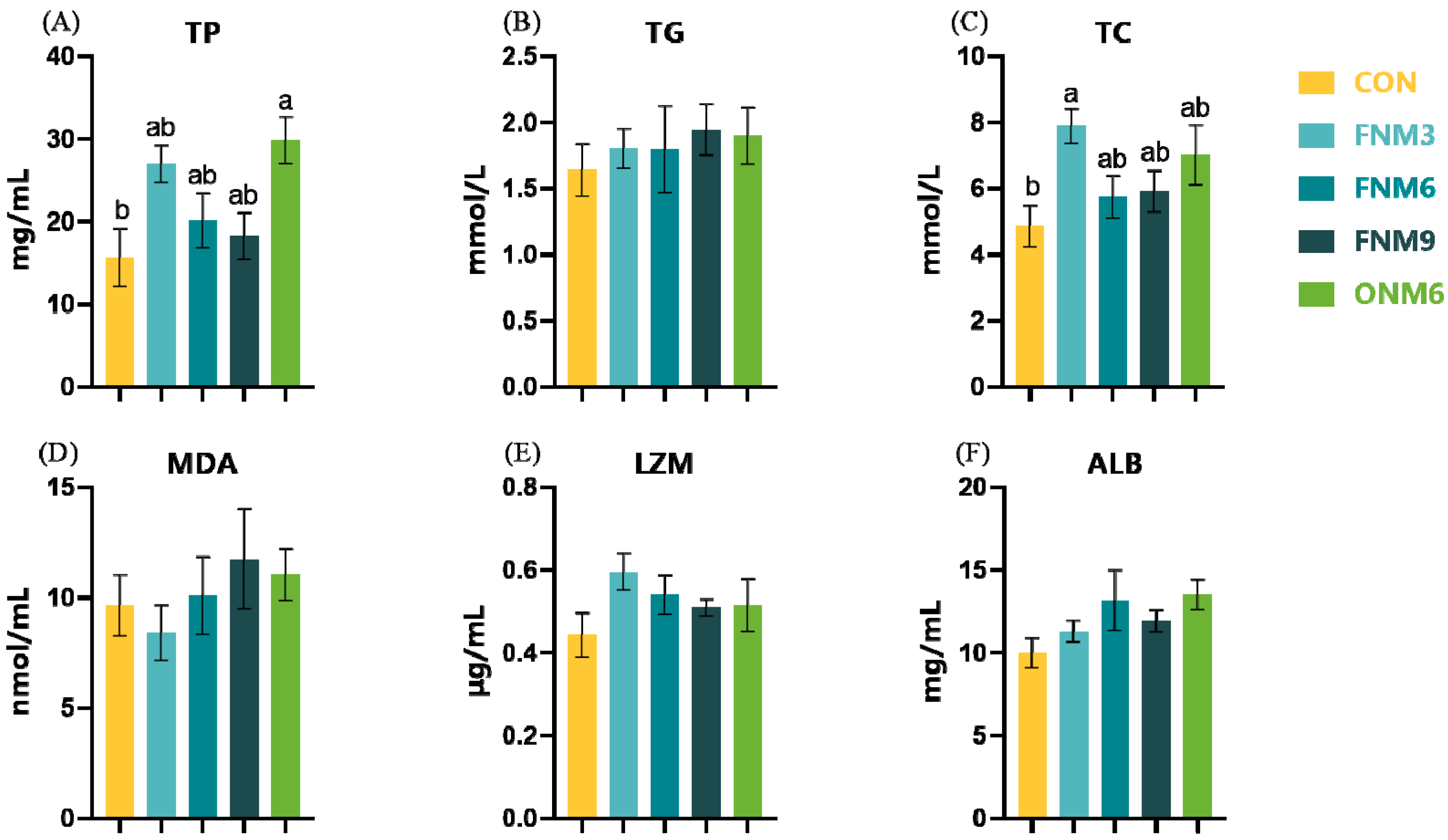
The serum biochemical indicators of fish reflect, to a certain extent, the physiological and health status of the fish. There is a close correlation between serum protein and the level of protein synthesis in the liver. Consequently, the content of serum protein is also a significant index reflecting protein metabolism, nutrition, and health within the organism [
52]. Albumin and globulin are essential components for maintaining cell nutrition and the osmotic pressure balance of blood, and their contents can indirectly indicate the status of protein synthesis in the liver [
53]. This study demonstrated that the supplementation of
N. japonica meal elevated the content of total serum protein. This indicates that the addition of
N. japonica meal may enhance the protein synthesis capacity of the fish liver and facilitate protein metabolism. The cholesterol content in serum can, to a certain extent, reflect the fat metabolism status of the liver. The supplementation of
N. japonica meal increased the content of total serum cholesterol, in particular at the 3% supplementation level. This suggested that the
N. japonica meal may be capable of regulating the lipid metabolism. Considering that the addition of
N. japonica meal in the feed had no significant impact on other serum indicators such as albumin, triglyceride, malondialdehyde, and lysozyme, the precise mechanism by which the
N. japonica meal regulated the fish physiological metabolism remains unclear and warrants further studies.
Despite the promising features, the application of
N. japonica was by no means devoid of negative consequences. In this study, the supplementation of
N. japonica meal in the feed reduced the activities of intestinal lipase and α-amylase. This could potentially be attributed to the existence of chitin in the
N. japonica meal. Chitin is a nitrogen-containing polysaccharide, constituted by a majority of N-acetylated D-glucosamine and a minority of D-glucosamine. As a highly insoluble compound, chitin could reduce the water-holding capacity of intestinal chyme. This characteristic not only shortens the transit time of chyme within the intestinal tract but also facilitates fecal expansion, thereby reducing the interaction duration between digestive enzymes and their substrates [
54]. The downregulation of digestive enzyme activity by
N. japonica may counteract its stimulatory effects on feed intake, consequently leading to no significant disparity in overall growth performance among the experimental groups.
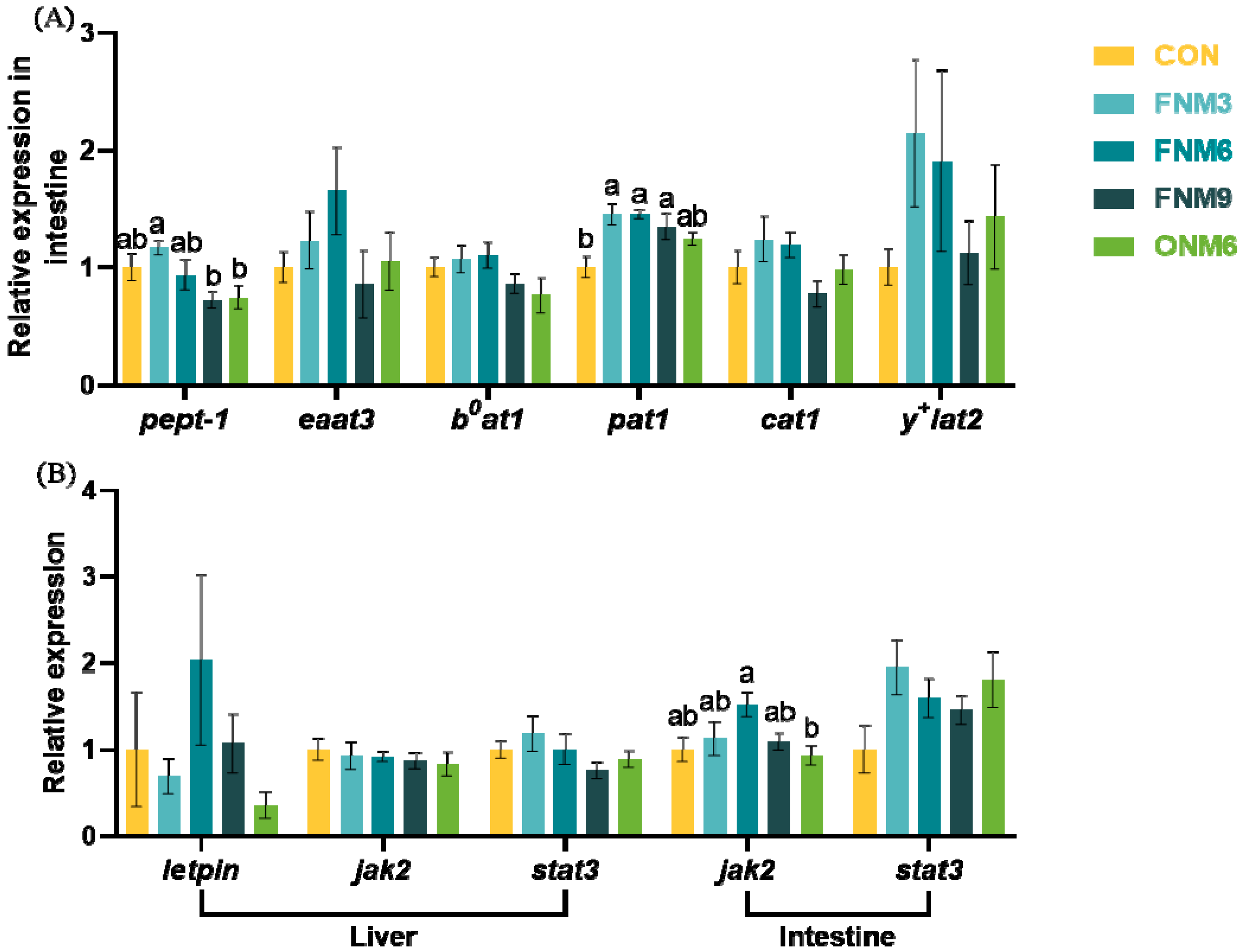
Besides the phenotypic parameters, this study also analyzed the expression of genes related to the absorption and transport of amino acids and peptides. Oligopeptides and amino acids derived from protein degradation in the intestine are transported into cells via transport carriers and subsequently utilized by the organism. The expression of oligopeptide and amino acid transport carriers in the intestinal tract of fish serves as a crucial indicator reflecting the intestinal absorptive capacity for oligopeptides and amino acids [
55,
56,
57]. The expression levels of amino acid transport carriers are prone to regulation by the nutritional status of the feed since the cells of the animal body generate an adaptive response to the ingested feed, and the amino acid transport carriers have the ability to sense amino acid levels. This experiment primarily investigated
pept-1 for transporting oligopeptides,
eaat3 for transporting acidic amino acids,
b0at1 for transporting neutral amino acids,
y+lat2 for transporting neutral and basic amino acids,
cat1 for transporting basic amino acids, and
pat1 for transporting amino-glycine. The supplementation of
N. japonica meal significantly up-regulated the relative mRNA expression level of intestinal
pat1. The free glycine in the diet increased with the escalating addition level of
N. japonica meal, and could subsequently stimulate the
pat1 expression. However, the addition of
N. japonica meal in the feed did not impact the relative mRNA expression of
pept-1,
eaat3,
b0at1,
y+lat2, and
cat1 in the intestine. Fish may have the capacity to regulate the expression of oligopeptide and amino acid transport carriers through a feedback mechanism, in order to control the absorption homeostasis of oligopeptides and amino acids in cells [
58,
59,
60].
Since the feed intake of fish in the treatment group was elevated, this experiment also investigated the impact of
N. japonica meal on the expression of appetite-related genes. In vertebrates, leptin is predominantly secreted by adipocytes, and functions to inhibit appetite for regulating energy balance and to decrease fat storage in adipocytes [
61]. Leptin primarily acts on the hypothalamus via the JAK2/STAT3 signaling pathway, suppressing the secretion of Neuropeptide Y by hunger neurons in the outer nucleus of the hypothalamus, thereby reducing appetite [
62,
63]. This experiment indicates that the addition of
N. japonica meal exerts no significant influence on the mRNA expression of
leptin (non-detectable in the intestine),
jak2, and
stat3 in the liver and intestine. Therefore, the
N. japonica meal may regulate feeding activities through alternative pathways.