Tracking Biomarkers for the Health and Welfare of Aquaculture Fish
Abstract
1. Introduction
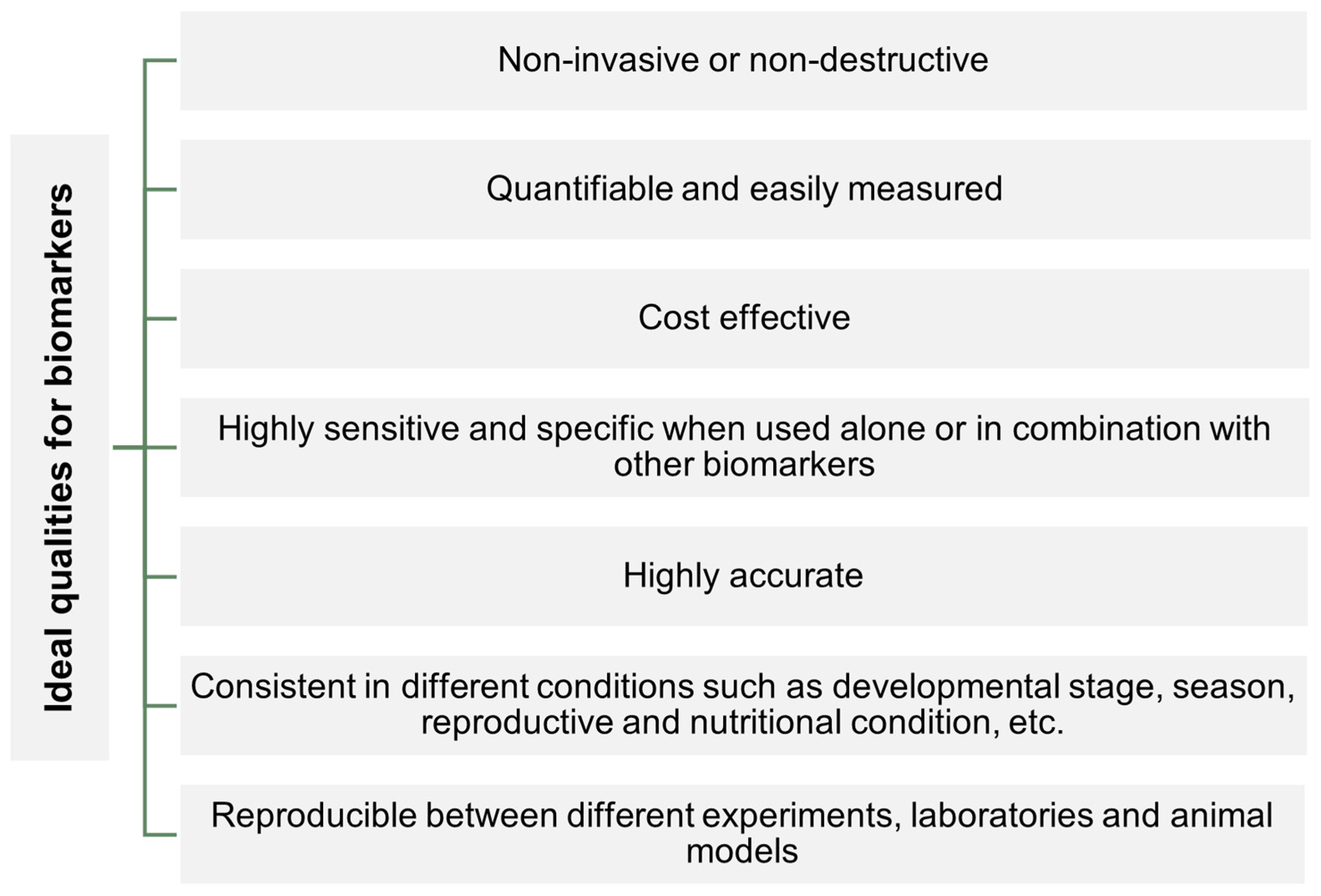
2. Biomarkers
3. Biomarkers Used for Fish Health and Welfare
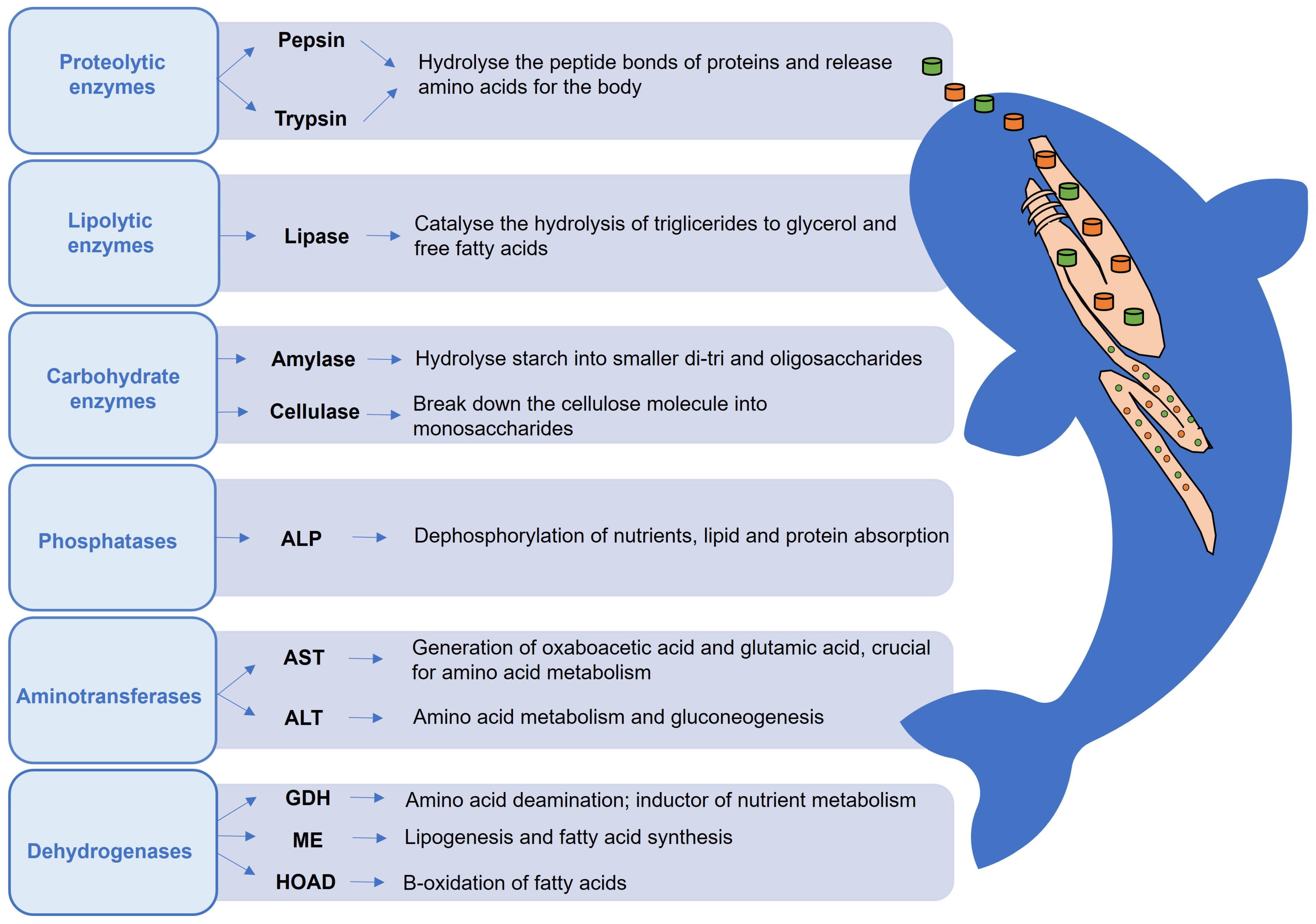
3.1. Metabolism Biomarkers
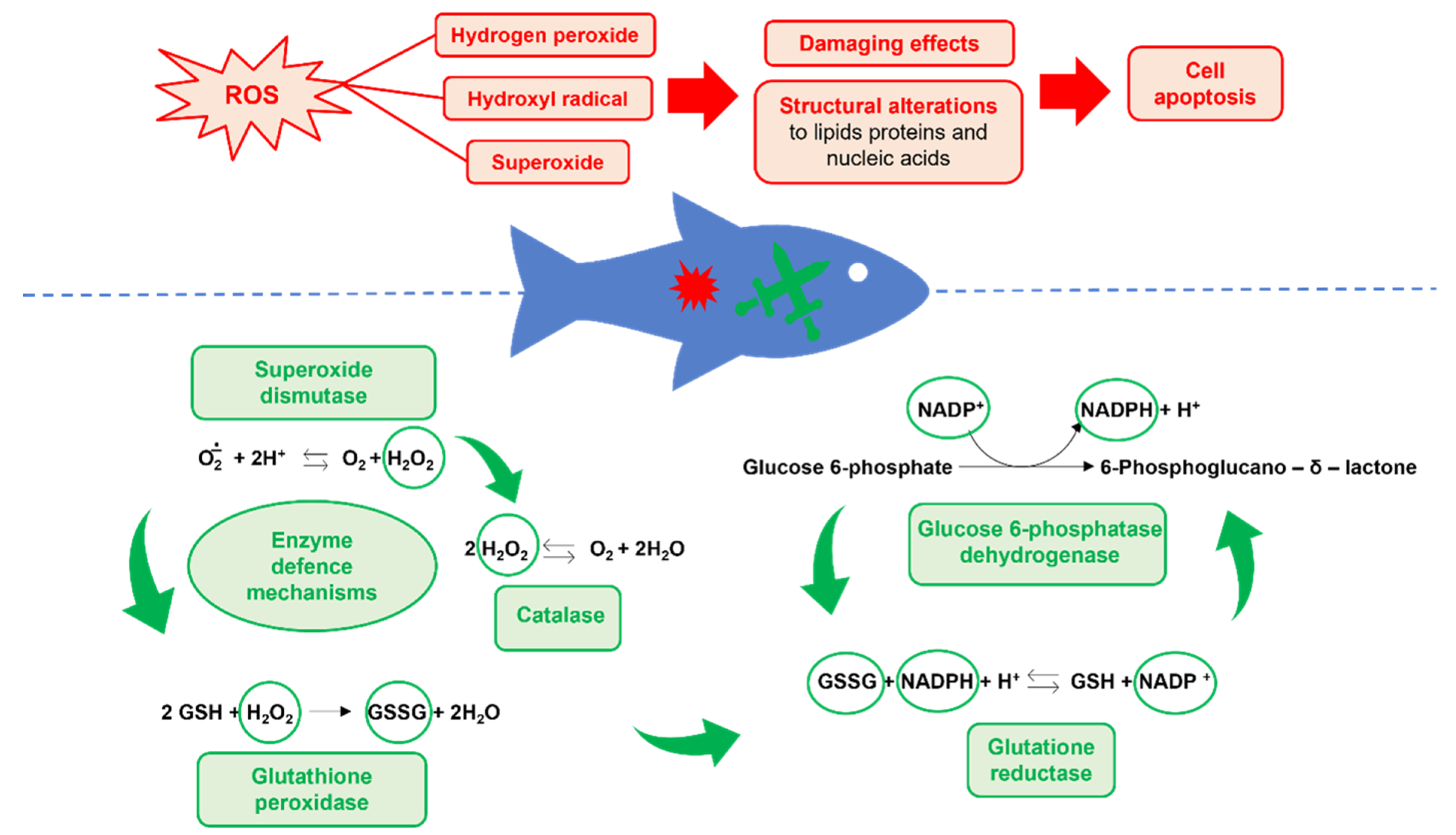
3.2. Oxidative Stress Biomarkers
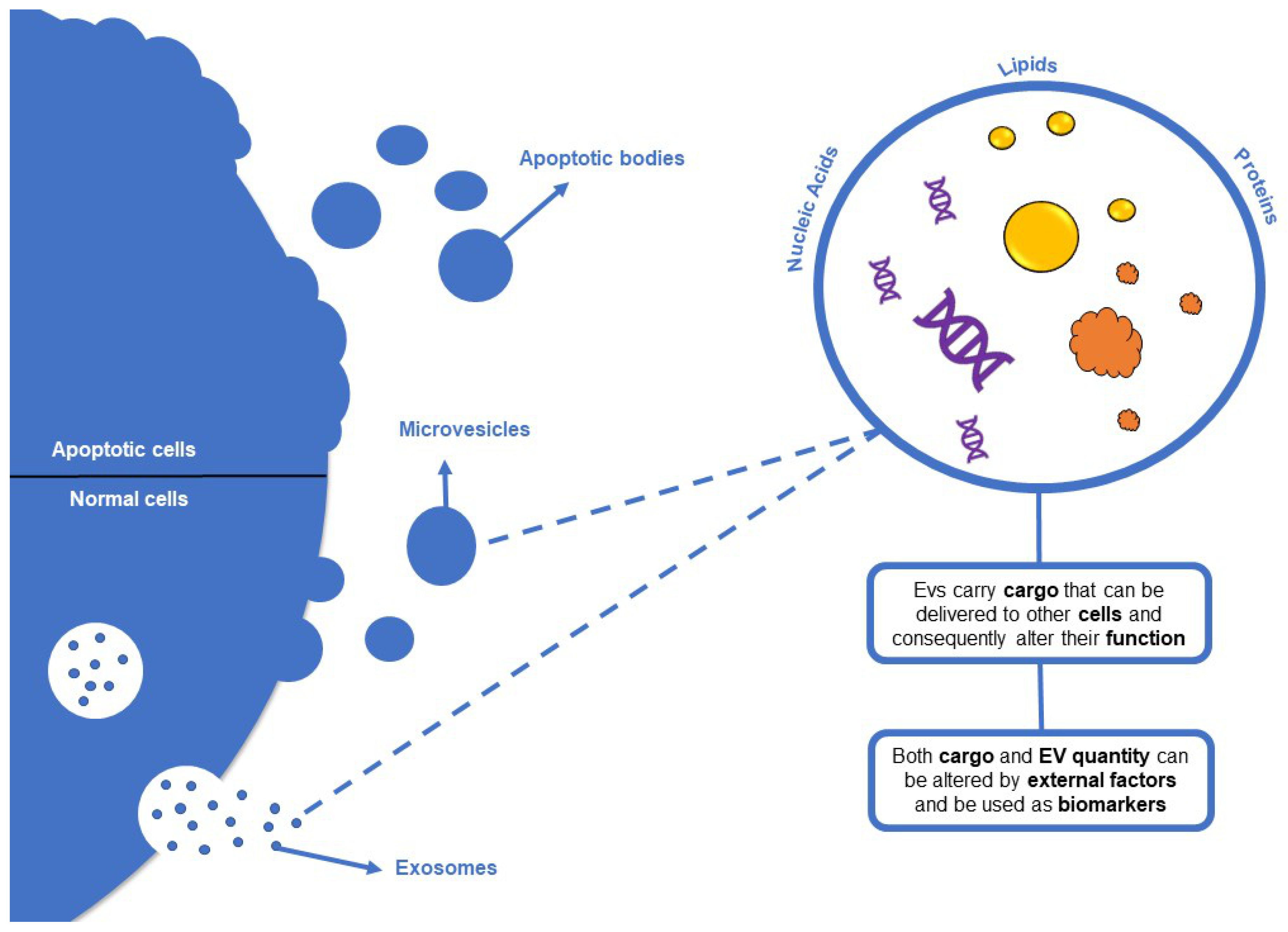
3.3. Immunological Biomarkers
3.4. Biochemical Biomarkers
3.5. Mucosal and Mucin-Associated Biomarkers
4. Application of Biomarkers in Aquaculture Studies
4.1. Nutrition
4.2. Stress
4.3. Infectious Diseases
4.4. Chemicals, Antibiotics and Vaccines
| Biomarker | Impact Studied | Organism Tissue | Species | Non-Lethal Potential | References |
|---|---|---|---|---|---|
| 3-hydroxysovaleric acid | Nutrition | Serum | Sparus aurata | Yes | [24] |
| 4- hydroxy-2-nonenal | Stress | Gill, Brain, Liver, Spleen | Dicentrarchus labrax | Unknown | [44] |
| Alanine transaminase (ALT) | Nutrition, Chemical, Disease | Serum, Mucus, Skin, Liver | Oreochromis niloticus, Oncorhynchus mykiss, Salmo salar, Argyrosomus regius | Yes | [43,83,90,105,113,115] |
| Alkaline phosphatase | Nutrition, Disease | Serum, Mucus, Skin, Intestine | Salmo salar, Argyrosomus regius | Yes | [91,105] |
| Alkaline protease | Nutrition | Intestine | Argyrosomus regius | Unknown | [84,90] |
| Amylase | Nutrition | Intestine | Cyprinus carpio, Argyrosomus regius | Unknown | [80,84,90] |
| Aspartate transaminase (AST) | Nutrition, Chemical, Disease | Serum, Mucus, Skin, Liver | Oreochromis niloticus, Oncorhynchus mykiss, Salmo salar, Argyrosomus regius | Yes | [43,83,90,105,113,115] |
| Carbonyl proteins | Nutrition, Chemical | Intestine, Gill, Liver, Brain, Muscle, Heart | Acipenser stellatus, Oncorhynchus mykiss | Yes | [40,43,116] |
| Catalase (CAT) | Nutrition, Chemical | Liver, Kidney, Intestine, Serum, Mucus, Skin, Heart | Cyprinus carpio, Oncorhynchus mykiss, Acipenser baerii, Acipenser stellatus, Oreochromis niloticus, Salmo salar, Cirrhinus mrigala | Yes | [33,34,40,80,81,82,88,105,112,116] |
| Catecholamines | Nutrition | Serum | Sparus aurata | Yes | [24] |
| Cholinesterases (ChE) | Chemicals | Brain, Muscle | Sparus aurata | Unknown | [12] |
| Complement system activity (ACH50) | Chemical | Serum, Intestine | Oreochromis niloticus, Argyrosomus regius | Yes | [91,115] |
| Cortisol | Nutrition, Stress | Serum | Oreochromis niloticus, Hypomesus transpacificus | Yes | [81,97] |
| C-reactive protein (CRP) | Chemical | Serum | Oreochromis niloticus | Yes | [113] |
| Creatine kinase | Disease, Chemical | Serum | Salmo salar, Oncorhynchus mykiss | Yes | [43,102] |
| Cytokine IL-22 | Disease | Gill | Salmo salar | Unknown | [103] |
| Enolase | Disease | Serum | Salmo salar | Yes | [101] |
| Ethoxyresorufin O-deethylase (EROD) | Nutrition | Liver, Kidney | Oncorhynchus mykiss | Unknown | [33,88] |
| Extracellular vesicles (EVs) (+ cargo) | Stress, Disease | Serum, Skin (Epidermal mucus) | Gadus morhua, Salmo salar, Cynoglossus semilaeuis | Yes | [61,106,107] |
| Glucose | Nutrition, Stress, Chemical | Serum | Oreochromis niloticus, Cirrhinus mrigala, Oncorhynchus mykiss, Argyrosomus regius | Yes | [34,43,81,83,113] |
| Glucose 6-phosphate dehydrogenase (G6PDH) | Nutrition | Intestine, Liver | Acipenser stellatus, Argyrosomus regius | Unknown | [40,83] |
| Glutamate dehydrogenase (GDH) | Nutrition | Liver | Argyrosomus regius | Unknown | [83,90] |
| Glutathione | Nutrition | Serum, Liver | Oreochromis niloticus | Yes | [82] |
| Glutathione peroxidase (GPx) | Nutrition, Stress, Chemical | Liver, Kidney, Intestine, Serum, Brain, Muscle, Gill, Heart | Cyprinus carpio, Oncorhynchus mykiss, Acipenser baerii, Acipenser stellatus, Oreochromis niloticus, Perca flavescens, Cirrhinus mrigala | Yes | [33,34,40,43,80,81,88,95,116] |
| Glutathione reductase (GR) | Nutrition, Stress | Liver, Kidney, Intestine, Brain, Gill, Muscle, Heart | Oncorhynchus mykiss, Acipenser baerii, Acipenser stellatus, Perca flavescens | Yes | [33,40,43,88,95,116] |
| Glutathione S-transferase (GST) | Nutrition, Chemical | Liver, Kidney, Intestine, Gill | Oncorhynchus mykiss, Acipenser baerii, Acipenser stellatus, Sparus aurata, Oreochromis niloticus | Yes | [12,33,40,88,112] |
| Goblet cells | Nutrition | Intestine | Oreochromis niloticus | Unknown | [81,82] |
| Hematocrit | Chemical | Plasma | Oreochromis niloticus, Cirrhinus mrigala | Yes | [34,112] |
| Hemoglobin concentration | Nutrition, Chemical | Plasma | Oreochromis niloticus, Cirrhinus mrigala | Yes | [34,81,112,115] |
| Heat shock protein (Hsp) | Nutrition, Stress, Chemical | Liver, Gills, Kidney, Serum, Muscle | Oreochromis niloticus, Dicentrarchus labrax, Salvelinus alpinus, Perca flavescens, Sparus aurata | Yes | [12,44,81,95,96] |
| Insulin-like growth factor (Igf1) | Stress | Serum, Liver | Perca flavescens | Yes | [95] |
| Intestinal pro-inflammatory cytokines (IL-1β, IL-8 and TNF-α) | Disease | Intestine | Ctenopharyngodon idella | Unknown | [109] |
| Lactate | Chemical | Serum | Oncorhynchus mykiss | Yes | [43] |
| Lactate dehydrogenase (LDH) | Disease, Chemical | Serum | Salmo salar, Oncorhynchus mykiss | Yes | [43,102] |
| Leukocyte respiratory burst activity | Chemical | Serum | Oreochromis niloticus | Yes | [115] |
| Lipase | Nutrition | Intestine | Cyprinus carpio, Argyrosomus regius | Unknown | [80,84,90] |
| Lysozyme | Nutrition, Disease, Chemical | Serum, Mucus, Skin, Intestine | Oreochromis niloticus, Salmo salar, Argyrosomus regius | Yes | [81,82,91,105,115] |
| Malondialdehyde (MDA) | Nutrition | Liver, Kidney, Intestine, Serum | Oncorhynchus mykiss, Acipenser stellatus, Oreochromis niloticus, Dicentrarchus labrax | Yes | [33,40,44,81,82,88] |
| Mucins | Nutrition, Stress, Disease | Intestine, Gill, Skin | Sparus aurata, Salmo salar, Oreochromis niloticus | Yes | [68,69,92,103] |
| Myeloperoxidase (MPO) | Disease | Intestine | Ctenopharyngodon idella | Unknown | [109] |
| Nitrotyrosine | Stress | Liver, Kidney, Muscle | Dicentrarchus labrax | Unknown | [44] |
| Peroxidase | Disease, Nutrition | Plasma, Mucus, Skin | Salmo salar, Argyrosomus regius | Yes | [91,105] |
| Peroxidation of lipids (LOP) | Chemical | Plasma, Liver, Intestine, Gill, Brain, Muscle | Cirrhinus mrigala, Oncorhynchus mykiss | Yes | [34,43] |
| Protease | Nutrition | Intestine | Cyprinus carpio | Unknown | [80] |
| Protein thiol groups | Nutrition | Intestine | Acipenser stellatus | Unknown | [40] |
| Red blood cells (RBCs) | Nutrition, Chemical | Plasma | Oreochromis niloticus, Cirrhinus mrigala, Argyrosomus regius | Yes | [34,81,91,112,115] |
| RNA/DNA ratio | Chemical | Muscle | Sparus aurata | Unknown | [12] |
| Rodlet cells | Stress | Intestine, Renal tubes, Gill | Dicentrarchus labrax | Unknown | [44] |
| Superoxide dismutase (SOD) | Nutrition, Stress, Disease, Chemical | Liver, Kidney, Intestine, Serum, Mucus, Skin, Brain, Muscle, Gill, Heart | Cyprinus carpio, Oncorhynchus mykiss, Acipenser baerii, Acipenser stellatus, Oreochromis niloticus, Perca flavescens, Salmo salar, Cirrhinus mrigala | Yes | [33,34,40,43,80,81,82,88,95,105,116] |
| Thiobarbituric acid reactive substances (TBARS) | Chemical | Brain, Heart | Sparus aurata, Oncorhynchus mykiss | Unknown | [12,116,117] |
| Trypsin | Nutrition | Intestine | Argyrosomus regius | Unknown | [84,90] |
| White blood cells (WBCs) | Nutrition, Chemical | Plasma | Oreochromis niloticus, Cirrhinus mrigala, Argyrosomus regius | Yes | [34,81,91,112] |
5. Advancing Biomarkers through Omics Technologies
| Omics | Analytical Method | Reference |
|---|---|---|
| Genomics | SNP technology | [122] |
| GWAS | ||
| Transcriptomics | RT-PCR | [44] |
| cDNA microarray | [13] | |
| RNA-seq technology | [74] | |
| Proteomics | 2D-PAGE | [124] |
| LC-MS/MS | [120,124] | |
| 1D-SDS-PAGE | [124] | |
| HPLC-ESI-MS/MS | [124] | |
| DIGE | [120,124] | |
| MALDI-TOF/TOF | [120,124] | |
| Immunohistochemistry | [44] | |
| Metabolomics | NMR | [129] |
| Mass spectrometry (MS)-based | [129] |
6. Conclusions and Future Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Standing Commitee on Agricultural Research. Strategic Research and Innovation Agenda: For the European Partnership on Animal Health and Welfare (EUP AH&W SRIA); Standing Commitee on Agricultural Research: Brussels, Belgium, 2023. [Google Scholar]
- Katharios, P. Disease Prevention in Farmed Fish: New Developments and Research Needs; Standing Commitee on Agricultural Research: Brussels, Belgium, 2019; p. 48. [Google Scholar]
- FAO. Fishery and Aquaculture Statistics—Yearbook 2021. In FAO Yearbook of Fishery and Aquaculture Statistics; Food and Agriculture Organization of United Nations: Rome, Italy, 2024. [Google Scholar]
- Manfrin, A.; Messori, S.; Arcangeli, G. Strengthening Fish Welfare Research through a Gap Analysis Study; Standing Committee on Agricultural Research: Brussels, Belgium, 2018. [Google Scholar]
- Barreto, M.O.; Planellas, S.R.; Yang, Y.; Phillips, C.; Descovich, K. Emerging indicators of fish welfare in aquaculture. Rev. Aquac. 2022, 14, 343–361. [Google Scholar] [CrossRef]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological methods in fish—Not only for beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Kiron, V. Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 2012, 173, 111–133. [Google Scholar] [CrossRef]
- WHO. Biomarkers and Risk Assessment: Concepts and Principles. Available online: http://www.inchem.org/documents/ehc/ehc/ehc155.htm#SectionNumber:1.2 (accessed on 31 May 2021).
- Sim, D.; Brothers, M.C.; Slocik, J.M.; Islam, A.E.; Maruyama, B.; Grigsby, C.C.; Naik, R.R.; Kim, S.S. Biomarkers and Detection Platforms for Human Health and Performance Monitoring: A Review. Adv. Sci. 2022, 9, 2104426. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.K.S. Use of biomarkers in environmental monitoring. Ocean Coast. Manag. 2009, 52, 348–354. [Google Scholar] [CrossRef]
- Sánchez, J.P.; Calduch-Giner, J.; Sitjà-Bobadilla, A.; Nácher-Mesyre, J.; Waagbo, R.; Berntssen, M.H.G.; Skiba, S.; Sándor, Z.; Montero, D.; Terova, G.; et al. Understanding Biomarkers in Fish Nutrition—Technical Booklet; ARRAINA—Advanced Research Initiatives for Nutrition and Aquaculture: Brussels, Belgium, 2016. [Google Scholar]
- Varó, I.; Navarro, J.C.; Nunes, B.; Guilhermino, L. Effects of dichlorvos aquaculture treatments on selected biomarkers of gilthead sea bream (Sparus aurata L.) fingerlings. Aquaculture 2007, 266, 87–96. [Google Scholar] [CrossRef]
- Raposo de Magalhães, C.S.F.; Cerqueira, M.A.C.; Schrama, D.; Moreira, M.J.V.; Boonanuntanasarn, S.; Rodrigues, P.M.L. A Proteomics and other Omics approach in the context of farmed fish welfare and biomarker discovery. Rev. Aquac. 2020, 12, 122–144. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Young, T. Showcasing metabolomic applications in aquaculture: A review. Rev. Aquac. 2016, 10, 135–152. [Google Scholar] [CrossRef]
- Benninghoff, A. Toxicoproteomics—The next step in the evolution of environmental biomarkers. Toxicol. Sci. 2007, 95, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.B. 7—Nutritional Physiology. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 367–452. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Assan, D.; Kuebutornye, F.K.A.; Hlordzi, V.; Chen, H.; Mraz, J.; Mustapha, U.F.; Abarike, E.D. Effects of probiotics on digestive enzymes of fish (finfish and shellfish); status and prospects: A mini review. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2022, 257, 110653. [Google Scholar] [CrossRef] [PubMed]
- Picha, M.E.; Turano, M.J.; Beckman, B.R.; Borski, R.J. Endocrine Biomarkers of Growth and Applications to Aquaculture: A Minireview of Growth Hormone, Insulin-Like Growth Factor (IGF)-I, and IGF-Binding Proteins as Potential Growth Indicators in Fish. North Am. J. Aquac. 2008, 70, 196–211. [Google Scholar] [CrossRef]
- Farrell, A.P. Encyclopedia of Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Beckman, B.R. Perspectives on concordant and discordant relations between insulin-like growth factor 1 (IGF1) and growth in fishes. Gen. Comp. Endocrinol. 2011, 170, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Capuzzo, A.; Moon, T.W. The role of circulating catecholamines in the regulation of fish metabolism: An overview. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 120, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Sopinka, N.M.; Donaldson, M.R.; O’Connor, C.M.; Suski, C.D.; Cooke, S.J. 11—Stress Indicators in Fish. In Fish Physiology; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 405–462. [Google Scholar]
- Gil-Solsona, R.; Nácher-Mestre, J.; Lacalle-Bergeron, L.; Sancho, J.V.; Calduch-Giner, J.A.; Hernández, F.; Pérez-Sánchez, J. Untargeted metabolomics approach for unraveling robust biomarkers of nutritional status in fasted gilthead sea bream (Sparus aurata). PeerJ 2017, 5, e2920. [Google Scholar] [CrossRef] [PubMed]
- Zambonino Infante, J.L.; Cahu, C.L. Dietary modulation of some digestive enzymes and Metabolic processes in developing marine fish: Applications to diet formulation. Aquaculture 2007, 268, 98–105. [Google Scholar] [CrossRef]
- Bakke, A.M.; Glover, C.; Krogdahl, Å. 2—Feeding, digestion and absorption of nutrients. In Fish Physiology; Grosell, M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 30, pp. 57–110. [Google Scholar]
- Metón Teijeiro, I.; Salgado Martín, M.d.C.; Anemaet, I.G.; González, J.D.; Fernández González, F.J.; Vázquez Baanante, M.I. Alanine aminotransferase: A target to improve utilisation of dietary nutrients in aquaculture. In Recent Advances in Pharmaceutical Sciences V; Torrero, D.M., Vinardell, M.P., Palazón, J., Eds.; Research Signpost: Thiruvananthapuram, India, 2015; pp. 133–148. [Google Scholar]
- Chimela, W.; Mesua, N.; Abdulraheem, B.-A. Aspartate Transaminase (AST) Activity in Selected Tissues & Organs of Clarias gariepinus Exposed to Different Levels of Paraquat. J. Environ. Anal. Toxicol. 2014, 4, 1000214. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Liu, S.; Zhong, H.; Zhang, C.; Kang, X.; Liu, Y. Characterization and dietary regulation of glutamate dehydrogenase in different ploidy fishes. Amino Acids 2012, 43, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Botham, K.M.; Mayes, P.A. Oxidation of Fatty Acids: Ketogenesis. In Harper’s Illustrated Biochemistry, 31st ed.; Rodwell, V.W., Bender, D.A., Botham, K.M., Kennelly, P.J., Weil, P.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Tocher, D.R.; Glencross, B.D. Lipids and Fatty Acids. In Dietary Nutrients, Additives, and Fish Health, 1st ed.; Lee, C.-S., Lim, C., III, D.M.G., Webster, C.D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Caimi, C.; Gasco, L.; Biasato, I.; Malfatto, V.; Varello, K.; Prearo, M.; Pastorino, P.; Bona, M.C.; Francese, D.R.; Schiavone, A.; et al. Could Dietary Black Soldier Fly Meal Inclusion Affect the Liver and Intestinal Histological Traits and the Oxidative Stress Biomarkers of Siberian Sturgeon (Acipenser baerii) Juveniles? Animals 2020, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Thilagavathi, T.; Rathika, R.; Poopal, R.K. Antioxidant status, biochemical, and hematological responses in a cultivable fish Cirrhinus mrigala exposed to an aquaculture antibiotic Sulfamethazine. Aquaculture 2018, 491, 10–19. [Google Scholar] [CrossRef]
- Quintas, A.; Freire, A.P.; Halpern, M.J. Bioquímica. Organização Molecular da Vida; LIDEL: Lisboa, Portugal, 2008. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Gregory, J.; Gatto, J.; Stryer, L. Biochemistry, 8th ed.; W. H. Freeman and Company: New York, NY, USA, 2015. [Google Scholar]
- Ciftci, M. Effects of some drugs on the activity of glucose 6-phosphate dehydrogenase from rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro. J. Enzym. Inhib. Med. Chem. 2005, 20, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Cox, M.; Hoskins, A. Lehninger Principles of Biochemistry, 8th ed.; W. H. Freeman and Company: New York, NY, USA; Macmillan Learning: New York, NY, USA, 2021. [Google Scholar]
- Dzoyem, J.P.; Kuete, V.; Eloff, J.N. 23—Biochemical Parameters in Toxicological Studies in Africa: Significance, Principle of Methods, Data Interpretation, and Use in Plant Screenings. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 659–715. [Google Scholar] [CrossRef]
- Florescu, I.E.; Georgescu, S.E.; Dudu, A.; Balaș, M.; Voicu, S.; Grecu, I.; Dediu, L.; Dinischiotu, A.; Costache, M. Oxidative Stress and Antioxidant Defense Mechanisms in Response to Starvation and Refeeding in the Intestine of Stellate Sturgeon (Acipenser stellatus) Juveniles from Aquaculture. Animals 2021, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.P.; Bhatnagar, A. Role of Thiols in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Frühbeck, G.; Gómez-Ambrosi, J. Chapter 8—Inflammatory and Oxidative Stress Markers in Skeletal Muscle of Obese Subjects. In Obesity; del Moral, A.M., Aguilera García, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 163–189. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Li, Z.-H.; Silovska, S.; Turek, J. Comparison of the effects of four anaesthetics on blood biochemical profiles and oxidative stress biomarkers in rainbow trout. Aquaculture 2011, 310, 369–375. [Google Scholar] [CrossRef]
- Fiocchi, E.; Civettini, M.; Carbonara, P.; Zupa, W.; Lembo, G.; Manfrin, A. Development of molecular and histological methods to evaluate stress oxidative biomarkers in sea bass (Dicentrarchus labrax). Fish Physiol. Biochem. 2020, 46, 1577–1588. [Google Scholar] [CrossRef]
- Iwama, G.; Thomas, P.; Forsyth, R.; Vijayan, M. Heat shock expression in fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Iwama, G.K.; Afonso, L.O.; Todgham, A.; Ackerman, P.; Nakano, K. Are hsps suitable for indicating stressed states in fish? J. Exp. Biol. 2004, 207, 15–19. [Google Scholar] [CrossRef]
- Gagnon, M.M.; Rawson, C.A. Bioindicator species for EROD activity measurements: A review with Australian fish as a case study. Ecol. Indic. 2017, 73, 166–180. [Google Scholar] [CrossRef]
- van Muiswinkel, W.B.; Vervoorn-Van der Wal, B. The immune system of fish. In Fish Diseases and Disorders: Protozoan and Metazoan Infections; Woo, P.T.K., Ed.; CAB International: Wallingford, UK, 2006; Volume 1, pp. 678–701. [Google Scholar]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Salinas, I.; Ding, Y.; Fernández-Montero, Á.; Sunyer, J.O. Mucosal Immunity in Fish. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Buchmann, K., Secombes, C.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 387–443. [Google Scholar] [CrossRef]
- Roy, S.; Kumar, V.; Kumar, V.; Behera, B.K. Acute Phase Proteins and their Potential Role as an Indicator for Fish Health and in Diagnosis of Fish Diseases. Protein Pept. Lett. 2017, 24, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Tort, L.; Balasch, J.; Mackenzie, S. Fish Immune System. A crossroads between innate and adaptive responses. Inmunologia 2003, 22, 277–286. [Google Scholar]
- Kreutz, L.C.; Gil Barcellos, L.J.; de Faria Valle, S.; de Oliveira Silva, T.; Anziliero, D.; Davi dos Santos, E.; Pivato, M.; Zanatta, R. Altered hematological and immunological parameters in silver catfish (Rhamdia quelen) following short term exposure to sublethal concentration of glyphosate. Fish Shellfish Immunol. 2011, 30, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.E. Immunity to bacteria in fish. Fish Shellfish Immunol. 1999, 9, 291–308. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Gruys, E.; Toussaint, M.J.; Niewold, T.A.; Koopmans, S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B 2005, 6, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Rieger, A.M.; Hall, B.E.; Barreda, D.R. Macrophage activation differentially modulates particle binding, phagocytosis and downstream antimicrobial mechanisms. Dev. Comp. Immunol. 2010, 34, 1144–1159. [Google Scholar] [CrossRef] [PubMed]
- Biller-Takahashi, J.D.; Takahashi, L.S.; Saita, M.V.; Gimbo, R.Y.; Urbinati, E.C. Leukocytes respiratory burst activity as indicator of innate immunity of pacu Piaractus mesopotamicus. Braz. J. Biol. 2013, 73, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Royan, M.R.; Gomes, A.S.; Espe, M.; Aksnes, A.; Norberg, B.; Gelebart, V.; Rønnestad, I. The stress response in Atlantic salmon (Salmo salar L.): Identification and functional characterization of the corticotropin-releasing factor (crf) paralogs. Gen. Comp. Endocrinol. 2021, 313, 113894. [Google Scholar] [CrossRef]
- Lemos, L.S.; Angarica, L.M.; Hauser-Davis, R.A.; Quinete, N. Cortisol as a Stress Indicator in Fish: Sampling Methods, Analytical Techniques, and Organic Pollutant Exposure Assessments. Int. J. Environ. Res. Public Health 2023, 20, 6237. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Uysal-Onganer, P.; Kraev, I.; Dodds, A.W.; Guðmundsdóttir, S.; Lange, S. Extracellular vesicles, deiminated protein cargo and microRNAs are novel serum biomarkers for environmental rearing temperature in Atlantic cod (Gadus morhua L.). Aquac. Rep. 2020, 16, 100245. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Andrews, A.M.; Paul, D.; Pachter, J.S. Extracellular vesicles: Mediators and biomarkers of pathology along CNS barriers. Fluids Barriers CNS 2018, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.; Balaj, L.; Breakefield, X.; Lai, C. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Cabillon, N.A.R.; Lazado, C.C. Mucosal Barrier Functions of Fish under Changing Environmental Conditions. Fishes 2019, 4, 2. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, Mucosal Immunity and Vaccination in Teleost Fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef] [PubMed]
- Caipang, C.M.A.; Lazado, C.C. 9—Nutritional impacts on fish mucosa: Immunostimulants, pre- and probiotics. In Mucosal Health in Aquaculture; Beck, B.H., Peatman, E., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 211–272. [Google Scholar] [CrossRef]
- Pérez-Sánchez, J.; Estensoro, I.; Redondo, M.J.; Calduch-Giner, J.A.; Kaushik, S.; Sitjà-Bobadilla, A. Mucins as Diagnostic and Prognostic Biomarkers in a Fish-Parasite Model: Transcriptional and Functional Analysis. PLoS ONE 2013, 8, e65457. [Google Scholar] [CrossRef] [PubMed]
- Sveen, L.R.; Grammes, F.T.; Ytteborg, E.; Takle, H.; Jørgensen, S.M. Genome-wide analysis of Atlantic salmon (Salmo salar) mucin genes and their role as biomarkers. PLoS ONE 2017, 12, e0189103. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I.; Parra, D. 6—Fish Mucosal Immunity: Intestine. In Mucosal Health in Aquaculture; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar] [CrossRef]
- Leino, R.L. Ultrastructure of immature, developing, and secretory rodlet cells in fish. Cell Tissue Res. 1974, 155, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Reite, O.B. The rodlet cells of teleostean fish: Their potential role in host defence in relation to the role of mast cells/eosinophilic granule cells. Fish Shellfish Immunol. 2005, 19, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.M.; Król, E. Nutrigenomics and immune function in fish: New insights from omics technologies. Dev. Comp. Immunol. 2017, 75, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.M.; Dehler, C.E.; Król, E. Transcriptomic responses in the fish intestine. Dev. Comp. Immunol. 2016, 64, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Mancera, J.M.; Costas, B. The Use of Dietary Additives in Fish Stress Mitigation: Comparative Endocrine and Physiological Responses. Front. Endocrinol. 2019, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Matias, A.C.; Soares, F.; Ribeiro, L.; Moreira, M.; Salamanca, N.; Jerez-Cepa, I.; Mancera, J.M.; Astola, A. Effect of amino acid supplementation and stress on expression of molecular markers in meagre (Argyrosomus regius). Aquaculture 2021, 534, 736238. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Sousa, C.; Coutinho, F.; Castro, C.; Fontinha, F.; Guerreiro, I.; Pousão, P.; Matos, E.; Díaz-Rosales, P.; Oliva-Teles, A.; et al. Functional Feeds to Tackle Meagre (Argyrosomus regius) Stress: Physiological Responses under Acute Stressful Handling Conditions. Mar. Drugs 2021, 19, 598. [Google Scholar] [CrossRef] [PubMed]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Monier, M.N.; Abdelrhman, A.M.; Dawood, M.A.O. Effect of dietary multi-stimulants blend supplementation on performance, digestive enzymes, and antioxidants biomarkers of common carp, Cyprinus carpio L. and its resistance to ammonia toxicity. Aquaculture 2020, 528, 735529. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Elbialy, Z.I.; Abdelhamid, A.I. Dietary sodium butyrate ameliorated the blood stress biomarkers, heat shock proteins, and immune response of Nile tilapia (Oreochromis niloticus) exposed to heat stress. J. Therm. Biol. 2020, 88, 102500. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.E.; Ahmed, S.A.A.; Amer, S.A.; Al-Gabri, N.A.; Ahmed, A.I.; Abdel-Warith, A.-W.A.; Younis, E.-S.M.I.; Metwally, A.E. Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020, 18, 100545. [Google Scholar] [CrossRef]
- Guerreiro, I.; Castro, C.; Antunes, B.; Coutinho, F.; Rangel, F.; Couto, A.; Serra, C.R.; Peres, H.; Pousão-Ferreira, P.; Matos, E.; et al. Catching black soldier fly for meagre: Growth, whole-body fatty acid profile and metabolic responses. Aquaculture 2020, 516, 734613. [Google Scholar] [CrossRef]
- Guerreiro, I.; Serra, C.R.; Coutinho, F.; Couto, A.; Castro, C.; Rangel, F.; Peres, H.; Pousão-Ferreira, P.; Matos, E.; Gasco, L.; et al. Digestive enzyme activity and nutrient digestibility in meagre (Argyrosomus regius) fed increasing levels of black soldier fly meal (Hermetia illucens). Aquac. Nutr. 2020, 27, 142–152. [Google Scholar] [CrossRef]
- Moutinho, S.; Pedrosa, R.; Magalhães, R.; Oliva-Teles, A.; Parisi, G.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae larvae meal in diets for European seabass (Dicentrarchus labrax) juveniles: Effects on liver oxidative status and fillet quality traits during shelf-life. Aquaculture 2021, 533, 736080. [Google Scholar] [CrossRef]
- Fawole, F.J.; Adeoye, A.A.; Tiamiyu, L.O.; Ajala, K.I.; Obadara, S.O.; Ganiyu, I.O. Substituting fishmeal with Hermetia illucens in the diets of African catfish (Clarias gariepinus): Effects on growth, nutrient utilization, haemato-physiological response, and oxidative stress biomarker. Aquaculture 2020, 518, 734849. [Google Scholar] [CrossRef]
- Fawole, F.J.; Labh, S.N.; Hossain, M.S.; Overturf, K.; Small, B.C.; Welker, T.L.; Hardy, R.W.; Kumar, V. Insect (black soldier fly larvae) oil as a potential substitute for fish or soy oil in the fish meal-based diet of juvenile rainbow trout (Oncorhynchus mykiss). Anim. Nutr. 2021, 7, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.C.; Capucchio, M.T.; Caldaroni, B.; Magara, G.; Dörr, A.J.M.; Biasato, I.; Biasibetti, E.; Righetti, M.; Pastorino, P.; Prearo, M.; et al. Influence of Hermetia illucens meal dietary inclusion on the histological traits, gut mucin composition and the oxidative stress biomarkers in rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 496, 50–57. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Couto, A.; Barroso, C.; Guerreiro, I.; Pousão-Ferreira, P.; Matos, E.; Peres, H.; Oliva-Teles, A.; Enes, P. Carob seed germ meal in diets for meagre (Argyrosomus regius) juveniles: Growth, digestive enzymes, intermediary metabolism, liver and gut histology. Aquaculture 2016, 451, 396–404. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Barroso, C.; Enes, P.; Couto, A.; Díaz-Rosales, P.; Afonso, A.; Kanashiro, E.; Peres, H.; Matos, E.; Oliva-Teles, A.; et al. Humoral and mucosal immune responses in meagre (Argyrosomus regius) juveniles fed diets with varying inclusion levels of carob seed germ meal. Fish Shellfish Immunol. 2018, 79, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Aanyu, M.; Betancor, M.B.; Monroig, O. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 488, 217–226. [Google Scholar] [CrossRef]
- Catalán, N.; Villasante, A.; Wacyk, J.; Ramírez, C.; Romero, J. Fermented Soybean Meal Increases Lactic Acid Bacteria in Gut Microbiota of Atlantic Salmon (Salmo salar). Probiotics Antimicrob. Proteins 2018, 10, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Monier, M.N.; Hoseinifar, S.H.; Faggio, C. Fish response to hypoxia stress: Growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 2019, 45, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Wang, H.-P.; Yao, H.; Shen, Z.-G.; Shaheen, A.A.; Abou-ElGheit, E.N. Expression of Hsp70, Igf1, and Three Oxidative Stress Biomarkers in Response to Handling and Salt Treatment at Different Water Temperatures in Yellow Perch, Perca flavescens. Front. Physiol. 2017, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.L.; McGowan, C.R.; Cooper, G.A.; Koop, B.F.; Davidson, W.S. Ribosomal genes and heat shock proteins as putative markers for chronic, sublethal heat stress in Arctic charr: Applications for aquaculture and wild fish. Physiol. Genom. 2011, 43, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Hasenbein, M.; Fangue, N.A.; Geist, J.P.; Komoroske, L.M.; Connon, R.E. Physiological stress biomarkers reveal stocking density effects in late larval Delta Smelt (Hypomesus transpacificus). Aquaculture 2016, 450, 108–115. [Google Scholar] [CrossRef]
- Waiho, K.; Afiqah-Aleng, N.; Iryani, M.T.M.; Fazhan, H. Protein–protein interaction network: An emerging tool for understanding fish disease in aquaculture. Rev. Aquac. 2021, 13, 156–177. [Google Scholar] [CrossRef]
- Mohanty, B.; Mohanty, S.; Mitra, T.; Mahanty, A.; Ganguly, S.; Singh, S. Omics Technology in Fisheries and Aquaculture. In Advances in Fish Research; Narendra Publishing House: Delhi, India, 2019; pp. 1–30. [Google Scholar]
- Braceland, M.; Bickerdike, R.; Tinsley, J.; Cockerill, D.; McLoughlin, M.F.; Graham, D.A.; Burchmore, R.J.; Weir, W.; Wallace, C.; Eckersall, P.D. The serum proteome of Atlantic salmon, Salmo salar, during pancreas disease (PD) following infection with salmonid alphavirus subtype 3 (SAV3). J. Proteom. 2013, 94, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Braceland, M.; McLoughlin, M.F.; Tinsley, J.; Wallace, C.; Cockerill, D.; McLaughlin, M.; Eckersall, P.D. Serum enolase: A non-destructive biomarker of white skeletal myopathy during pancreas disease (PD) in Atlantic salmon Salmo salar L. J. Fish Dis. 2015, 38, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.N.; Powell, M.D. The effects of heart and skeletal muscle inflammation and cardiomyopathy syndrome on creatine kinase and lactate dehydrogenase levels in Atlantic salmon (Salmo salar L.). Sci. World J. 2012, 2012, 741302. [Google Scholar] [CrossRef] [PubMed]
- Gjessing, M.C.; Krasnov, A.; Timmerhaus, G.; Brun, S.; Afanasyev, S.; Dale, O.B.; Dahle, M.K. The Atlantic Salmon Gill Transcriptome Response in a Natural Outbreak of Salmon Gill Pox Virus Infection Reveals New Biomarkers of Gill Pathology and Suppression of Mucosal Defense. Front. Immunol. 2020, 11, 2154. [Google Scholar] [CrossRef] [PubMed]
- Pionnier, N.; Adamek, M.; Miest, J.J.; Harris, S.J.; Matras, M.; Rakus, K.; Irnazarow, I.; Hoole, D. C-reactive protein and complement as acute phase reactants in common carp Cyprinus carpio during CyHV-3 infection. Dis. Aquat. Org. 2014, 109, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yi, M.; Xiao, P.; Meng, L.; Li, X.; Sun, G.; Liu, Y. The impact of Aeromonas salmonicida infection on innate immune parameters of Atlantic salmon (Salmo salar L). Fish Shellfish Immunol. 2015, 44, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Lagos, L.; Tandberg, J.; Kashulin-Bekkelund, A.; Colquhoun, D.J.; Sørum, H.; Winther-Larsen, H.C. Isolation and Characterization of Serum Extracellular Vesicles (EVs) from Atlantic Salmon Infected with Piscirickettsia Salmonis. Proteomes 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, B.; Xu, Z.; Jia, L.; Li, M.; He, X.; Bao, B. Detecting Cynoglossus semilaevis infected with Vibrio harveyi using micro RNAs from mucous exosomes. Mol. Immunol. 2020, 128, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Huang, X.-Y.; Yang, M.-J.; Wang, S.; Ren, S.-T.; Li, H.; Peng, X.-X. GC/MS-based metabolomics approach to identify biomarkers differentiating survivals from death in crucian carps infected by Edwardsiella tarda. Fish Shellfish Immunol. 2014, 39, 215–222. [Google Scholar] [CrossRef]
- Song, X.; Zhao, J.; Bo, Y.; Liu, Z.; Wu, K.; Gong, C. Aeromonas hydrophila induces intestinal inflammation in grass carp (Ctenopharyngodon idella): An experimental model. Aquaculture 2014, 434, 171–178. [Google Scholar] [CrossRef]
- Okoroiwu, H.U.; Iwara, I.A. Dichlorvos toxicity: A public health perspective. Interdiscip. Toxicol. 2018, 11, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Awaisheh, S.S.; Khalifeh, M.S.; Rahahleh, R.J.; Al-Khaza’leh, J.M.; Algroom, R.M. Sulfamethazine contamination level and exposure assessment in domestic and imported poultry meats in Jordan. Vet. World 2019, 12, 1992–1997. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, F.G.; Carra, M.L.; Jonsson, C.M.; Gonçalves, V.T.; Dal’Bo, G.; Nunes, K.S.D.; Valim, J.H.; Dallago, B.S.L.; do Nascimento de Queiroz, S.C.; Reyes, F.G.R. Effects of Dietary Exposure to Sulfamethazine on the Hematological Parameters and Hepatic Oxidative Stress Biomarkers in Nile Tilapia (Oreochromis niloticus). Bull. Environ. Contam. Toxicol. 2016, 97, 528–535. [Google Scholar] [CrossRef]
- Julinta, R.B.; Abraham, T.J.; Roy, A.; Singha, J.; Boda, S.; Patil, P.K. Dietary influences of oxytetracycline on the growth and serum biomarkers of Oreochromis niloticus (L.). Ecotoxicol. Environ. Saf. 2019, 186, 109752. [Google Scholar] [CrossRef] [PubMed]
- Shiogiri, N.S.; Ikefuti, C.V.; Carraschi, S.P.; da Cruz, C.; Fernandes, M.N. Effects of azithromycin on tilapia (Oreochromis niloticus): Health status evaluation using biochemical, physiological and morphological biomarkers. Aquac. Res. 2017, 48, 3669–3683. [Google Scholar] [CrossRef]
- de Sousa, E.L.; Assane, I.M.; Santos-Filho, N.A.; Cilli, E.M.; de Jesus, R.B.; Pilarski, F. Haematological, biochemical and immunological biomarkers, antibacterial activity, and survival in Nile tilapia Oreochromis niloticus after treatment using antimicrobial peptide LL-37 against Streptococcus agalactiae. Aquaculture 2021, 533, 736181. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kurhaluk, N.; Grudniewska, J. Biomarkers of oxidative stress and antioxidant defences as indicators of different disinfectants exposure in the heart of rainbow trout (Oncorhynchus mykiss Walbaum). Aquac. Res. 2015, 46, 679–689. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kurhaluk, N.; Grudniewska, J.; Andriichuk, A. Tissue-specific responses of oxidative stress biomarkers and antioxidant defenses in rainbow trout Oncorhynchus mykiss during a vaccination against furunculosis. Fish Physiol. Biochem. 2014, 40, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. Toward a road map for global -omics: A primer on -omic technologies. Am. J. Epidemiol. 2014, 180, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Chandhini, S.; Rejish Kumar, V.J. Transcriptomics in aquaculture: Current status and applications. Rev. Aquac. 2019, 11, 1379–1397. [Google Scholar] [CrossRef]
- Forné, I.; Abián, J.; Cerdà, J. Fish proteome analysis: Model organisms and non-sequenced species. Proteomics 2010, 10, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Primrose, S.B.; Twyman, R. Principles of Genome Analysis and Genomics; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Huete-Pérez, J.A.; Quezada, F. Genomic approaches in marine biodiversity and aquaculture. Biol. Res. 2013, 46, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.M.; Silva, T.S.; Dias, J.; Jessen, F. PROTEOMICS in aquaculture: Applications and trends. J. Proteom. 2012, 75, 4325–4345. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Kumar, G.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. Proteomics for understanding pathogenesis, immune modulation and host pathogen interactions in aquaculture. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 32, 100625. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Ellis, D.I. Metabolomics: Current analytical platforms and methodologies. TrAC Trends Anal. Chem. 2005, 24, 285–294. [Google Scholar] [CrossRef]
- Young, T.; Alfaro, A.C. Metabolomic strategies for aquaculture research: A primer. Rev. Aquac. 2018, 10, 26–56. [Google Scholar] [CrossRef]
- Canellas, A.L.B.; Costa, W.F.; Freitas-Silva, J.; Lopes, I.R.; de Oliveira, B.F.R.; Laport, M.S. In sickness and in health: Insights into the application of omics in aquaculture settings under a microbiological perspective. Aquaculture 2022, 554, 738132. [Google Scholar] [CrossRef]
- Miller, M.G. Environmental metabolomics: A SWOT analysis (strengths, weaknesses, opportunities, and threats). J. Proteome Res. 2007, 6, 540–545. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.; Oliva-Teles, A.; Couto, A. Tracking Biomarkers for the Health and Welfare of Aquaculture Fish. Fishes 2024, 9, 289. https://doi.org/10.3390/fishes9070289
Oliveira J, Oliva-Teles A, Couto A. Tracking Biomarkers for the Health and Welfare of Aquaculture Fish. Fishes. 2024; 9(7):289. https://doi.org/10.3390/fishes9070289
Chicago/Turabian StyleOliveira, Joana, Aires Oliva-Teles, and Ana Couto. 2024. "Tracking Biomarkers for the Health and Welfare of Aquaculture Fish" Fishes 9, no. 7: 289. https://doi.org/10.3390/fishes9070289
APA StyleOliveira, J., Oliva-Teles, A., & Couto, A. (2024). Tracking Biomarkers for the Health and Welfare of Aquaculture Fish. Fishes, 9(7), 289. https://doi.org/10.3390/fishes9070289