Abstract
The European eel encounters challenges in achieving sexual maturation in captivity, which has been a concern for researchers. This study explores surrogate broodstock technology as an alternative approach for eel production. The present study aimed to evaluate zebrafish and European sea bass as potential recipients for European eel spermatogonia transplantation, given the abundance of eel type A spermatogonia (SPGA). Immature European eel testes were dissected and maintained at 4 °C or cryopreserved. SPGA were obtained by dissociation of fresh or post-thawed tissue, employing an enzymatic solution, and then labelled with fluorescent membrane marker PKH26. SPGA from fresh tissue were transplanted into wild-type zebrafish larvae and triploid European sea bass larvae, while SPGA from cryopreserved testis were transplanted into vasa::egfp transgenic zebrafish larvae. One-and-a-half months post-transplantation (mpt), fluorescent donor cells were not detected in the gonads of zebrafish or European sea bass. Molecular qPCR analyses at 1.5 or 6 mpt did not reveal European eel-specific gene expression in the gonads of any transplanted fish. The findings suggest that the gonadal microenvironments of zebrafish and European sea bass are unsuitable for the development of European eel spermatogonia, highlighting distinctive spermatogonial stem cell migration mechanisms within teleost species
Keywords:
transplantation; surrogate production; surrogacy; spermatogonial stem cells; testis; cryopreservation Key Contribution:
Surrogate broodstock technology is being explored for European eel using zebrafish and European sea bass larvae as recipients. After xenotransplantation, eel spermatogonia were not detected in their gonads, indicating an unsuitable microenvironment.
1. Introduction
The European eel (Anguilla anguilla) is a commercial fish species, the life cycle of which includes two transoceanic migrations. After spending an average of 5–25 years as yellow eels in continental waters, they gradually transition to silver eels while migrating to seawater. The sexual maturation of both males and females occurs during their journey to the Sargasso Sea, their spawning area [1,2,3]. The eels are exploited at all life stages, but their associated aquaculture production is based on the catch of glass eels and their subsequent rearing, since the life cycle of the eels has not yet been closed in captivity [4,5]. Moreover, the indices of glass and yellow eel recruitment have suffered a decline of 95–99% compared to the mean levels of 1960–1976, and they have remained at a critical level since then [6]. The causes of the European eel decline are numerous, including barriers to migrations, pathogens, overfishing, habitat loss, and pollutants [7,8]. For these reasons, the species is currently classified as “critically endangered” according to the IUCN [9], and the European Union has established measures for recovering the population (Regulation 1100/2007, 18 September 2007). To meet the growing demand for eels, captive reproduction seems the only viable long-term alternative which can reduce pressure on their natural populations. However, the European eel is not able to mature sexually in captivity because there is a blockage in the hypothalamus–pituitary–gonad axis and a dopaminergic inhibition of the gonadotropins-releasing hormone [10,11]. Thus, to overcome the lack of normal spawning stimuli in captivity, the maturation of both males [12,13] and females [14,15,16] must be induced by long-term hormonal treatments. Nonetheless, there is a high degree of divergence relative to the hormonal treatment response. This is especially the case for females, with significant variability in the percentage of spawning, egg quality, and hatching larvae [17]. Thus far, conventional reproduction methods have been predominantly employed for the sexual maturation of European eel (hypophysation of females [18]; human chorionic gonadotropin injections for males [19]). Alternative methods and technologies for eel breeding have been assessed, such as forced swimming, simulating migration [20]; the use of androgen-releasing implants [21]; specific-recombinant gonadotropins [22,23]; thermal pretreatments [24,25]; osmotic pumps [26]; and gonadotropin plasmid gene therapy [27], resulting in some encouraging results for European eel reproduction.
In general, the study of reproductive techniques has been crucial in the technological improvement of aquaculture, and recently, the development of surrogate broodstock technology in fish seems to be proving a useful method to produce certain species [28,29]. The surrogate broodstock technology in aquaculture produces donor-derived gametes in a recipient fish (surrogate individual) by transplanting germline stem cells of the donor into the recipient from a different strain or species [30,31]. Surrogacy offers numerous benefits to fish production, such as (i) the reduction of space required for breeding large species [32,33], (ii) the preservation and restoration of genetic resources using cryopreserved cells, and (iii) the control of egg and sperm production [30,34,35,36]. There are different types of germ cells that might be considered for xenotransplantation. In fish, the undifferentiated type A spermatogonia (SPG Aund) (putative spermatogonial stem cells, SSCs) are defined as subpopulations of type A spermatogonia (SPGA) that will undergo several mitotic divisions, the number of which is teleost species-specific, before differentiating throughout the spermatogenesis [37,38]. The plasticity of SPGAund in fish allows them to differentiate into sperm or eggs, depending on the sex of the surrogate recipient [39,40]. Recently, the first inter-family xenotransplantation in marine fish species was achieved, in which Japanese flounder (Paralichthys olivaceus) produced functional turbot (Scophtalmus maximus) spermatozoa [41]. Although the transplant success rate had initially been high, the survival rate of the recipients at 2 and 3 years post-transplantation was reported to be very low; therefore, gametes were obtained from only a small number of animals.
It appears that, among the major concerns in xenotransplantation, it is not only important to choose a host species that is compatible with the donor, but also to ensure that its reproduction is advantageous for the production of the donor species [36]. Hattori et al. [42] reported that xenotransplantation of SPG from Atlantic salmon into triploid rainbow trout produced donor-derived sperm and oocytes in 10% and 12.1% of the transplanted fishes, respectively. The recipient fish required between 1–2 years (for the males) and 3 years (for the females) to mature; hence, the xenotransplantation shortened the normal maturation time of Atlantic salmon by 1 year. Regarding xenotransplantation between phylogenetically more-distant species, Saito et al. [43,44] performed single primordial germ cell (PGC) xenotransplantation between Japanese eel (A. japonica) and zebrafish (Danio rerio); hybrid beluga (Huso huso) × sterlet (Acipenser ruthenus) into goldfish (Carassius auratus); and sterlet into goldfish. In these cases, donor cells were incorporated in the recipient embryos, but production of donor-gametes was not yielded. Finally, similar results occurred in other species when xenotransplantation of SSCs was attempted into recipient larvae. SSCs from southern bluefin tuna (Thunnus maccoyii) [45], Pacific blue tuna (T. orientalis) [46,47], American paddlefish (Polyodon spathula) [48], Japanese yellowtail (Seriola quinqueradiata) [49], and nibe croaker (Nibea mitsukurii) [50] were transplanted into larvae from yellowtail kingfish (S. lalandi) [45], Dabry’s sturgeon (A. dabryanus) [48], nibe croaker [49], chub mackerel (Scomber japonicus) [46,50] or hybrid mackerel (S. australasicus × S. japonicus) [47]. In those cases, donor cells were incorporated [45,46,47,48,49,50], but donor gamete production was not obtained. Nevertheless, Silva et al. [51] showed that adult Nile tilapia (Oreochromis niloticus) was a suitable recipient species for SSCs from Jundia catfish (Rhamdia quelen), reporting donor-derived sperm 4 months post-transplantation in the testis of the recipient adult tilapia. Regarding the selection of the host, it is necessary to consider their sterilization in order to avoid competition among donor germ cells for the niche, which could lower efficiency in transplantation. There are several sterilization strategies, such as triploidization [52], knockdown of genes involved in germ cell development [53], or the use of hybrid species [54]. However, it has been demonstrated that donor-derived gametes could be also obtained from non-sterilized recipients, although with limited results [55,56].
Therefore, the present study has aimed to evaluate two teleost species with well-known life-cycles for captive reproduction, but which are evolutionarily distant from the eel, as potential recipients for the xenotransplantation of European eel spermatogonia: the European sea bass (Dicentrarchus labrax) and the zebrafish.
2. Materials and Methods
2.1. European Eel Handling and Sampling
Several batches of live immature European eels ranging between 68–187 g and 30–46 cm were bought at the local market and transported to the Laboratory of Fish Reproduction facilities at the Universitat Politècnica de València (UPV, Valencia, Spain). A total of 36 eels were placed into 150 L freshwater aquaria equipped with separate recirculation systems and thermostats. The fish were fasted during the experiment, and the aquaria were covered to maintain constant shade and reduce fish stress. Fish were sacrificed via a benzocaine overdose followed by decapitation. Testis were extracted and weighed to calculate the gonadosomatic index (GSI = 100 · gonad weight/total body weight) and to process them for use in the consecutive experimental stages (Figure 1).
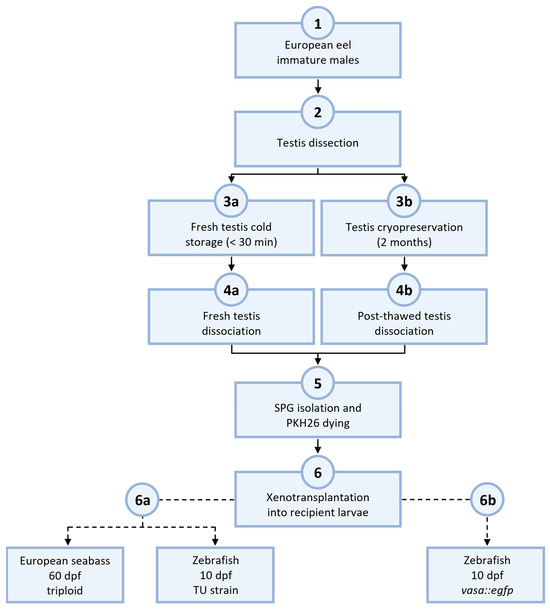
Figure 1.
Experimental design of European eel spermatogonial transplantation. Testis from immature eels (1) were dissected (2) and kept in cold storage for less than 30 min (3a) or cryopreserved for storage of more than 2 months (3b). The same dissociation protocol was applied for both groups of preserved testis (4a and 4b). After the spermatogonia isolation and dyeing via the use of fluorescent linker-dye PKH26 (5), the SPG obtained were utilized for the xenotransplantation into recipient larvae (6). Eel SPG from fresh testis was transplanted into triploid European sea bass or TU zebrafish larvae (6a). Eel SPG from thawed testis was transplanted into transgenic vasa::egfp zebrafish larvae (6b).
2.2. European Sea Bass Broodstock and Larvae Sterilization
European sea bass individuals were reared in the Aquaculture Experimental Station at the Institut Français de Recherche pour l’Exploitation de la Mer (IFREMER, Palavas-les-Flots, France). Sea bass broodstock originated from domesticated stocks at IFREMER, were maintained in a closed recirculating system under controlled conditions, and fed on a commercial diet. A total of 50 5-year-old mature male and 5-year-old mature female European sea bass were kept in fiberglass tanks (10 m3) with a continuous supply of seawater (700 L/h) maintained at natural water temperature. Five batches of larvae were sterilized by triploidization to be used as sterile recipients in the xenotransplantation. In order to pressurize the eggs, they were placed in a 200 mm stainless steel cylinder full of water. The cylinder was closed by a piston before receiving a 2 min hydraulic pump pressure shock (Enerpac BBS 1212) (a pressure shock of 8500 psi) applied 6 min after fertilization [57]. Immediately after the pressure treatment, the eggs were divided into two groups and transferred to cylindroconical black gel-coated 0.5 m3 tanks with a closed recirculating system. Larvae were reared at 16 °C from hatching to 71 days post-hatching (dph), and then at 20 °C until the end of the experiment. Larvae were fed daily with a commercial diet, and the rearing method was as described by Peruzzi et al. [58].
2.3. Zebrafish Broodstock and Larvae Collection
2.3.1. Experiment 1
Tübingen (TU) zebrafish individuals were reared at the Laboratory of Fish Reproduction in the UPV. Zebrafish broodstock were maintained under controlled photoperiod (14L:10D) and temperature (27.5 °C) conditions in a Tecniplast Zebtect recirculating system. Fish were fed twice daily with a commercial diet (ZEBRAFEED, Sparos LDA, Olhão, Portugal). Approximately 15 h before spawning, adult zebrafish (sex ratio 1:1) were divided into four 1.7 L sloping breeding tanks separated by a barrier (Tecniplast, UK). The wall separating the adults was removed 15 min before the lights were turned on, and upon lighting, it was observed that the adults began spawning. The eggs were collected immediately after spawning and placed in a nursery tank within 3.5 L tanks and inside a recirculating system without water flow until hatching. After 24 h, eggs with an opaque appearance (dead or unfertilized) were removed. Larvae were fed daily with Artemia nauplii and twice a day with a commercial diet of different pellet sizes according to the recommended feeding described in the ZEBRAFEED protocol. Zebrafish larvae were fasted for two days before xenotransplantation to facilitate the microinjection.
2.3.2. Experiment 2
Zebrafish from the vasa::egfp (ddx4sa6158/sa6158) transgenic line were reared at the facilities of the Institute of Aquaculture and Environmental Safety at the Hungarian University of Agriculture and Life Sciences (Gödöllő, Hungary). The zebrafish broodstock were maintained under a controlled photoperiod (14L:10D) and temperature (25 °C) conditions and were fed twice a day with SDS Small Gran granulated feed and with Artemia nauplii. The spawning procedure was very similar to the one performed at UPV. Namely, approximately 16 h before spawning, adult zebrafish (sex ratio 1:1) were divided into four 1.7 L sloping breeding tanks separated by a barrier (Tecniplast, IT). The barrier was removed after the lights were turned on, and the eggs were collected immediately after spawning and placed in a Petri dish. After 24 h, eggs with an opaque appearance (dead or unfertilized) were removed. Larvae were fed daily with Artemia nauplii and twice a day with a commercial diet of different pellet sizes according to the recommended feeding described in the ZEBRAFEED protocol. Larvae were not fasted before the injection, in order to improve larvae viability.
2.4. Cryopreservation of European Eel Testes
The cryopreservation protocol of immature eel testes has previously been optimized [59]. Namely, testes from immature eels (n = 10 mean body weight = 100 ± 7 g; GSI = 0.011 ± 0.001) were dissected and washed in L-15 medium three times. Testes were then cut into pieces of approximately 3–4 mm and placed in cryotubes with cryopreservation medium (10% DMSO, 0.1 M glucose, and 1.5% BSA in L-15) for a 15 min equilibration on ice. The cryotubes were transferred to a CoolCellTM box (Corning) and then placed into a deep freezer at −80 °C for approximately 90 min. This enabled a cooling rate of ~1 °C/min. Subsequently, cryotubes were plunged into liquid nitrogen and were kept under that condition for 2 months until tissue dissociation.
2.5. Enzymatic Dissociation of Gonad Cells
For the European sea bass experiment, testes from immature European eels (n = 14, mean body weight = 122 ± 6 g; GSI = 0.040 ± 0.003) were dissected, washed three times in a 24-well plate with L-15 and kept at 4 °C until fresh tissue dissociation. The zebrafish experiments used cells isolated from both frozen and fresh European eel testes. Cryotubes were immersed into a 15 °C water bath until completely thawed. The thawed testes pieces were transferred to a 24-well plate with Leibovitz medium (L-15; Merck, Darmstadt, Germany) and washed 3 times for 5 min. Similarly, fresh testes from immature eels (n = 7, mean body weight = 129 ± 12 g; GSI = 0.062 ± 0.006) were dissected and washed three times in a 24-well plate with L-15 and kept on ice until tissue dissociation.
Regarding tissue dissociation, fresh or thawed testes were placed into tubes containing 5 mL of an enzymatic solution composed of L-15 with 0.15% trypsin, 0.2% collagenase and 0.01% DNase. Tissue pieces were cut and minced into small fragments and then incubated in an orbital shaker for 1.5 h at 23 °C, applying repeated trituration every 30 min. After incubation, the enzymatic reaction was stopped by the addition of 10% FBS. The obtained suspension was filtered through a 20 µm filter (CellTrics®, Sysmex, Barcelona, Spain). The tubes with gonad cell suspension were centrifuged at 250× g for 15 min at 23 °C. The pellets were resuspended in L-15 medium, and cells were counted using a Neubauer haemocytometer. The gonad cell suspensions were labelled using the PKH26 Red Fluorescent Cell Linker Kit (Merck) according to the manufacturer’s protocol (1:20 final dye concentration) and stored at 4 °C until further use (between 24 and 96 h) (Figure 2).
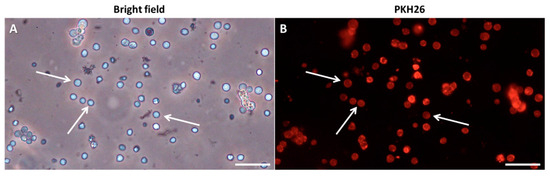
Figure 2.
Testicular cell suspensions from immature European eel labelled with PKH-26 under phase contrast (A) and under a fluorescent filter (B). Arrows indicate spermatogonia. Scale bar: 50 µm.
2.6. Transplantation of Eel Spermatogonia into Triploid European Sea Bass Larvae
Glass capillaries (borosilicate glass, GC100F-10, Harvard Apparatus Ltd., Kent, UK) were pulled into microinjection needles by use of a vertical puller (PC-10, Narishigue). The needles were ground with a microgrinder (EG-45, Narishigue) under a 30° angle to create a sharp edge. For transplantation, 400 recipient larvae (60 dph, [60]) were anesthetized with MS222 (25 mg/L) diluted in isosmotic water and transferred onto glass sheets covered with disposable paper to prevent the larvae from slipping. Larvae were divided into two transplantation groups according to the number of injected cells. Glass needles were loaded with the cell suspension via negative pressure. Approximately 0.5 µL, containing 37,500 cells (high concentration group, HC) or 3000 cells (low concentration group, LC), was injected into the peritoneal cavity close to the presumptive genital ridge using a mechanical micromanipulator (Leica Leitz, Wetzlar, Germany) and a microinjector (CellTram® Eppendorf, Hamburg, Germany) under a Leica M80 stereomicroscope. After transplantation, recipient sea bass larvae were transferred to a bucket with an aeration pipe to check their survival before being transferred into their rearing tanks. Control larvae (triploids but non-transplanted) were also stocked into separate rearing tanks under the same conditions as the transplanted larvae.
2.7. Transplantation of Eel Spermatogonia into Zebrafish Larvae
2.7.1. Experiment 1. Transplantation of Fresh Eel Spermatogonia into TU Zebrafish
Glass capillaries (borosilicate glass, GC100F-10, Harvard Apparatus Ltd., Kent, UK) were pulled into microinjection needles using a vertical puller (PC-10, Narishigue). The tips of the needles were ground with a microgrinder (EG-45, Narishigue) to a 35° angle and with an opening of 20–30 µm. For transplantation, recipient larvae (10 dpf) were anesthetized with 0.03% 2-phenoxyethanol and transferred into a Petri dish coated with 2% agar. Glass needles were loaded with the cell suspension using microloader tips. Approximately 0.3 µL of cell suspension, containing 15,000 cells, was manually injected into the peritoneal cavity between the swim bladder and the intestines (Video S1), using a microinjector (FemtoJet® 4x, Eppendorf, Hamburg, Germany). Following transplantation, recipient larvae were transferred to a 1.7 L recovery tank filled with system water before being transferred into their rearing tanks. One hundred and fifty-two fish were xenotransplanted with eel spermatogonia from fresh testis, while cell medium was injected into one hundred and ten larvae as operational control, and one hundred and seventy individuals were kept as intact control.
2.7.2. Experiment 2. Transplantation of Thawed Eel Spermatogonia into Transgenic vasa::egfp Zebrafish
Glass capillaries (borosilicate glass, G-1, Narishigue Scientific, Tokyo, Japan) were pulled using a horizontal needle puller (PN-31, Narishigue). The tips of the needles were broken with the use of curved tweezers to obtain a sharp edge. For transplantation, recipient larvae (10 days post-fertilization, dpf) were anesthetized with 0.03% 2-phenoxyethanol and transferred into a Petri dish coated with 2% agar. Glass needles were loaded with the cell suspension using microloader tips (Eppendorf, Hamburg, Germany). Approximately 0.3 µL of cell suspension, containing 3000 cells, was manually injected into the peritoneal cavity of each larva between the swim bladder and the intestines [61], using a microinjector (MINJ-1 microINJECTORTM system, Tritech Research, Los Angeles, CA, USA) employed under a binocular stereomicroscope. Following transplantation, recipient larvae were placed into a Petri dish filled with system water in an incubator at 26 °C. After 2 days, larvae were transferred into their rearing tanks. One hundred larvae were xenotransplanted with eel cells from thawed testes, while cell medium was injected into fifty larvae as operational control, and thirty individuals were kept as intact control.
2.8. Detection of Donor European Eel Spermatogonia in Transplanted Larvae
2.8.1. Verification of the Presence of Transplanted Eel Cells in European Sea Bass and Zebrafish by Fluorescent Labelling and qPCR
In the case of the European sea bass, larvae were anesthetized, and fluorescence was checked 24 h following the microinjection in the control and transplanted fish. At 1.5 months post-transplantation (mpt), these were euthanized via an anesthetic overdose, and gonads (n = 15, 15 and 17 from the HC, LC and control groups, respectively) were dissected to observe their fluorescence under a Leica DM2000 LED microscope and a Leica MC190 HD camera.
Moreover, gonads were also sampled for Quantitative Polymerase Chain Reactions (qPCR) analyses. Due to their small size (1.5 mpt), the viscera were removed and the whole-body mid-segment of the larvae containing the gonads was taken (n = 15 HC group, n = 15 LC group, n = 8 control group) and kept in RNAlater at −20 °C until RNA extraction. At 6 mpt, gonads and muscle from each group (n = 20) were dissected for qPCR analyses and tissues were kept in RNAlater at −20 °C until RNA extraction.
For histology analyses, gonads (n = 5/group) were dissected and fixed in 10% NBF for 24 h. Samples were then dehydrated with increasing percentages of ethanol and embedded in resin (Technovit 7100), as per the instructions of the manufacturer. Sections measuring 5 µm were cut with a Microm HM325 microtome and stained with hematoxylin and eosin. The slides were observed with a Nikon Eclipse E-400 microscope and pictures were taken with a FullHD camera (Moticam 1080).
In the case of the zebrafish, a sampling for both experiments was performed 1.5 mpt. Animals were euthanized with an anesthetic overdose and dissected to observe the fluorescence in their gonads under a fluorescent stereomicroscope. Samples of gonad and muscle were collected from the control (experiment 1, n = 10; experiment 2, n = 4) and transplanted (experiment 1, n = 15 fish; experiment 2, n = 20 fish) groups and preserved in RNAlater at −20 °C until qPCR analyses.
2.8.2. Identifying Species-Specific Genes
The qPCR analyses were undertaken using species-specific primers (Table 1). The target genes were vasa and dnd1. qPCR primers for vasa1, vasa2 and dnd1 from European eel were previously described by Blanes-García et al. [62]. Primers for European sea bass (SB-vasa, accession number: XM_051427266.1; SB-dnd1, accession number: XM_051421257.1) and zebrafish (ZF-vasa, accession number: XM_005156453.4; ZF-dnd1, accession number: NM_212795.1) were designed based on the published sequences for each species and employing the Primer-Blast primer-designing tool from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/, accessed on 2 August 2022). Primers were purchased from Integrate DNA Technology Inc. (IDT; Coralville, IA, USA). Before carrying out these analyses, primers were tested on European eel, European sea bass and zebrafish gonads and muscle to confirm that there was no cross-reaction between the eel and the recipient species.

Table 1.
Specific primer sets used for qPCR. AA: European eel; ZF: zebrafish; SB: European sea bass; FW: Forward primer; RV: Reverse primer.
2.8.3. RNA Extraction and Reverse Transcription
The total RNA from preserved tissue samples from the European sea bass and zebrafish was isolated using phenol/chloroform extraction in the Trizol reagent (Life Technologies, Inc.; Carlsbad, CA, USA), as described by Morini et al. [63]. The RNA concentration and 280/260 and 280/230 ratios were determined using a NanoDrop 2000C Spectrophotometer (Fisher Scientific SL; Valencia, Spain). A Deoxyribonuclease I treatment and first-strand complementary DNA (cDNA) synthesis were performed from 500 ng of total RNA using a QuantiTect Reverse Transcription kit (Qiagen; Hilden, Germany), following the manufacturer’s instructions.
2.8.4. Gene Expression by Quantitative Real-Time PCR
The expression levels of vasa and dnd1 genes in the samples for control and xenotransplanted European sea bass and zebrafish were measured by performing qPCR assays using a model StepOnePlusTM (Applied Biosystems; Foster City, CA, USA) with Maxima SYBR Green/ROX qPCR MasterMix (ThermoFisher Scientific; Waltham, MA, USA). The qPCR protocol was performed, comprising an initial step of 95 °C for 10 min and 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s. To evaluate assay specificity, the machine performed a melting curve analysis directly after PCR by slowly (0.3 °C/s) increasing the temperature from 60 to 95 °C, with continuous registration of any changes in fluorescent emission intensity. The total volume for each qPCR reaction was 20 µL, with 5 µL of previously diluted cDNA (1:20) template, forward and reverse primers (250 nM each) and SYBR Green/ROX Master Mix (12 µL). Serial dilutions of the cDNA pool of gonad tissues were run in duplicate and used as a standard curve to measure vasa and dnd1 efficiency in adult European sea bass gonads, zebrafish gonads, and European eel testis. As a calibrator, a 1:20 dilution was included in each run for the respective gene. Absolute expression of mRNA levels was quantified by using an efficiency-corrected expression [64]. Target genes in samples were run in duplicate qPCR reactions. A non-template control (cDNA replaced by water) for each primer pair was replicated in all plates.
2.9. Statistical Analysis
The mean ± standard error (SEM) was calculated for gene expression data. The Shapiro–Wilk test and Levene’s test were used to check the normality of data distribution and the variance homogeneity, respectively. Kruskal–Wallis tests were used to compare differences between expression levels in each experimental group. In all cases, significant differences were detected when the p-value < 0.05. All statistical analyses were performed using the statistical package SPS version 24.0 for Windows software (SPSS Inc., Chicago, IL, USA). Data graphs were created using the ggplot2 package from R [65].
3. Results
3.1. Eel Spermatogonia Xenotransplantation into European Sea Bass Larvae
European sea bass larvae were xenotransplanted with fresh cells (Table 2). A day after microinjection, a low mortality rate was observed among the larvae from both HC and LC groups (94 and 92% of larvae survival, respectively). Moreover, observation was made of the area around the injection point (Figure 3A) in the transplanted (Figure 3C,D) and control (Figure 3B) larvae. Fluorescence was observed in the transplanted larvae around the swim bladder (Figure 3F–I) but was not visible in the control larvae (Figure 3E). However, at 1.5 mpt, PKH26-labelled cells were not observed, either in the gonads from the control group (Figure 4G) or in those of the transplanted groups (Figure 4H,I). The sampled sea bass from both groups presented rudimentary gonads (Figure 4A–F). At 6 mpt, the gonads of both control and transplanted groups consisted mainly of somatic cells, along with the presence of some germ cells (SPGA and type B spermatogonia; Figure S1).

Table 2.
Summary of the survival rate (%) from transplanted and control groups just before the samplings at 1.5 and 6 months post-transplantation.
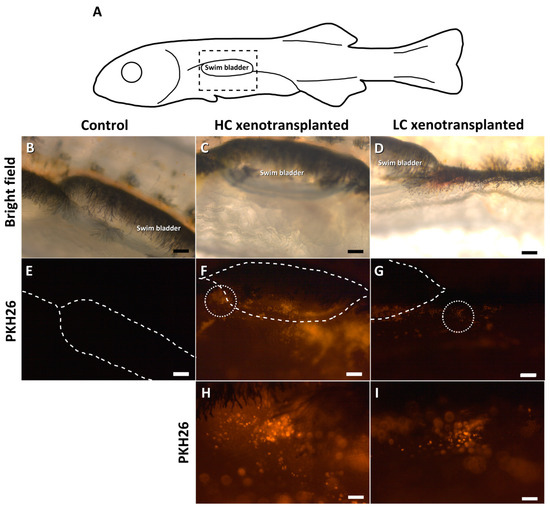
Figure 3.
Detailed pictures of the injection point (A) in European sea bass 24 h after the xenotransplantation: control group (B,E), high cell-concentration group (C,F,H) and low cell-concentration group (D,G,I). The dashed rectangle in A indicates the enlarged area (B–D). The swim bladder position is illustrated with dashed lines (E–G). The dotted circles in F and G indicate the enlarged area, within which the injected eel cells are shown (H,I). Scale bars: (B–G): 200 µm; (H,I): 50 µm.
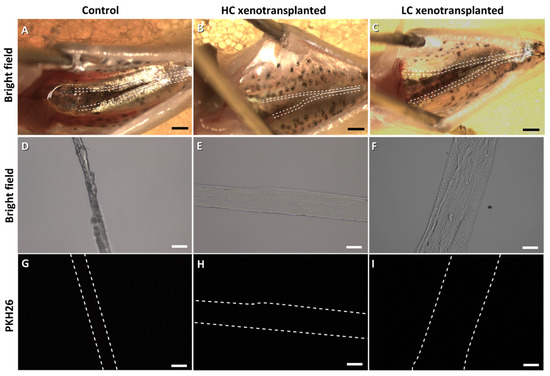
Figure 4.
European sea bass gonads 1.5 months post-xenotransplantation from the control group (A,D,G), high cell-concentration group (HC: (B,E,H)) and low cell-concentration group (LC: (C,F,I)). The gonads’ positions are shown with dashed lines. Scale bars: (A–C): 200 µm; (D–I): 50 µm.
3.2. Eel Spermatogonia Xenotransplantation into Zebrafish Larvae
TU zebrafish larvae were xenotransplanted with fresh cells (Table 2; experiment 1). The area around the injection point (Figure 5A) was observed in the transplanted (Figure 5B) and control (Figure 5C) larvae 1 week after transplantation. Fluorescence was observed in the transplanted larvae, with high intensity around the swim bladder (Figure 5E,G), while no fluorescence was found in the control larvae (Figure 5D,F).
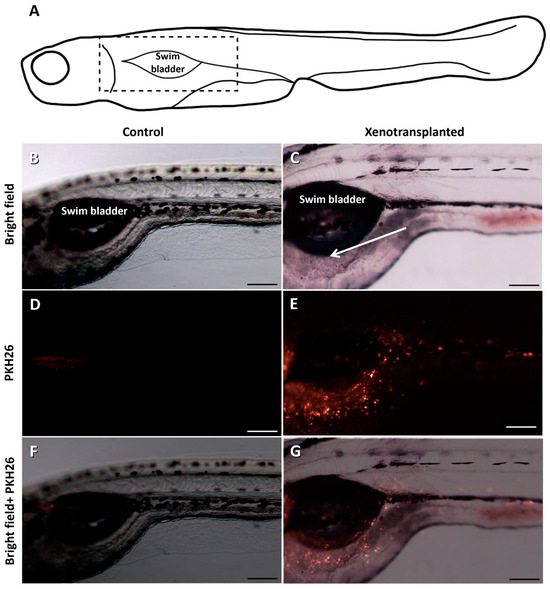
Figure 5.
Detailed pictures of the area around the injection point (A) in zebrafish 7 days after the xenotransplantation: control group (B,D,F) and xenotransplanted group (C,E,G). Fluorescence can be observed next to the swim bladder in the xenotransplanted fish. The arrow indicates the injection point. The dashed rectangle in (A) indicates the enlarged area (B–G). Scale bars: 200 µm.
Thawed eel SPG were xenotransplanted into vasa::egfp zebrafish larvae (Table 2; experiment 2). The survival rate among those injected with spermatogonia or with L-15 (operational control) was similar to the control group in both experiments. At 1.5 mpt, recipient gonads were identified visually in the TU zebrafish (Figure 6A,B) and by their green fluorescence in transgenic vasa::egfp (Figure 6E,F). However, the incorporation of PKH26-labelled cells was not observed in the gonads from the control (Figure 6C,G) or transplanted groups (Figure 6D,H) in either of the two zebrafish experiments. The zebrafish experiments were not extended until 6 mpt due to the results obtained in the first sampling at 1.5 mpt and given the results of the European sea bass experiment.

Figure 6.
Zebrafish gonads 1.5 months post-transplantation in both experiments: control group (experiment 1: (A,C); experiment 2: (E,G)) and transplanted group (experiment 1: (B,D); experiment 2: (F,H)). Expression of the vasa gene in the gonads from transgenic vasa::egfp: control (E) and xenotransplanted (F) zebrafish. Red fluorescent-labelled cells were not observed in the control or xenotransplanted groups from either experiment (C,D,G,H). The swim bladder position is shown with dashed lines. Scale bars: 100 µm.
3.3. Gene Expression in Samples from Zebrafish and European Sea Bass
In relation to the detection of European eel genes AA-vasa1, AA-vasa2 or AA-dnd1, none of the genes were detected in the control or transplanted groups in xenotransplanted species.
The levels of expression of the species-specific vasa and dnd1 were compared in different tissues of European sea bass and zebrafish. In the case of the European sea bass, there were no differences in expression between the control and transplanted groups. At 1.5 mpt, a very weak SB-vasa expression was detected in the mid-part samples that contained the gonads, while there was no expression of SB-dnd1 (Figure 7A). However, significant expression was detected in gonad samples at 6 mpt for both genes without apparent differences between SB-vasa or SB-dnd1 (Figure 7B). As for the muscle, SB-vasa expression was significantly lower than that of SB-dnd1, although expression levels were still very weak compared to the gonads (Figure 7B).
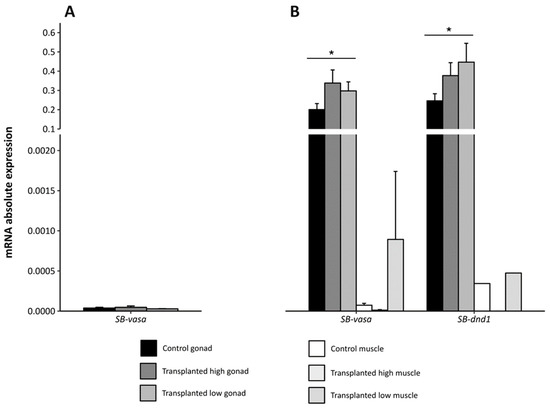
Figure 7.
Absolute expression of the vasa and dnd1 genes in European sea bass (A) in the mid-body segment, from control and 1.5 month-post-transplant larvae, and (B) in the gonad and muscle, from control and 6 month-post-transplant larvae, with high or low concentrations of cells. Values are presented as the mean ± SEM. Asterisks indicate significantly higher expression levels of both genes in the gonads, independently of the experimental group (control or transplanted). SB: European sea bass.
In the cases of both zebrafish experiments, there were no differences in expression between the control and transplanted groups. The gonads were the predominant site of expression of ZF-vasa and ZF-dnd1, compared to the expression in the muscle (Figure 8). In addition, expression of ZF-vasa was significantly higher than the expression of ZF-dnd1 in the gonad for both experiments.
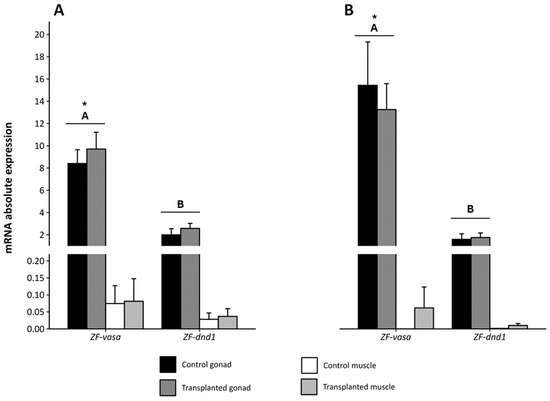
Figure 8.
Absolute expression of the vasa and dnd1 genes in zebrafish in the gonad and muscle from control and 1.5 month-post-transplant larvae from (A) experiment 1, using fresh European eel spermatogonia, and (B) experiment 2, using thawed European eel spermatogonia. Values are presented as the mean ± SEM. Different upper-case letters indicate significantly different expression levels between vasa and dnd1. Asterisks indicate significantly higher expression of vasa in the gonads, independently of the experimental group (control or transplanted). ZF: Zebrafish.
4. Discussion
Spermatogonia seem to be the most reliable option for xenotransplantation, because of their high abundance, suitability for cryopreservation, and sexual plasticity. Furthermore, the transplantation of SPG has been extensively investigated in multiple studies, and it represents the dominant cell type for allo- or xenotransplantation strategies [66,67,68,69]. Numerous studies have reported successful data on gamete production in intraspecific transplantations [39,70,71]. However, the number of cases successful in obtaining donor-derived gametes from recipient fish is broadly inferior in interspecific transplantations, and it is limited to trials between evolutionarily close species [56,72,73,74]. In the case of the European eel, there are no closely-related species for which captive breeding and aquaculture production have been extensively developed. Nevertheless, there are studies in which incorporation and proliferation of donor-derived cells in the recipient species have been achieved between phylogenetically distant species (S. quinqueradiata and Trachurus japonicus [55]; Brycon orbygnyanus and Astyanax altiparanae [75]), even those belonging to different families (Solea senegalensis and S. maximus [76]; R. quelen and Cyprinus carpio [77]; R. quelen and Oreochromis niloticus [51]; and S. maximus and P. olivaceus [41]).
To improve the success rate of xenotransplantation, several steps need to be optimized, such as the sterilization of recipients, the isolation of donor cells and the labelling and identification of donor cells. Regarding sterilization, the most widely applied methods are the use of triploid and/or hybrid recipients, where it has been demonstrated that the transplanted cells have the capacity to differentiate into gametes from the donor species [73,78,79,80]. However, in the present study, triploidy-sterilized European sea bass showed initial colonization of donor SPGA at 1.5 mpt, since no fluorescent-labelled cells were observed in the gonads. Franěk et al. [56] tested different methods using zebrafish as a model and demonstrated that employing dnd1-knockdown recipient fish was the best option for transplanted cells. In that study, triploids or hybrids (D. rerio × D. albolineatus) showed colonization of the gonads from transplanted cells, but the adults presented a lower quality of donor-derived sperm in terms of quantity and motility, compared to dnd1-knockdown recipients. Furthermore, the use of dnd1-knockdown recipients could prove to provide a better recipient for eel spermatogonia.
According to other reports, vasa and dnd1 are considered essential markers for identifying germ cells, and species-specific expression patterns of both have been observed in primordial germ cells (PGCs), as well as in germ cells of the testis and ovaries of diverse fish species [62,81,82]. Blázquez et al. [83] characterized a vasa homolog in European sea bass and proved that it was differentially expressed in the adult gonads, compared to other tissues. Moreover, the lowest values of vasa were found in 1-month-old larvae, and then gradually increased because of gametogenesis. In fact, our analysis in triploid individuals showed a higher expression of vasa in the gonads of 9.5-month-old sea bass than in the muscle at that time, and in the mid-segment of the juveniles at 3.5 months old. Mazón et al. [84] showed that diploid 10-month-old sea bass displayed a similar degree of testis development, mostly composed of SPGA, but differing in the number of germ cells, which was higher than in our triploid sea bass. Other studies have shown that until 1 year of age, triploid sea bass have a similar degree of gonad maturation compared to diploids, containing up to secondary spermatocytes in the testes, and primary oocytes in the ovaries [85]. In the case of the dnd1 expression, Djellata et al. [86] characterized a dnd homolog in European sea bass and reported a similar expression pattern as that outlined for vasa, being high in the early stages of development due to the inheritance of maternal mRNA, and this is followed by a decrease to a minimum, before an increase during the gametogenesis. In the present study, European sea bass dnd1 was not expressed in 3.5-month-old triploid fish, but it was expressed at 9.5 months, showing no differences, compared to vasa, at that time. Most research on the consequences of triploidization in teleosts are focused on survival, growth, gonadal development and reproductive physiology [87,88], but little is known about how triploidy affects gene expression [89,90,91,92].
In the case of the non-sterilized recipient zebrafish used by Franěk et al. [56], whilst the number of successful cases was low, donor-derived sperm and eggs were obtained. Nevertheless, it has been reported that triploidy or germ-cell depletion in zebrafish mostly leads to the production of sterile males [93,94]; thus, only sperm can be obtained by using these animals as recipients in transplantations. In the present study, the zebrafish used in both experiments were not triploidized or germ-cell depleted, so they might have been able to produce both sperm and oocytes following the transplantation procedure. Similarly to the European sea bass experiment, eel-specific genes were not expressed in either the control or the 1.5 month-post-transplant zebrafish, while vasa and dnd1 from zebrafish were significantly expressed in the gonads, compared to the muscle [95,96], in both zebrafish experiments.
Regarding the type of germ cell being transplanted, Saito et al. [43] tested the Japanese eel as a donor of PGC and used zebrafish as a recipient species. Following their results, it was suggested that at least the migration mechanism of PGC is well conserved between the Japanese eel and the zebrafish (median divergence time: 250 MYA; [97]), but transplanted PGCs did not develop into more advanced stages a few days after hatching, as was the case when using different Cypriniforms and loach as donors and zebrafish as recipient species [98]. Yoshizaki and Lee [99] showed that the migration ability of SPGA isolated from adult rainbow trout testes might be weaker than that of PGCs, even though they maintained a certain ability to migrate toward the recipient gonads. Thus, the gonadal microenvironment of the larvae was suitable for interacting with the transplanted cells [100]. It is possible that using PGCs instead of spermatogonia for transplantation between European eel and zebrafish could improve the success of cell migration, as was reported in Japanese eel, since it seems that the mechanism of migration of these cells is well conserved among the fish species [44,101,102]. However, the technique to identify and obtain PGCs from European eel has not yet been developed.
Furthermore, it has been shown that the number of gonad cells used, in particular, the proportion of SPGA in the donor cells, is positively correlated with the success rates of transplantation [36]. However, several studies have demonstrated that even if the number of SPG injected into the larval cavity is relatively small (ranging from 3000 to 5000), it is enough to induce positive results of donor cell incorporation into the recipient gonads, or even the production of donor-derived gametes [53,65,103,104]. Thus, as similar or higher concentrations of cells per larvae were used in the current study, other factors must influence the cell migration process. The present study did not use any enrichment method apart from centrifuging the cell solution, since it is known that the immature testes of European eel contain mostly SPG Aund [25,63]. Thus, enrichment of spermatogonia after isolation does not seem to be necessary for the European eel xenotransplants.
Transplanting SPGA from one species into another can result in inadequate interaction with the specialized cells and structures in the gonadal microenvironment of the recipient species. This would lead to an incorrect performance of the numerous endocrine regulatory cascades affecting the donor germ cells, jeopardizing the processes of proliferation and differentiation into spermatozoa or oocytes [81]. The gonadal microenvironment of the SPGA regulates their activity in the testes by providing growth factors and paracrine interactions [105]. There is not one factor alone which can be considered preeminent in the gonad microenvironment of fish, as it is a complex and dynamic system with multiple interacting factors. Therefore, choosing a compatible recipient species for xenotransplantation is challenging due to the need for an appropriate niche to support the survival of donor cells, proliferation and maturation [106]. The species in the present study (zebrafish and European sea bass) were chosen because they have well-known life-cycles and have demonstrated their capacity for reproduction under controlled aquaculture conditions [107,108], which would contribute to the promotion of eel reproduction. Additionally, current studies with fish transplantation show that spermatogonia physiology is well-conserved in phylogenetically distant groups, as shown between perciformes and siluriformes [51]. The use of European sea bass and zebrafish as surrogate recipients has shed light on the distinctive SPGA migration mechanisms within teleost species and the importance of the gonadal microenvironment for the migration, development and proliferation of xenotransplanted cells. The lack of settled European eel SPGA in the gonads of both species suggests that the physiological mechanisms that control the cell incorporation have evolutionary diverged among the three species. Alternatively, the existence of an immune response to xenotransplantation, especially among distant species, could have been a contributing factor [109]. Thus, the SPGA isolated from European eel seemed unable to migrate to the genital ridges of the zebrafish and European sea bass larvae; hence, these two species cannot be considered as candidate species for the surrogate broodstock production of the European eel. In future experiments, different approaches could be taken. In this sense, sterilization of recipients using dnd1-knockdown could be an option to improve transplantation efficiency [56], although a host species for which sterilization does not result in the production of single-sex individuals should be chosen. Finally, other phylogenetically closer species could be used. The idea of identifying a species phylogenetically close to the European eel and the reproduction in captivity of which is well-established presents a complex issue. Therefore, the most promising option appears to be the use of the Japanese eel, which has a fully documented life-cycle in captivity [110].
5. Conclusions
The present study explored the potential for xenotransplantation as an innovative approach for European eel reproduction. The results obtained with the two chosen species, the European sea bass and zebrafish, suggested that spermatogonial migration mechanisms could not be well-conserved. Additionally, there is a potential rejection of the xenotransplanted cells due to the immune system response. Therefore, the development of surrogate broodstock technology for the European eel would necessitate finding a phylogenetically closer species with a suitable gonadal microenvironment which would support the incorporation and differentiation of European eel SPGA.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9070290/s1, Figure S1: European sea bass gonads 6 months post-xenotransplantation from the control group (A, D), high cell-concentration group (HC: B, E) and low cell-concentration group (LC: C, F). SPGAund: undifferentiated type A spermatogonia; SPGB: type B spermatogonia. The dashed rectangle indicates the enlarged area. Scale bars: A–C: 100 µm; D–F: 50 µm; Video S1: manual injection of a zebrafish larvae into the peritoneal cavity between the swim bladder and the intestines, as observed under a stereomicroscope.
Author Contributions
Conceptualization, M.B.-G., Z.M. and J.F.A.; Methodology, M.B.-G. and Z.M.; Formal Analysis, M.M.; Investigation, M.B.-G., Z.M., M.M. and A.V.; Data Curation, M.B.-G.; Writing—Original Draft Preparation, M.B.-G.; Writing—Review and Editing, Z.M., M.M., Á.H., A.V. and J.F.A.; Supervision, Z.M., M.M., Á.H., A.V. and J.F.A.; Funding Acquisition, J.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministry of Science, Innovation and Universities (EELGONIA; RTI2018-096413-B-I00) and the ThinkInAzul Project, supported by the Spanish Ministry of Science and Innovation (MCIN) with funding from the European Union NextGenerationEU (PRTR-C17.I1) and the Generalitat Valenciana to SEASPERM (THINKINAZUL/2021/012), including the Marina Morini postdoctoral contract. The study was partially funded by the EU H2020 Research Innovation Program (AQUAEXCEL3.0; grant agreement No. 871108). Marta Blanes-García had a PhD contract from the UPV (PAID-01-20). Zoran Marinović and Ákos Horváth were supported by the National Research, Development and Innovation Office of Hungary (NKFIH K138425 and FK142933 grants), the National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation fund (ÚNKP-23-4-II-MATE-10 grant) and the KKP program of the Hungarian University of Agriculture and Life Sciences, as well as the Flagship Research Groups Programme of the Hungarian University of Agriculture and Life Sciences and the Research Excellence Programme of the Hungarian University of Agriculture and Life Sciences.
Institutional Review Board Statement
Experimental animals were handled following the European Union regulations concerning the protection of experimental animals (Dir 86/609/EEC) and the recommendations given in the “Guide for the Care and Use of Laboratory Animals” in accordance with the Spanish Royal Decree 53/2013 regarding the protection of animals used for scientific purposes (BOE 2013). The applied protocols were approved by the Experimental Animal Ethics Committee from the Universitat Politècnica de València, the local government (Generalitat Valenciana, permit number: 2019/VSC/PEA/0073), and the Languedoc-Roussillon French ethics committee (permit number #40759). All efforts were made to minimize fish suffering.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Palstra, A.; van Ginneken, V.; van den Thillart, G.E.E.J.M. Effects of swimming on silvering and maturation of the European eel, Anguilla anguilla L. In Spawning Migration of the European Eel: Reproduction Index, a Useful Tool for Conservation Management; van den Thillart, G., Dufour, S., Rankin, J.C., Eds.; Springer: Berlin, Germany, 2009; pp. 229–252. [Google Scholar]
- Righton, D.; Westerberg, H.; Feunteun, E.; Økland, F.; Gargan, P.; Amilhat, E.; Metcalfe, J.; Lobon-Cervia, J.; Sjöberg, N.; Simon, J.; et al. Empirical observations of the spawning migration of European eels: The long and dangerous road to the Sargasso Sea. Sci. Adv. 2016, 2, e1501694. [Google Scholar] [CrossRef] [PubMed]
- van Ginneken, V.; Antonissen, E.; Müller, U.K.; Booms, R.; Eding, E.; Verreth, J.; van den Thillart, G. Eel migration to the Sargasso: Remarkably high swimming efficiency and low energy costs. J. Exp. Biol. 2005, 208, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Asturiano, J.F. Improvements on the reproductive control of the European eel. In Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology; Yoshida, M., Asturiano, J.F., Eds.; Springer Nature: Singapore, 2020; pp. 293–320. [Google Scholar] [CrossRef]
- Ottolenghi, F.; Silvestri, C.; Giordano, P.; Lovatelli, A.; New, M.B. Eels—Anguilla spp. In Capture-Based Aquaculture: The Fattening of Eels, Groupers, Tunas and Yellowtails; FAO: Rome, Italy, 2004; pp. 21–68. [Google Scholar]
- ICES. European eel (Anguilla anguilla) throughout its natural range. In Report of the ICES Advisory Committee; ICES Advice on fishing opportunities and conservation, ele.2737.nea; ICES: Copenhagen, Denmark, 2022. [Google Scholar] [CrossRef]
- Bertucci, A.; Hoede, C.; Dassié, E.; Gourves, P.Y.; Suin, A.; Le Menach, K.; Budzinski, H.; Daverat, F. Impact of environmental micropollutants and diet composition on the gut microbiota of wild European eels (Anguilla anguilla). Environ. Pollut. 2022, 314, 120207. [Google Scholar] [CrossRef] [PubMed]
- Parchemin, C.; Tapissier-Bontemps, N.; Sasal, P.; Faliex, E. Anguilla sp. diseases diagnoses and treatments: The ideal methods at the crossroads of conservation and aquaculture purposes. J. Fish Dis. 2022, 45, 943–969. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.; Crook, V.; Gollock, M. Anguilla anguilla. The IUCN Red List of Threatened Species. Int. Union Conserv. Nat. 2020, 2020, T60344A152845178. [Google Scholar] [CrossRef]
- Dufour, S.; Weltzien, F.A.; Sebert, M.E.; Le Belle, N.; Vidal, B.; Vernier, P.; Pasqualini, C. Dopaminergic inhibition of reproduction in teleost fishes: Ecophysiological and evolutionary implications. Ann. N. Y. Acad. Sci. 2005, 1040, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.; Pasqualini, C.; Le Belle, N.; Holland, M.C.H.; Sbaihi, M.; Vernier, P.; Zohar, Y.; Dufour, S. Dopamine inhibits luteinizing hormone synthesis and release in the juvenile European eel: A neuroendocrine lock for the onset of puberty. Biol. Reprod. 2004, 71, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Gallego, V.; Mazzeo, I.; Vílchez, M.C.; Peñaranda, D.S.; Carneiro, P.C.F.; Pérez, L.; Asturiano, J.F. Study of the effects of thermal regime and alternative hormonal treatments on the reproductive performance of European eel males (Anguilla anguilla) during induced sexual maturation. Aquaculture 2012, 354–355, 7–16. [Google Scholar] [CrossRef]
- Pérez, L.; Asturiano, J.F.; Tomás, A.; Zegrari, S.; Barrera, R.; Espinós, F.J.; Navarro, J.C.; Jover, M. Induction of maturation and spermiation in the male European eel: Assessment of sperm quality throughout treatment. J. Fish Biol. 2000, 57, 1488–1504. [Google Scholar] [CrossRef]
- Butts, I.A.E.; Sørensen, S.R.; Politis, S.N.; Pitcher, T.E.; Tomkiewicz, J. Standardization of fertilization protocols for the European eel, Anguilla anguilla. Aquaculture 2014, 426–427, 9–13. [Google Scholar] [CrossRef]
- Palstra, A.; Cohen, E.G.H.; Niemanstverdriet, P.R.W.; van Ginneken, V.; van den Thillart, G.E.E.J.M. Artificial maturation and reproduction of European silver eel: Development of oocytes during final maturation. Aquaculture 2005, 249, 533–547. [Google Scholar] [CrossRef]
- Pedersen, B.H. Induced sexual maturation of the European eel Anguilla anguilla and fertilization of the eggs. Aquaculture 2003, 224, 323–338. [Google Scholar] [CrossRef]
- Pérez, L.; Peñaranda, D.S.; Dufour, S.; Baloche, S.; Palstra, A.P.; van den Thillart, G.E.E.J.M.; Asturiano, J.F. Influence of temperature regime on endocrine parameters and vitellogenesis during experimental maturation of European eel (Anguilla anguilla) females. Gen. Comp. Endocrinol. 2011, 174, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kottmann, J.S.; Jørgensen, M.G.P.; Bertolini, F.; Loh, A.; Tomkiewicz, J. Differential impacts of carp and salmon pituitary extracts on induced oogenesis, egg quality, molecular ontogeny and embryonic developmental competence in European eel. PLoS ONE 2020, 15, e0235617. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Duncan, N.J.; Asturiano, J.F. Hormonal manipulations for the enhancement of sperm production in cultured fish and evaluation of sperm quality. Aquaculture 2017, 472, 21–44. [Google Scholar] [CrossRef]
- Palstra, A.; Guerrero, M.A.; de Laak, G.; Breteler, J.P.G.K.; van den Thillart, G.E.E.J.M. Temporal progression in migratory status and sexual maturation in European silver eels during downstream migration. Fish Physiol. Biochem. 2011, 37, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, A.; Lokman, P.M.; Govoni, N.; Casalini, A.; Emmanuele, P.; Parmeggiani, A.; Mordenti, O. Co-treatment with androgens during artificial induction of maturation in female eel, Anguilla anguilla: Effects on egg production and early development. Aquaculture 2017, 479, 508–515. [Google Scholar] [CrossRef]
- Jéhanette, P.; Palstra, A.P.; Giménez, I.; Schipper, H.; Swinkels, W.; Henisbroek, L.T.N.; Komen, H. Recombinant gonadotropins to induce oocyte development in vitro and in vivo in the European eel Anguilla anguilla. Fishes 2023, 8, 123. [Google Scholar] [CrossRef]
- Peñaranda, D.S.; Gallego, V.; Rozenfeld, C.; Herranz-Jusdado, J.G.; Pérez, L.; Gómez, A.; Giménez, I.; Asturiano, J.F. Using specific recombinant gonadotropins to induce spermatogenesis and spermiation in the European eel (Anguilla anguilla). Theriogenology 2018, 107, 6–20. [Google Scholar] [CrossRef]
- Ferrão, L.; Morini, M.; Gallego, V.; Felip, A.; Gómez, A.; Pérez, L.; Asturiano, J.F. Cold seawater pre-treatment affects the spermatogenesis and the reproductive performance of male European eels. In Proceedings of the Aquaculture Europe, Funchal, Madeira, Portugal, 4–7 October 2021; pp. 397–398. [Google Scholar]
- Rozenfeld, C.; García-Carpintero, V.; Pérez, L.; Gallego, V.; Herranz-Jusdado, J.G.; Tveiten, H.; Johnsen, H.K.; Fontaine, R.; Weltzien, F.A.; Cañizares, J.; et al. Cold seawater induces early sexual developmental stages in the BPG axis of European eel males. BMC Genom. 2019, 20, 597. [Google Scholar] [CrossRef]
- Blanes-García, M.; García-Salinas, P.; Morini, M.; Pérez, L.; Asturiano, J.F.; Gallego, V. Using osmotic pumps to induce the production of gametes in male and female European eels. Animals 2022, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Zapater, C.; Morini, M.; González-López, W.A.; Borges, L.P.; Pérez, L.; Asturiano, J.F.; Gómez, A. Gonadotropin plasmid gene therapy triggers spermatogenesis in European eel (Anguilla anguilla). In Proceedings of the XIV Congress of the Iberian Association of Comparative Endocrinology, Bilbao, Spain, 11–13 September 2023; p. 37. [Google Scholar]
- Yamaha, E.; Saito, T.; Goto-Kazeto, R.; Arai, K. Developmental biotechnology for aquaculture, with special reference to surrogate production in teleost fishes. J. Sea Res. 2007, 58, 8–22. [Google Scholar] [CrossRef]
- Goto, R.; Saito, T. A state-of-the-art review of surrogate propagation in fish. Theriogenology 2019, 133, 216–227. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Yazawa, R. Application of surrogate broodstock technology in aquaculture. Fish. Sci. 2019, 85, 429–437. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Yazawa, R.; Yoshizaki, G. Intraperitoneal germ transplantation technique in marine teleosts. In Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology; Yoshida, M., Asturiano, J.F., Eds.; Springer Nature: Singapore, 2020; pp. 357–379. [Google Scholar] [CrossRef]
- Pšenička, M.; Saito, T.; Rodina, M.; Dzyuba, B. Cryopreservation of early stage Siberian sturgeon Acipenser baerii germ cells, comparison of whole tissue and dissociated cells. Cryobiology 2016, 72, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Takeuchi, Y.; Du, H.; Yue, H.; Ruan, R.; Li, C.; Wei, Q. Spermatogonia from cryopreserved testes of critically endangered Chinese sturgeon efficiently colonized and preferentially proliferated in the recipient gonads of Yangtze sturgeon. Mar. Biotechnol. 2022, 24, 136–150. [Google Scholar] [CrossRef]
- Jin, Y.H.; Robledo, D.; Hickey, J.M.; McGrew, M.J.; Houston, R.D. Surrogate broodstock to enhance biotechnology research and applications in aquaculture. Biotechnol. Adv. 2021, 49, 107756. [Google Scholar] [CrossRef]
- Lacerda, S.M.S.N.; Costa, G.M.J.; Campos-Junior, P.H.A.; Segatelli, T.M.; Yazawa, R.; Takeuchi, Y.; Morita, T.; Yoshizaki, G.; de França, L.R. Germ cell transplantation as a potential biotechnological approach to fish reproduction. Fish Physiol. Biochem. 2013, 39, 3–11. [Google Scholar] [CrossRef]
- Ryu, J.H.; Xu, L.; Wong, T.T. Advantages, factors, obstacles, potential solutions, and recent advances of fish germ cell transplantation for aquaculture—A practical review. Animals 2022, 12, 423. [Google Scholar] [CrossRef]
- Lacerda, S.M.S.N.; Costa, G.M.J.; de França, L.R. Biology and identity of fish spermatogonial stem cells. Gen. Comp. Endocrinol. 2014, 207, 56–65. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Okutsu, T.; Suzuki, K.; Takeuchi, Y.; Takeuchi, T.; Yoshizaki, G. Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc. Natl. Acad. Sci. USA 2006, 103, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Okutsu, T.; Ichikawa, M.; Hayashi, M.; Takeuchi, Y. Sexual plasticity of rainbow trout germ cells. Anim. Reprod. 2010, 7, 187–196. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, X.; Liu, Q.; Yang, J.; Xu, S.; Wu, Z.; Wang, Y.; You, F.; Song, Z.; Li, J. Successful spermatogonial stem cells transplantation within Pleuronectiformes: First breakthrough at inter-family level in marine fish. Int. J. Biol. Sci. 2021, 17, 4426–4441. [Google Scholar] [CrossRef] [PubMed]
- Hattori, R.S.; Yoshinaga, T.T.; Katayama, N.; Hattori-Ihara, S.; Tsukamoto, R.Y.; Takahashi, N.S.; Tabata, Y.A. Surrogate production of Salmo salar oocytes and sperm in triploid Oncorhynchus mykiss by germ cell transplantation technology. Aquaculture 2019, 506, 238–245. [Google Scholar] [CrossRef]
- Saito, T.; Goto-Kazeto, R.; Kawakami, Y.; Nomura, K.; Tanaka, H.; Adachi, S.; Arai, K.; Yamaha, E. The mechanism for primordial germ-cell migration is conserved between Japanese eel and zebrafish. PLoS ONE 2011, 6, e24460. [Google Scholar] [CrossRef]
- Saito, T.; Pšenička, M.; Goto, R.; Adachi, S.; Inoue, K.; Arai, K.; Yamaha, E. The origin and migration of primordial germ cells in sturgeons. PLoS ONE 2014, 9, e86861. [Google Scholar] [CrossRef] [PubMed]
- Bar, I.; Smith, A.; Bubner, E.; Yoshizaki, G.; Takeuchi, Y.; Yazawa, R.; Chen, B.N.; Cummis, S.; Elizur, A. Assessment of yellowtail kingfish (Seriola lalandi) as a surrogate host for the production of southern bluefin tuna (Thunnus maccoyii) seed via spermatogonial germ cell transplantation. Reprod. Fertil. Dev. 2015, 28, 2015–2064. [Google Scholar] [CrossRef][Green Version]
- Yazawa, R.; Takeuchi, Y.; Morita, T.; Ishida, M.; Yoshizaki, G. The Pacific bluefin tuna (Thunnus orientalis) dead end gene is suitable as a specific molecular marker of type A spermatogonia. Mol. Reprod. Dev. 2013, 80, 871–880. [Google Scholar] [CrossRef]
- Kawamura, W.; Tani, R.; Yahagi, H.; Kamio, S.; Morita, T.; Takeuchi, Y.; Yazawa, R.; Yoshizaki, G. Suitability of hybrid mackerel (Scomber australasicus x S. japonicus) with germ cell-less sterile gonads as a recipient for transplantation of bluefin tuna germ cells. Gen. Comp. Endocrinol. 2020, 295, 113525. [Google Scholar] [CrossRef]
- Ye, H.; Takeuchi, Y.; Wu, M.; Yue, H.; Ruan, R.; Du, H.; Zhou, C.; Xiang, H.; Li, C.; Wei, Q. Assessment of Yangtze sturgeon as recipient for the production of American paddlefish gametes through spermatogonia transplantation. Theriogenology 2020, 158, 168–179. [Google Scholar] [CrossRef]
- Higuchi, K.; Takeuchi, Y.; Miwa, M.; Yamamoto, Y.; Tsunemoto, K.; Yoshizaki, G. Colonization, proliferation, and survival of intraperitoneally transplanted yellowtail Seriola quinqueradiata spermatogonia in nibe croaker Nibea mitsukurii recipient. Fish. Sci. 2011, 77, 69–77. [Google Scholar] [CrossRef]
- Yazawa, R.; Takeuchi, Y.; Higuchi, K.; Yatabe, T.; Kabeya, N.; Yoshizaki, G. Chub mackerel gonads support colonization, survival, and proliferation of intraperitoneally transplanted xenogenic germ cells. Biol. Reprod. 2010, 82, 896–904. [Google Scholar] [CrossRef]
- Silva, M.A.; Costa, G.M.J.; Lacerda, S.M.S.N.; Brandão-Dias, P.F.P.; Kalapothakis, E.; Silva Júnior, A.F.; Alvarenga, E.R.; França, L.R. Successful xenogeneic germ cell transplantation from Jundia catfish (Rhamdia quelen) into adult Nile tilapia (Oreochromis niloticus) testes. Gen. Comp. Endocrinol. 2016, 230–231, 48–56. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Yatabe, T.; Yoshikawa, H.; Ino, Y.; Kabeya, N.; Yazawa, R.; Yoshizaki, G. Production of functionally sterile triploid Nibe croaker Nibea mitsukurii induced by cold-shock treatment with special emphasis on triploid aptitude as surrogate broodstock. Aquaculture 2018, 494, 45–56. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Ino, Y.; Kishimoto, K.; Koyakumaru, H.; Saito, T.; Kinoshita, M.; Yoshiura, Y. Induction of germ cell-deficiency in grass puffer by dead end 1 gene knockdown for use as a recipient in surrogate production of tiger puffer. Aquaculture 2020, 526, 735385. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Xu, D.; Ino, Y.; Yoshino, T.; Hayashida, T.; Wang, J.; Yazawa, R.; Yoshizaki, G.; Takeuchi, Y. Hybrid sterility in fish caused by mitotic arrest of primordial germ cells. Genetics 2018, 209, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Morishima, K.; Miwa, M.; Kumakura, N.; Kudo, S.; Ichida, K.; Mitsuboshi, T.; Takeuchi, Y.; Yoshizaki, G. Functional sperm of the yellowtail (Seriola quinqueradiata) were produced in the small-bodied surrogate, jack mackerel (Trachurus japonicus). Mar. Biotechnol. 2015, 17, 644–654. [Google Scholar] [CrossRef]
- Franěk, R.; Cheng, Y.; Fučíková, M.; Kašpar, V.; Xie, X.; Shah, M.A.; Linhart, O.; Šauman, I.; Pšenička, M. Who is the best surrogate for germ stem cell transplantation in fish? Aquaculture 2022, 549, 737759. [Google Scholar] [CrossRef]
- Peruzzi, S.; Chatain, B. Pressure and cold shock induction of meiotic gynogenesis and triploidy in the European sea bass, Dicentrarchus labrax L.: Relative efficiency of methods and parental variability. Aquaculture 2000, 189, 23–37. [Google Scholar] [CrossRef]
- Peruzzi, S.; Chatain, B.; Saillant, E.; Haffray, P.; Menu, B.; Falguière, J.C. Production of meiotic gynogenetic and triploid sea bass, Dicentrarchus labrax L.: 1. Performances, maturation and carcass quality. Aquaculture 2004, 230, 41–64. [Google Scholar] [CrossRef]
- Marinović, Z.; Blanes-García, M.; Šćekić, I.; Lujić, J.; Ferrão, L.; Morini, M.; Balogh, R.; Urbányi, B.; Horváth, Á.; Asturiano, J.F. Department of Aquaculture, Institute of Aquaculture and Environmental Safety, Hungarian University of Agriculture and Life Sciences. Páter Károly u. 1, 2100, Gödöllő, Hungary. 2024; manuscript in preparation. [Google Scholar]
- Vergnet, A.; Clota, F.; Lallement, S.; Blanca, M.; Vandeputte, M.; Allal, F.; Lareyre, J. Development of a germ stem cell grafting procedure in European sea bass as an innovative practical approach for broodstock management. In Proceedings of the Aquaculture Europe, Rimini, Italy, 27–30 September 2022; pp. 1377–1378. [Google Scholar]
- Marinović, Z.; Lujić, J.; Li, Q.; Iwasaki, Y.; Urbányi, B.; Yoshizaki, G.; Horváth, Á. Cryopreservation and transplantation of spermatogonial stem cells. In Germline Development in Zebrafish: Methods and Protocols; Dosch, R., Ed.; Humana Press: New York, NY, USA, 2021; pp. 37–47. [Google Scholar] [CrossRef]
- Blanes-García, M.; Marinović, Z.; Herranz-Jusdado, J.-G.; Ferrão, L.; Gallego, V.; Pérez, L.; Pšenička, M.; Asturiano, J.F.; Morini, M. Characterization of potential spermatogonia biomarker genes in the European eel (Anguilla anguilla). Fish Physiol. Biochem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.; Peñaranda, D.S.; Vílchez, M.C.; Nourizadeh-Lillabadi, R.; Lafont, A.G.; Dufour, S.; Asturiano, J.F.; Weltzien, F.A.; Pérez, L. Nuclear and membrane progestin receptors in the European eel: Characterization and expression in vivo through spermatogenesis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 207, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Pflaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 26 January 2024).
- Sato, M.; Morita, T.; Katayama, N.; Yoshizaki, G. Production of genetically diversified fish seeds using spermatogonial transplantation. Aquaculture 2014, 422–423, 218–224. [Google Scholar] [CrossRef]
- Shang, M.; Su, B.; Perera, D.A.; Alsaqufi, A.; Lipke, E.A.; Cek, S.; Dunn, D.A.; Qin, Z.; Peatman, E.; Dunham, R.A. Testicular germ line cell identification, isolation, and transplantation in two North American catfish species. Fish Physiol. Biochem. 2018, 44, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Tani, R.; Yazawa, R.; Kamio, S.; Kawamura, W.; Morita, T.; Takeuchi, Y.; Yoshizaki, G. Establishment of surrogate broodstock technology in Scombridae species by germ cell transplantation. Aquac. Res. 2022, 53, 2760–2771. [Google Scholar] [CrossRef]
- Farlora, R.; Hattori-Ihara, S.; Takeuchi, Y.; Hayashi, M.; Octavera, A.; Alimuddin; Yoshizaki, G. Intraperitoneal germ cell transplantation in the Nile tilapia Oreochromis niloticus. Mar. Biotechnol. 2014, 16, 309–320. [Google Scholar] [CrossRef]
- Morita, T.; Kumakura, N.; Morishima, K.; Mitsuboshi, T.; Ishida, M.; Hara, T.; Kudo, S.; Miwa, M.; Ihara, S.; Higuchi, K.; et al. Production of donor-derived offspring by allogeneic transplantation of spermatogonia in the yellowtail (Seriola quinqueradiata). Biol. Reprod. 2012, 86, 1–11. [Google Scholar] [CrossRef]
- Franěk, R.; Marinović, Z.; Lujić, J.; Urbányi, B.; Fučíková, M.; Kašpar, V.; Pšenička, M.; Horváth, Á. Cryopreservation and transplantation of common carp spermatogonia. PLoS ONE 2019, 14, e0205481. [Google Scholar] [CrossRef]
- Hamasaki, M.; Takeuchi, Y.; Yazawa, R.; Yoshikawa, S.; Kadomura, K.; Yamada, T.; Miyaki, K.; Kikuchi, K.; Yoshizaki, G. Production of tiger puffer Takifugu rubripes offspring from triploid grass puffer Takifugu niphobles parents. Mar. Biotechnol. 2017, 19, 579–591. [Google Scholar] [CrossRef]
- Perera, D.A.; Alsaqufi, A.; Shang, M.; Wade, D.C.; Su, B.; Elaswad, A.; Fobes, M.; Beam, R.; Garcia, G.; Dunn, D.A.; et al. Xenogenesis-production of channel catfish × blue catfish hybrid progeny by fertilization of channel catfish eggs with sperm from triploid channel catfish males with transplanted blue catfish germ cells. N. Am. J. Aquac. 2017, 79, 61–74. [Google Scholar] [CrossRef]
- Xu, D.; Yoshino, T.; Konishi, J.; Yoshikawa, H.; Ino, Y.; Yazawa, R.; Dos Santos Nassif Lacerda, S.M.; De França, L.R.; Takeuchi, Y. Germ cell-less hybrid fish: Ideal recipient for spermatogonial transplantation for the rapid production of donor-derived sperm. Biol. Reprod. 2019, 101, 492–500. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira-Silva, D.H.; dos Santos Silva, A.P.; da Silva Costa, R.; Senhorini, J.A.; Ninhaus-Silveira, A.; Veríssimo-Silveira, R. Preliminary study on testicular germ cell isolation and transplantation in an endangered endemic species Brycon orbignyanus (Characiformes: Characidae). Fish Physiol. Biochem. 2021, 47, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarini, T.; Sarasquete, C.; Cabrita, E. Development of interspecies testicular germ-cell transplantation in flatfish. Reprod. Fertil. Dev. 2013, 26, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.F.; Martinez, E.R.M.; Digmayer, M.; Doretto, L.B.; Nóbrega, R.H. Successful cryopreservation of spermatogonia stem cells of Neotropical catfish (Rhamdia quelen) and enriched germ cell transplantation into common carp (Cyprinus carpio) testes. Fishes 2023, 8, 478. [Google Scholar] [CrossRef]
- Lee, S.; Bang, W.Y.; Yang, H.-S.; Lee, D.-S.; Song, H.Y. Production of juvenile masu salmon (Oncorhynchus masou) from spermatogonia-derived sperm and oogonia-derived eggs via intraperitoneal transplantation of immature germ cells. Biochem. Biophys. Res. Commun. 2021, 535, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-T.; Saito, T.; Crodian, J.; Collodi, P. Zebrafish germline chimeras produced by transplantation of ovarian germ cells into sterile host larvae. Biol. Reprod. 2011, 84, 1190–1197. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Ino, Y.; Shinegaga, K.; Katayama, T.; Kuroyanagi, M.; Yoshiura, Y. Production of tiger puffer Takifugu rubripes from cryopreserved testicular germ cells using surrogate broodstock technology. Aquaculture 2018, 493, 302–313. [Google Scholar] [CrossRef]
- Begum, S.; Gnanasree, S.M.; Anusha, N.; Senthilkumaran, B. Germ cell markers in fishes—A review. Aquac. Fish. 2022, 7, 540–552. [Google Scholar] [CrossRef]
- Sharma, P.; Purohit, S.; Kothiyal, S.; Bhattacharya, I. Germ cell development in teleost gonads. Aquac. Fish. 2024, 9, 422–436. [Google Scholar] [CrossRef]
- Blázquez, M.; González, A.; Mylonas, C.C.; Piferrer, F. Cloning and sequence analysis of a vasa homolog in the European sea bass (Dicentrarchus labrax): Tissue distribution and mRNA expression levels during early development and sex differentiation. Gen. Comp. Endocrinol. 2011, 170, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Mazón, M.J.; Gómez, A.; Yilmaz, O.; Carrillo, M.; Zanuy, S. Administration of follicle-stimulating hormone in vivo triggers testicular recrudescence of juvenile European sea bass (Dicentrarchus labrax). Biol. Reprod. 2014, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Felip, A.; Zanuy, S.; Carrillo, M.; Piferrer, F. Growth and gonadal development in triploid sea bass (Dicentrarchus labrax L.) during the first two years of age. Aquaculture 1999, 173, 389–399. [Google Scholar] [CrossRef]
- Djellata, A.; Zapater, C.; Ibañez, S.; Gómez, A. Molecular characterization and gonad expression pattern of dead-end (dnd) in European sea bass (Dicentrarchus labrax). In Proceedings of the XIV Congress of the Iberian Association of Comparative Endocrinology, Bilbao, Spain, 11–13 September 2023; p. 44. [Google Scholar]
- Felip, A.; Zanuy, S.; Carrillo, M.; Piferrer, F. Induction of triploidy and gynogenesis in teleost fish with emphasis on marine species. Genetica 2001, 111, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Piferrer, F.; Beaumont, A.; Falguière, J.-C.; Flajšhans, M.; Haffray, P.; Colombo, L. Polyploid fish and shellfish: Production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 2009, 293, 125–156. [Google Scholar] [CrossRef]
- Beato, S.; Sánchez-Baizán, N.; Felip, A.; Piferrer, F. DNA methylation during early development in diploid and triploid European sea bass. In Proceedings of the 12th International Symposium on Reproductive Physiology of Fish, Crete, Greece, 15–19 May 2023; p. 99. [Google Scholar]
- Christensen, K.A.; Sakhrani, D.; Rondeau, E.B.; Richards, J.; Koop, B.F.; Devlin, R.H. Effect on triploidy on liver gene expression in coho salmon (Oncorhynchus kisutch) under different metabolic states. BMC Genom. 2019, 20, 336. [Google Scholar] [CrossRef]
- van de Pol, I.L.E.; Flik, G.; Verberk, W.C.E.P. Triploidy in zebrafish larvae: Effects on gene expression, cell size and cell number, growth, development and swimming performance. PLoS ONE 2020, 15, e0229468. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huang, T.; Jin, X.; Cui, C.; Li, D.; Sun, C.; Han, Y.; Mu, Z. Morphology, sex steroid level and gene expression analysis in gonadal sex reversal of triploid female (XXX) rainbow trout (Onchorhynchus mykiss). Fish Physiol. Biochem. 2016, 42, 193–202. [Google Scholar] [CrossRef]
- Delomas, T.A.; Dabrowski, K. Why are triploid zebrafish all males? Biol. Reprod. 2018, 99, 302–312. [Google Scholar] [CrossRef]
- Tzung, K.-W.; Goto, R.; Saju, J.M.; Sreenivasan, R.; Saito, T.; Arai, K.; Yamaha, E.; Hossain, M.S.; Calvert, M.E.K.; Orbán, L. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Rep. 2015, 4, 61–73. [Google Scholar] [CrossRef]
- Braat, A.K.; Speksnijder, J.E.; Zivkovic, D. Germ line development in fishes. Int. J. Dev. Biol. 1999, 43, 745–760. [Google Scholar] [PubMed]
- Braat, A.K.; van de Water, S.; Goos, H.; Bogerd, J.; Zivkovic, D. Vasa protein expression and localization in the zebrafish. Mech. Dev. 2000, 95, 271–274. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A resource for timelines, timetrees and divergence times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Goto-Kazeto, R.; Arai, K.; Yamaha, E. Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol. Reprod. 2008, 78, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Lee, S. Production of live fish derived from frozen germ cells via germ cell transplantation. Stem Cell Res. 2018, 29, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Okutsu, T.; Morita, T.; Terasawa, M.; Yazawa, R.; Takeuchi, Y. Biological characteristics of fish germ cells and their application to developmental biotechnology. Reprod. Domest. Anim. 2012, 47, 187–192. [Google Scholar] [CrossRef]
- Saito, T.; Goto-Kazeto, R.; Fujimoto, T.; Kawakami, Y.; Arai, K.; Yamaha, E. Inter-species transplantation and migration of primordial germ cells in cyprinid fish. Int. J. Dev. Biol. 2010, 54, 1481–1486. [Google Scholar] [CrossRef]
- Robles, V.; Riesco, M.F.; Pšenička, M.; Saito, T.; Valcarce, D.G.; Cabrita, E.; Herráez, P. Biology of teleost primordial germ cells (PGCs) and spermatogonia: Biotechnological applications. Aquaculture 2017, 472, 4–20. [Google Scholar] [CrossRef]
- Kise, K.; Yoshikawa, H.; Sato, M.; Tashiro, M.; Yazawa, R.; Nagasaka, Y.; Takeuchi, Y.; Yoshizaki, G. Flow-cytometric isolation and enrichment of teleost type a spermatogonia based on light-scattering properties. Biol. Reprod. 2012, 86, 1–12. [Google Scholar] [CrossRef]
- Marinović, Z.; Li, Q.; Lujić, J.; Iwasaki, Y.; Csenki, Z.; Urbányi, B.; Yoshizaki, G.; Horváth, Á. Preservation of zebrafish genetic resources through testis cryopreservation and spermatogonia transplantation. Sci. Rep. 2019, 9, 13861. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Nóbrega, R.; Pšenička, M. Spermatogonial stem cells in fish: Characterization, isolation, enrichment, and recent advances of in vitro culture systems. Biomolecules 2020, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Yoshizaki, G. Germline stem cells: Basic studies and applications in conservation and aquaculture. In Encyclopedia of Fish Physiology; Alderman, S.L., Gillis, T.E., Eds.; Academic Press: Toronto, ON, Canada, 2024; pp. 660–670. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J. Vis. Exp. 2012, 69, e4196. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Sether, B.S.; Albrektsen, S.; Noble, C. Review on European sea bass (Dicentrarchus labrax, Linnaeus, 1758) nutrition and feed management: A practical guide for optimizing feed formulation and farming protocols. Aquac. Nutr. 2015, 21, 129–151. [Google Scholar] [CrossRef]
- Yoshinaga, T.T. Development of Immunosuppressive Methods for Gonadal Grafting and Xenogeneic Germ Cell Transplantation in Rainbow Trout (Onchorhynchus mykiss). Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2022. [Google Scholar] [CrossRef]
- Masuda, Y.; Imaizumi, H.; Oda, K.; Hashimoto, H.; Usuki, H.; Teruya, K. Artificial completion of the Japanese eel, Anguilla japonica, life cycle: Challenge to mass production. Bull. Fish. Res. Agen. 2012, 35, 111–117. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).