Abstract
Egyptian Red Sea fisheries face the same challenges as most of the world’s fisheries, including overexploitation, habitat loss, IUU fishing, pollution, and climate change. These fisheries are highly diverse with multiple species targeted by multiple fleets, using different fishing gears. Much work has been performed in recent years to assess Red Sea fish stocks. However, not all fish stocks in the Egyptian Red Sea are assessed, and those that are assessed only cover 30% of landings. The assessments are unbalanced by area, with the Gulf of Suez being much better covered than the southern Red Sea and Gulf of Aqaba. The results show that most of the analyzed stocks are overexploited. There is an urgent need to take action to protect, conserve, and restore the different fish stocks in different fishing grounds. These actions will ensure the sustainability of the fisheries, making them ecologically friendly and economically and socially efficient.
Key Contribution:
Population dynamics parameters are valuable for understanding fish populations and assessing stock health, enabling the development of sustainable management measures for commercially exploited species.
1. Introduction
The Red Sea, a tropical sea with a rich history and unique marine ecosystem, is home to a wide variety of fish, many of which are of great commercial importance [1,2,3]. However, the unsustainable exploitation and illegal, unreported, and unregulated fishing (IUU) of these species threatens the health of the ecosystem and the long-term viability of the fishery [1,2,4]. Despite a growing demand for fishing resources due to population increase and a hard economic situation, sustainable management remains a major challenge for Egypt [5,6,7]. Understanding the biology of these species is essential to ensure the sustainability of fishing and the conservation of the marine ecosystem.
The Red Sea (Figure 1) is an elongated narrow sea between Northeastern Africa and the Arabian Peninsula, between 30° N and 12°30′ N and from 32° E to 43° E, with a straight-line length of about 2000 km and an average width of 208 km [5]. The Red Sea is connected to the Indian Ocean in the south through the narrow strait of Bab al Mandab. It has an average depth of 491 m, with a maximum depth of 2850 m. In the north, the Red Sea is divided into the Gulfs of Suez and Aqaba. The Red Sea is characterized by a few unique oceanographic and biological structures and is considered a hotspot for coral reef ecology. It also boasts high fish diversity, with more than 1400 species of fish reported in FishBase (www.fishbase.org, accessed on 1 April 2024).
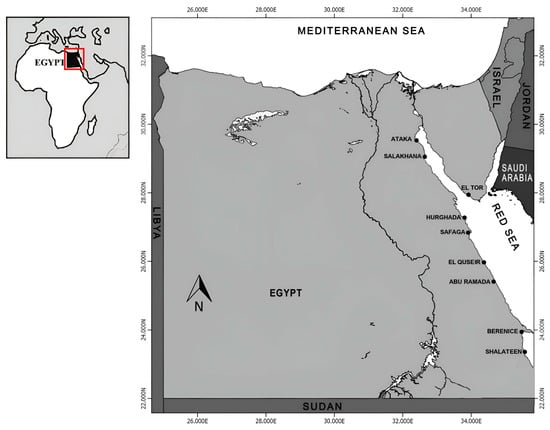
Figure 1.
Egyptian Red Sea with the different fishing ports.
Three main fishing methods are deployed in Red Sea fisheries: bottom trawl, purse seine, and artisanal ones. Bottom trawls constitute about 20% of Egyptian Red Sea fisheries, forming about 54% of the gross revenue due to the high price of some demersals caught by it, such as shrimp and cephalopods. Purse seine constitutes about 68%, and the artisanal fishery contributes the remaining 12% of the Red Sea catch in Egypt [1].
This study aims to provide up-to-date scientific information on the biological parameters of more than 50 commercial fish species from the Red Sea, including data on catch, areas fished, as well as basic data on the biology and dynamics of these species, thereby contributing to sustainable fisheries management and the conservation of marine biodiversity in this unique region. In the absence of information in this regard, this contribution is the first attempt to compile population dynamics parameters and the exploitation status of 55 fish species in the Egyptian Red Sea. This information will provide a solid basis for assessing the current status of fish stocks and establishing reasonable catch limits.
2. Material and Methods
The Egyptian sector of the Red Sea (Figure 1) is about 1080 km long, extending from Suez City in the north to Mersa Halayeb in the south. Numerous fishing grounds are found along the Egyptian Red Sea, yielding an average annual catch of about 48 thousand tons [8]. The Egyptian Red Sea is divided into three main fishing grounds: the Gulf of Suez, the proper Red Sea (from Hurghada to Halayeb, including Foul Bay), and the Gulf of Aqaba. Several landing sites are located along the Egyptian Red Sea, including Ataka, El-Tor, Nuweiba, Hurghada, Safaga, Quseir, Berenice, Shalateen, and Abu Ramad. Additionally, Egypt has signed treaties with its neighbors to allow fishing in their territories (Figure 2).
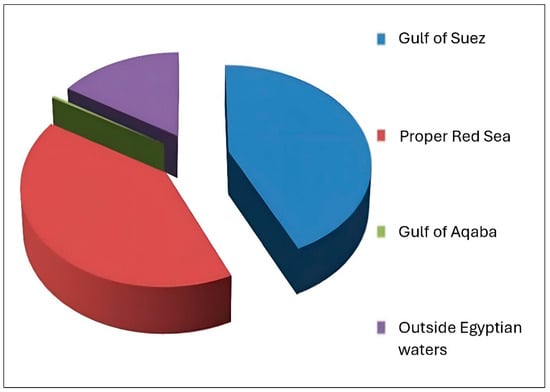
Figure 2.
Contribution (%) of the main fishing grounds to the Red Sea’s total catch.
The most common fishing gears used in the Red Sea by Egyptian fishermen are bottom trawls and purse seines (industrial fishery), hand lines, long lines, and gillnets (artisanal fishing), along with a variety of gears used by some of the traditional coastal communities. The industrial fishing fleets operate primarily in the Gulf of Suez and its neighboring areas, as well as Foul Bay. Semi-industrial fleets concentrate near Ataka, Salakhana, Hurghada, and El-Tor. Small-scale fisheries are common in Safaga, Quseir, Shalateen, and Abu Ramad [1].
This study relies on fishery statistics from the General Authority for Fish Resources Development (GAFRD) annual statistical book of 2021. Bimonthly field trips lasting at least seven days each have been carried out routinely since 1999 by the Fish Population Dynamics Lab, National Institute of Oceanography and Fisheries (NIOFs), to cover all fishing grounds along the Egyptian Red Sea. During these field trips, fish samples were collected directly from commercial fishing boats, and interviews were conducted with fishermen working in the area. This study analyzes data collected over the past ten years (2014–2024) in the Egyptian Red Sea with its two gulfs (Figure 1). This study focused on landings at the following locations: Ataka and El-Tor in the Gulf of Suez; Hurghada, Berenice, Shalateen, and Abu Ramad in the proper Red Sea; and Nuweiba and Dahab in the Gulf of Aqaba. In addition, the population parameters were compiled from two sources: previously published works and new estimations by the authors of this study for specific species.
Estimates of the asymptotic length (L∞) and the growth rate (K) were obtained using the ELEFAN program (Electronic Length Frequency Analysis) within TropfishR [9] for R statistical program version 3.6.3 [10].
Analysis of the cumulative catch curve [11] and of the length-converted catch curve [12] was used to estimate the total mortality coefficient (Z).
Natural mortality (M) per age was estimated using an online tool (barefootecologist.com) with three empirical formulae. Fishing mortality (F) was then determined as Z − M.
The exploitation status (E) was calculated as E = F/Z, where F is the fishing mortality rate.
The exploitation rate (E) is crucial for evaluating a fish stock’s status as optimal, underexploited, or overexploited. According to Gulland (1971), a stock is considered optimally fished when fishing mortality (F) equals natural mortality (M). In simpler terms, an exploitation rate (E) of 0.5 indicates optimal fishing pressure.
3. Results and Discussion
3.1. Landings and Catch Composition
Official annual landings of the Egyptian Red Sea fluctuated between 43.6 and 51.5 thousand tons from 2012 to 2021, averaging around 48 thousand tons. Red Sea fisheries account for about 11.5% of Egypt’s total landed catch from natural resources, generating an estimated revenue of EGP 6.35 billion. Figure 2 and Figure 3 illustrate the contribution of different fishing grounds to the Red Sea’s total catch. Table 1 presents catch data from the GAFRD’s 2021 statistical book. It is worth mentioning that the data recording system in Egypt is inaccurate. Many commercial species are not recorded separately but mixed in an “others” group. Also, there is no recorded data about bycatch and discards in the Egyptian marine fisheries. Therefore, urgent improvements are needed for the current recording system, as well as capacity-building programs to raise the qualifications of recorders at the different landing sites.
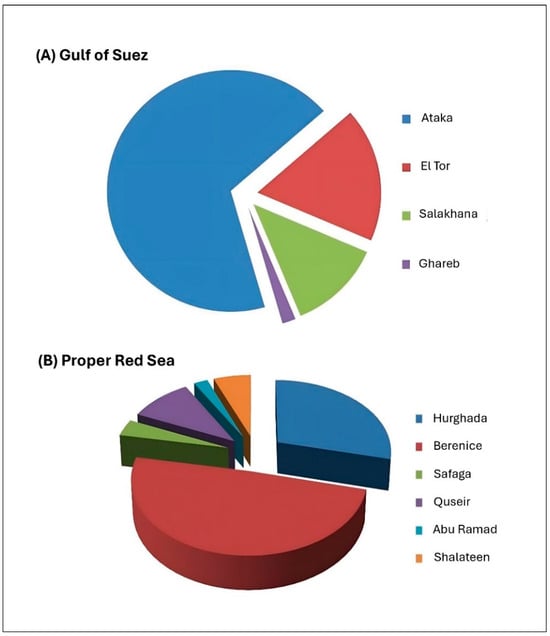
Figure 3.
Contribution (%) of the main fishing ports to the Red Sea’s total catch.

Table 1.
Available catch data based on GAFRD statistical book 2021 [8].
The field trips revealed a high diversity and abundance of fish species, especially in the Gulf of Aqaba, which the current catch statistics may not fully capture. Additionally, catch data are often recorded for groups of species rather than individual species.
The trawl fishery primarily catches fish from groups such as Synodontidae (lizardfish), Lutjanidae (snapper), Penaeidae (large shrimp), Mullidae (red mullet), Nemipteridae (breams), and Siganidae (rabbitfish). It is worth noting that large shrimp and cuttlefish are the most commercially valuable species in the trawl fishery, commanding high prices between EGP 300 and 500 per kilogram and representing about 45% of the trawl fishery’s gross revenue.
While the dominant fish families in the artisanal catch include groupers (Serranidae), emperors (Lethrinidae), and longspine bream (Sparidae), other Scombridae (little tuna and Spanish mackerel) are also present. More than 100 fish species from up to 20 families, like needlefish, squirrelfish, goatfish, and rabbitfish, were grouped together in the “others” category without any sorting or identification [1,13,14]. This “others” group constitutes about 46.5 percent of the total artisanal landings, highlighting the lack of accuracy and detail in the current fishery statistics collection and recording system.
Unlike the Gulf of Suez, where industrial fisheries dominate, artisanal fishing is the primary activity in the proper Red Sea and Gulf of Aqaba. It supplies approximately 40% of the total fish production from the proper Red Sea and constitutes the total of the Gulf of Aqaba’s catch [8,13,14,15,16,17,18,19].
3.2. Population Parameters and Exploitation Level
As shown in Table 2, the population parameters and status of 55 fish, crustacean, and mollusk species from the Egyptian Red Sea are presented. Despite the fact that both of Mehanna’s (2001) [20,21] studies might be outdated, they are included in Table 2 to capture all the available data. Mehanna’s (2001) [20] study is the only one that has assessed Rhabdosargus haffara in the Gulf of Suez, although Osman et al. (2020) [22] more recently evaluated the same species in the Red Sea off the Hurghada fishing area. Similarly, Mehanna’s (2001) [21] study remains the sole one on Rastrelliger kanagurta in the Gulf of Suez. Based on Gulland’s (1971) interpretation of the exploitation level, only five fish species are maintained at below slightly optimum exploitation levels: Lethrinus borbonicus, L. variegatus, and Euthynnus affinis from the Gulf of Aqaba and Monotaxis grandoculis and Parupeneus forsskali from Hurghada in the proper Red Sea. The remaining species, 50 out of the 55 assessed commercial species (90.9%), were found to be overexploited.

Table 2.
Growth parameters and mortality estimates of fish species from Red Sea, Egypt.
4. Conclusions
Our analysis of the Egyptian Red Sea’s most commercially important fish species reveals a high fishing mortality, which indicates a high exploitation level. Therefore, a reduction in exploitation rate and consequently the fishing mortality by about 20 to 70% is suggested to reach the optimum level (Eopt), which would help ensure the sustainability of the fish stocks. This can be achieved through enforcement of the closed fishing season and enforcement of the minimum legal size to conserve the spawning stock. The basic biological information generated from this study will be valuable for further population studies and stock assessment. These findings, in turn, can be applied to develop sustainable management measures for these commercially exploited fish species.
Author Contributions
S.F.M.: conceptualization, data analysis, writing—original draft, writing—review and editing. M.S.-K.: conceptualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mehanna, S.F. Egyptian Marine Fisheries and its sustainability. In Sustainable Fish Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2021; pp. 111–140. [Google Scholar]
- Samy-Kamal, M.; Mehanna, S.F. Evolution of fishing effort and fishing capacity during the last two decades (2000–2019) in Egypt’s marine fisheries: Spotting the fleet overcapacity. Reg. Environ. Chang. 2023, 23, 113. [Google Scholar] [CrossRef]
- Samy, M.; Lizaso, J.S.; Forcada, A. Status of marine protected areas in Egypt. Biodivers. Conserv. 2011, 34, 165–177. [Google Scholar] [CrossRef]
- Samy-Kamal, M. Insights on illegal, unreported and unregulated (IUU) fishing activities by Egyptian vessels in neighbouring countries. Fishes 2022, 7, 288. [Google Scholar] [CrossRef]
- Samy-Kamal, M. Status of fisheries in Egypt: Reflections on past trends and management challenges. Rev. Fish Biol. Fish. 2015, 25, 631–649. [Google Scholar] [CrossRef]
- Samy-Kamal, M. Outlook on the fisheries policy reform in Egypt and the draft of the new fisheries law. Mar. Policy 2020, 120, 104136. [Google Scholar] [CrossRef]
- Samy-Kamal, M. Prices in the Egyptian seafood market: Insights for fisheries management and food security. Fish. Res. 2021, 233, 105764. [Google Scholar] [CrossRef]
- GAFRD Annual Reports 2012–2021; GAFRD Fisheries Statistics Yearbook; General Authority for Fish Resources Development: Cairo, Egypt, 2022.
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR: An R package for fisheries analysis with length-frequency data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef]
- R Core Team. R A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 April 2024).
- Jones, R.; van Zalinge, N.P. Estimates of mortality rate and population size for shrimp in Kuwait waters. Kuwait Bull. Mar. Sci. 1981, 2, 273–288. [Google Scholar]
- Pauly, D. Length converted catch curves: A powerful tool for fisheries research in the tropics (Part 1). Fishbyte 1983, 1, 9–13. [Google Scholar]
- Mehanna, S.F. An assessment and management of the coral reef fish stocks in the Gulf of Suez. Egypt. J. Aquat. Biol. Fish. 1999, 3, 103–114. [Google Scholar]
- Mehanna, S.F.; El-Gammal, F.I. Gulf of Suez fisheries: Current status, assessment and management. J. King Abdulaziz Univ. Mar. Sci. 2007, 18, 3–18. [Google Scholar] [CrossRef]
- Mehanna, S.F.; Hegazi, M.M.; El-Sherbeny, A.S. Stock assessment and management of the cuttlefish Sepia pharaoni (Mollusca: Cephalopoda) in the Gulf of Suez. Egypt. J. Aquat. Biol. Fish. 2009, 13, 421–431. [Google Scholar]
- Mehanna, S.F. Population dynamics and management of snubnose emperor Lethrinus bungus (L. borbonicus) from the Foul Bay, Red Sea. In Proceedings of the INOC-XI International Symposium, Bogor, Indonesia, 25–27 October 2011; pp. 121–129. [Google Scholar]
- Mehanna, S.F. Population Dynamics and Assessment of the Snubnose Emperor Lethrinus borbonicus (L. bungus) from the Gulf of Aqaba, Egypt. Egypt. J. Aquat. Biol. Fish. 2023, 27, 643–654. [Google Scholar] [CrossRef]
- Mehanna, S.F. Life history parameters and stock status of kawakawa, Euthynnus affinis (Cantor, 1849) from the Gulf of Aqaba, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2024, 28, 299–312. [Google Scholar] [CrossRef]
- Mohammad, A.S.; Mehanna, S.F.; Osman, Y.A.; El-Mahdy, S.M. Age, growth and population parameters of the spiny squirrelfish, Sargocentron spiniferum (Forsskål, 1775) from Shalateen fishing area, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 469–480. [Google Scholar] [CrossRef][Green Version]
- Mehanna, S.F. Growth, mortality and yield per recruit of Rhabdosargus haffara (Sparidae) from the Suez Bay. Egypt. J. Aquat. Biol. Fish. 2001, 5, 31–46. [Google Scholar] [CrossRef][Green Version]
- Mehanna, S.F. Dynamics and management of the Indian mackerel Rastrelliger kanagurta (Cuvier, 1816) in the Gulf of Suez, Egypt. Egypt. J. Aquat. Biol. Fish. 2001, 5, 179–194. [Google Scholar] [CrossRef][Green Version]
- Osman, Y.A.; Mehanna, S.F.; El-Mahdy, S.M.; Mohammad, A.S.; Mahe, K. Age precision and growth rate of Rhabdosargus haffara (Forsskål, 1775) from Hurghada fishing area, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 341–352. [Google Scholar] [CrossRef][Green Version]
- Zaahkouk, S.A.; Khalaf-Allah, H.M.; Mehanna, S.F.; El-Gammal, F.I.; Makkey, A.F. Studies on age, growth, and mortality rates for management of the redspot emperor, Lethrinus lentjan (Lacepède, 1802) in the Egyptian sector of Red Sea. Egypt. J. Aquat. Biol. Fish. 2017, 21, 63–72. [Google Scholar] [CrossRef]
- Mehanna, S.F.; El-Gammal, F.I.; Zaahkouk, S.A.; Khalaf-Allah, H.M.; Makkey, A.F. Population dynamics of the small tooth emperor, Lethrinus microdon (Valenciennes, 1830) from the Egyptian Red Sea. Int. J. Fish. Aquat. Stud. 2017, 5, 158–163. [Google Scholar]
- El-Mahdy, S.M.; Mehanna, S.F.; Mohammad, A.S.; Osman, Y.A. Age, growth, mortality and yield per recruit of humpnose big-eye bream Monotaxis grandoculis (Forsskål, 1775) from Hurghada, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2022, 26, 857–868. [Google Scholar] [CrossRef]
- Mehanna, S.F.; Osman, Y.A.A.; Khalil, M.T.; Hassan, A. Age and growth, mortality and exploitation ratio of Epinephelus summana (Forsskål, 1775) and Cephalopholis argus (Schneider, 1801) from the Egyptian Red Sea coast, Hurghada fishing area. Egypt. J. Aquat. Biol. Fish. 2019, 23, 65–75. [Google Scholar] [CrossRef]
- Mehanna, S.F. Population dynamics of the areolate grouper Epinephelus areolatus from the Egyptian sector of Red Sea. In Proceedings of the 12th International Conference of Union of Arab Biologists, El-Hodeida University, Al Hudaydah, Yemen, 19–24 November 2005. [Google Scholar]
- Mohammad, A.S. Population Dynamics and Stock Assessment of Some Species of Genus Cephalopholis and Genus Variola from the Red Sea, Egypt. MSc Thesis, Assiut University, Asyut, Egypt, 2007; 178p. [Google Scholar]
- Mehanna, S.F.; Soliman, F.M.; Soliman, H.A.; Baker, T.S. Age, growth and length-weight relationship of Lutjanus quinqelineatus (Boch, 1790) and Lutjanus ehrenbergii (Petere, 1869) from the Red Sea, Egypt. In Proceedings of the Al-Azhar University, Assiut Branch Annual Conference, Hurghada, Egypt, 5–7 August 2017. [Google Scholar]
- Baker, T.S.; Mehanna, S.F. Some biological aspects and life history parameters of common bluestripe snapper Lutjanus kasmira (Family: Lutjanidae) from Shalatein, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2024, 28, 411–422. [Google Scholar] [CrossRef]
- Mehanna, S.F.; Ali, M.M.; Abdella, A.N.E. Evaluation of fishery status and some biological aspects of striped piggy, Pomadasys stridens from Gulf of Suez, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2023, 27, 791–803. [Google Scholar] [CrossRef]
- Mehanna, S.F. Population dynamics and stock assessment of two Lizardfish species in the southern Red Sea coasts, Foul Bay, Egypt. Egypt. J. Aquat. Biol. Fish. 2022, 26, 299–311. [Google Scholar] [CrossRef]
- Farrag, M.M.; Osman, A.G.M.; Mehanna, S.F.; Osman, Y.A. Fisheries status of the common species of family Mullidae in the Southern Red Sea, Hurghada, Egypt. Egypt. J. Aquat. Biol. Fish. 2018, 22, 249–265. [Google Scholar]
- El-Mahdy, S.M.; Mohammad, A.S.; Osman, Y.A.; Hassanein, E.M.; Mehanna, S.F. Age, Growth, Mortality and exploitation level of Squirrelfish, Neoniphon sammara (Forsskal, 1775) from Hurghada, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2023, 27, 51–63. [Google Scholar] [CrossRef]
- Mohammad, A.S.; Mehanna, S.F.; Mahmoud, U.M. Age and growth based on the scale readings of the two carangid species Carangoides bajad and Caranx melampygus from Shalateen fishing area, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2022, 26, 399–411. [Google Scholar] [CrossRef]
- El-Mahdy, S.M.; Mehanna, S.F.; Mahmoud, U.M.; El-Gammal, F.I. Population Dynamics and Management of Two-barred Seabream Acanthopagrus Bifasciatus in the Red Sea, Egypt. Int. J. Ecotoxicol. Ecobiol. 2019, 4, 80–87. [Google Scholar]
- Osman, Y.A. Population dynamics and stock assessment of the commercial species of family Scaridae in the Southern Red Sea, Egypt. MSc Thesis, Port Said University, Port Fuad, Egypt, 2015. [Google Scholar]
- Mehanna, S.F. Fisheries management of the slimy mackerel Scomber japonicus in the Gulf of Suez based on relative yield per recruit analysis. Egypt. J. Aquat. Biol. Fish. 2002, 6, 217–232. [Google Scholar] [CrossRef][Green Version]
- Mehanna, S.F.; El-Gammal, F.I. Stock assessment of the round herring, Etrumeus teres (DeKay, 1842) from the Egyptian sector of Red Sea. Indian J. Fish. 2005, 52, 377–383. [Google Scholar]
- Mehanna, S.F. Population dynamics of Penaeus semisulcatus in the Gulf of Suez, Egypt. Asian J. Fish. 2000, 13, 127–137. [Google Scholar] [CrossRef]
- Mehanna, S.F.; El-Gammal, F.I. Growth and population dynamics of cuttlefish Sepia savignyi in the Gulf of Suez, Red Sea. Indian J. Fish. 2010, 57, 1–6. [Google Scholar]
- Mehanna, S.F.; Amin, A.M. Population dynamics of the cuttlefish Sepia dollfusi (Adam, 1941) from the Gulf of Suez, Egypt. Egypt. J. Aquat. Biol. Fish. 2005, 9, 1–14. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).