1. Introduction
Freshwater ecosystems worldwide are facing mounting ecological stress related to anthropogenic activity, resulting in the rapid loss of biodiversity [
1]. Stream-fish assemblages across North America have been particularly impacted by stream fragmentation due to flow modifications and habitat degradation [
2,
3]. For instance, the construction or modification of bridges and culverts can impact stream habitats through alterations in the flow gradients, which are detrimental to fish species by impairing passage for seasonal and daily movements and generating downstream scour [
4,
5]. Indeed, the improper design of culverts could lead to water flow velocities that prevent the passage of resident or migrating fishes by exceeding their swimming capabilities [
6]. This could lead to the extirpation of resident fishes by limiting recolonization or inhibiting/preventing migrations critical to their life history [
7]. Moreover, successful culvert passage can be influenced by environmental parameters such as temperature, which can profoundly affect fish physiology and performance [
8]. Therefore, the impacts of such barriers and temperature on fish passage/migration have ecological implications for species distribution and abundance and are believed to contribute to the global decline in freshwater species [
6,
9,
10,
11,
12].
Many taxa native to the Edwards Plateau Ecoregion region of Texas, which is synonymous with the Texas Hill Country (Edwards Plateau hereafter), rely on the stable spring complexes fed by the Edwards Aquifer as refugia and have evolved highly restricted ranges found in only a few rivers such as the Guadalupe River [
13]. New urban developments including transportation improvements are being planned to address the high human population growth in this region, many of which are likely to increase groundwater withdrawal, resulting in decreased spring flow. Such efforts will include building new stream crossings, thus presenting a risk of exacerbating the existing habitat fragmentation to the species therein [
14]. The Edwards Plateau is home to several species deemed species of greatest conservation need (SGCN) by the Texas Parks and Wildlife Department (TPWD). However, little is known regarding the swimming performance of many of these species. Thus, there is a clear need to gain knowledge of the swimming performance of SGCN fishes of the region at relevant life stages and temperatures to help inform stream-crossing designs and barrier modifications.
To address these critical knowledge gaps, we sought to characterize various aspects of the swimming performance of four fish SGCN: the Guadalupe Bass (
Micropterus treculii), Guadalupe Roundnose Minnow (
Dionda nigrotaeniata), Guadalupe Darter (
Percina apristis), and Plateau Shiner (
Cyprinella lepida). The Guadalupe Bass is the state fish of Texas and is a popular sport fish found only in the Edwards Plateau. The 2018 Annual Report of the Guadalupe Bass Restoration Initiative stated that the economic value of fishing in the region was estimated to be USD 71 million over a 16-month period, with Guadalupe Bass being explicitly targeted by half of the anglers [
15]. Conservation efforts in preventing introgressive hybridization with Smallmouth Bass are ongoing, but perhaps a more considerable concern to the species is their vulnerability to habitat alterations and loss [
7,
16]. This is likely due in part to the fact that during their early life stages, a shift in habitat preference occurs toward increased current and depth. However, the habitat associations of juvenile Guadalupe Bass remain poorly understood [
14,
17]. Guadalupe Roundnose Minnow, Plateau Shiner, and Guadalupe Darter are at risk of habitat fragmentation as all have restricted ranges and rely on spring complexes for habitats found within a few rivers in the region [
13,
18,
19,
20,
21]. The movement of darter species is characterized by long-term residence in relatively small areas and is generally low compared to other fish families [
5]. Habitat modification could be problematic as darter swimming activity often occurs in short “burst-and-coast” movements on the benthic surface, allowing individuals to take refuge in lower-velocity water while bracing against the substrate. Poorly designed culverts could thus present a barrier to migration by not permitting this strategy [
10].
Specifically, our primary goal was to measure the maximum sustained swimming speed (Ucrit) of the previously mentioned species under a range of relevant temperatures (15, 22.5, and 30 °C) to be used in site specific calculations of culvert water velocities (Vf). Efforts were focused on the adult and juvenile (Guadalupe Bass only) life stages. A secondary objective was to collect additional physiological endpoints of relevance to overall swimming performance including the maximum burst swimming speeds (Umax), metabolic rate measurements (i.e., standard metabolic rate (SMR), maximum metabolic rate (MMR) and aerobic scope (AS)), minimum cost of transport (COTmin), cost of transport at Ucrit (COTUcrit), and optimal swimming speed (Uopt).
2. Materials and Methods
2.1. Experimental Animals
Adult Guadalupe Bass (Nueces River, Edwards County), Guadalupe Roundnose Minnow (Guadalupe River, Kerr County), Guadalupe Darter (San Marcos River, Hays County), and Plateau Shiner (Nueces River, Uvalde/Real Counties) were collected from river systems within the Edwards Plateau of TX. Guadalupe Roundnose Minnow, Guadalupe Darter, and Plateau Shiner were captured using a 3 m × 1.5 m seine net with 3/8 inch (9.5 mm) knotless mesh. Guadalupe Bass adults were collected via angling with a soft plastic lure with a single hook, or with an inline spinner with an individual treble hook (i.e., no multi-hook lures were used to avoid injury to the fish). Fish were immediately (<30 s after capture) placed in an oxygenated vessel and water changes were carried out approximately every 30 min [
22]. Fish that took longer to unhook were released. Fish were transported to the Heart of the Hills (HOH) Fisheries Science Center in Kerr County, TX in an oxygenated hauling tank and then “tempered” to the HOH water via water exchanges over ~1 h. Fish were collected from all habitat types available in the stream (run, riffle, pool). Juvenile Guadalupe Bass were obtained from HOH. Except for the adult Guadalupe Bass, fish were promptly transported in well-aerated coolers to the University of North Texas (UNT) in Denton County, TX, where they were acclimated and maintained for use in experiments. The experimentation of adult Guadalupe Bass was initiated at the HOH Fisheries Science Center; however, due to mechanical issues with the swim tunnel, testing was completed at UNT, as described in more detail below. Each species was collected, acclimated, and tested separately in succession.
For each species and life stage, fish were distributed evenly among the three treatment tanks upon arrival, each initially matched to the temperature of the transport water from the respective field collection. To ensure sufficient sample sizes for the swimming trials (
n = 8) and account for any potential mortality from handling stress (or otherwise), ~11–12 fish were included in each treatment. Final acclimation temperatures of 15, 22.5, and 30 °C were achieved by raising or lowering each tank’s temperature at a rate not exceeding 1 °C per hour and no more than 2–3 °C total change per day. Water temperatures selected either approximated spring (i.e., hydrologic feature, not the season) temperatures in the Edwards Plateau (22–23 °C) or represented the typical low and high temperatures experienced in Edwards Plateau streams. Once the target temperatures were reached, fish were held at their respective acclimation temperatures for a minimum of two weeks before experimentation. The temperatures in the 22.5 and 30 °C tanks were achieved using aquarium heaters, whereas the temperature in the 15 °C tank was achieved using a chiller unit (Frigid Units, Inc., Toledo, OH, USA). Fish held at UNT were maintained in living stream tanks (Frigid Units, Inc., Toledo, OH, USA) filled with ~500 L of reconstituted hard water (RHW) or dechlorinated City of Denton tap water [
23]. Large cylindrical fiberglass tanks filled with ~1800 L of water were used to hold adult Guadalupe Bass tested at HOH. Water used for fish culture and testing at HOH was directly spring fed from an adjacent property and used for all fisheries-related work at the facility. Water quality in all holding tanks at both locations was maintained using individual recirculating filtration systems per tank. Temperature, dissolved oxygen (DO), pH, conductivity, and ammonia were recorded at least twice per week from each tank. Temperature and DO were measured using a YSI ProODO oxygen meter (YSI Incorporated, Yellow Springs, OH, USA), and pH was measured with an Orion Star A121 portable pH meter (Thermo Fisher Scientific, Waltham, MA, USA). Conductivity was monitored using an Oakton CON 6+ conductivity meter (Thermo Fisher Scientific, Waltham, MA, USA), and ammonia was monitored using an aquarium test kit (Mars, Inc., Chalfont, PA, USA). Fish were fed a combination of defrosted bloodworms and Tetramin fish flakes ad libitum once daily and maintained under a 14:10 light:dark schedule.
2.2. Ucrit and Metabolic Rate Measurements
Maximum sustained swimming speed (Ucrit) and metabolic rate (ṀO2) measurements of juvenile Guadalupe Bass, Guadalupe Roundnose Minnow, and Plateau Shiner were conducted in a 1.5 L Blazka-style swim tunnel (Loligo Systems, Viborg, Denmark). Adult Guadalupe Bass swimming performance trials were conducted in a 180 L Brett-style swim tunnel (Loligo Systems, Viborg, Denmark) housed at the HOH facility to accommodate their larger size. However, this large volume precluded the ability to collect ṀO2 measurements due to an excessive volume-to-fish mass ratio; thus, no ṀO2-related measurements are provided for this group of fish. Additionally, the 180 L swim tunnel required a significant repair toward the end of testing, with ~2–3 fish remaining to be tested per treatment. The remaining fish were transferred to UNT to complete the trials in a smaller 10 L swim Brett-style tunnel (Loligo Systems, Viborg, Denmark), focusing on the smaller of the remaining fish that would fit comfortably within the tunnel. To this end, the remaining fish were reacclimated at UNT to their respective temperatures for a minimum of two weeks prior to completing the swim trials to account for transport stress.
Oxygen and temperature readings were collected using a mini oxygen dipping probe and Pt1000 temperature probe, respectively (Loligo Systems, Viborg, Denmark). Before each experiment, a two-point calibration at the appropriate acclimation temperature was performed on the oxygen probe. The 100% air saturation calibration value was achieved by vigorously aerating the water with an air stone, and the 0% O2 saturation value was obtained using a 10 g/L sodium sulfite solution (Avantor, Allentown, PA, USA). The temperature during the experiments was maintained using a submerged heating coil attached to an Alpha 8 heating/cooling circulating water bath (LAUDA-Brinkman, LP, Delran, NJ, USA). Experimental data were collected using AutoResp version 2.1.2 (Loligo Systems, Viborg, Denmark).
Prior to initiating swim trials, preliminary tests in size-matched static respirometers (Loligo Systems, Viborg, Denmark) were performed on each species and life stage (minimum of four individuals) to establish the period of time needed to permit recovery from handling stress and swim chamber habituation. Fish were held in static respirometers until their metabolic rates stabilized to a routine metabolic rate (RMR), defined by at least three consecutive measurements not deviating by more than ~10%. For the Plateau Shiner, the period of habituation appeared substantial (~11 h), and therefore an overnight habituation period was employed for this species. The final habituation periods for the other species were determined as follows: Guadalupe Roundnose Minnow ~5 h, juvenile Guadalupe Bass ~4 h, adult Guadalupe Bass ~4.5 h, and Guadalupe Darter ~1.5 h.
Swim trials were performed following previously established methods [
24,
25]. Food was withheld for all fish for at least 24 h prior to introduction into a swim chamber to eliminate specific dynamic action as a confounding factor. Approximate body length (BL) was quickly estimated using a ruler, and approximate mass was estimated based on prior experience to minimize handling stress. Final definitive measurements of these parameters were collected after each swim trial, and the data were mass and length corrected. Fish were gently transferred into the swim tunnel, maintained at their respective acclimation temperatures, and allowed to habituate to the chamber for the pre-established period of habituation at a minimal flow speed (~0.5 BL/s) prior to initiating the
Ucrit test. Measurements of ṀO
2 were initiated during the habituation period immediately following transfer to the chamber. Fish monitoring and recording during the tests were conducted remotely using a small video camera connected to a computer to avoid disturbing the fish. Each test was performed by forcing the fish to swim for a series of 20-min intervals, each consisting of a flush, wait, and measurement period until the fish failed to continue swimming. Wait periods were set to 30 s while both flush and measurement periods were optimized to balance obtaining sufficient O
2 measurements for a high
r2 value (see below) and sufficient time to replenish the chamber with near 100% air saturated water. Each interval progressively increased in velocity by increments of ~0.5 BL until failure. Failure was determined when the fish became fatigued and impinged on the rear screen or showed prolonged resting against the rear screen two consecutive times following a temporary reduction in flow after the first occurrence. Failure was confirmed by using video analysis of swim trial recordings.
Ucrit was calculated using the following equation originally described by Brett:
where
Uf (cm/s) represents the highest swimming speed maintained for a complete interval, T (s) is the time spent at the final velocity, t is the time interval (s), and d
U is the increment in swim speed (cm/s) [
26]. Following the completion of the swim trial, fish were removed from the swim tunnel and euthanized using a buffered 10 g/L methanesulfonate (MS-222) solution, and biometric data were collected. The empty chamber was resealed to measure and correct for potential background oxygen consumption due to microbial respiration. Note that
Ucrit measurements for Guadalupe Darter were not possible due to their behavior, which typically consisted of extending their pectoral fins and resting on or near the mesh at the back of the swim chamber for the duration of the swim trial. This behavior has been observed in other darter species and is likely due to their ecology as a benthic species [
27].
Metabolic rate analyses were performed using ṀO
2 data collected during the swim trials except for the Guadalupe Darter and adult Guadalupe Bass for reasons described above. The ṀO
2 measurements of the last three intervals prior to ramp-up were averaged together to better represent the initial metabolic rate before initiating the experiment. Both least squares linear regression and exponential regression have been used to model data of this nature in fish swimming performance studies [
25]. By applying both, it was found that exponential regression better modeled the data (higher
r2 value) 82% of the time. Standard metabolic rate (SMR; y-intercept) and maximum metabolic rate (MMR; extrapolated at
Ucrit) were therefore derived for each fish using an exponential regression of the logarithm of ṀO
2 (mgO
2/g/h) versus swimming speed. Only individuals with ṀO
2 regressions yielding
r2 values ≥ 0.7 were used for the metabolic analyses. Aerobic scope (AS) was calculated by subtracting the SMR from the MMR. Given that Guadalupe Darter would not swim in the swim tunnel, SMR, MMR, and AS were determined using static respirometers. This approach was achieved by employing the standard “chase” method to obtain the MMR and taking the mean of the lowest 10th percentile values following habituation to obtain the SMR [
28,
29]. Additionally, as described earlier, the large volume-to-mass ratio for adult Guadalupe Bass precluded ṀO
2 assessments for this life stage. For the swimming performance trials, sample sizes ranged from 7 to 12 individuals. For the metabolic rate measurements, sample sizes typically ranged from 5 to 10. An exception was the Plateau Shiner, which had low sample sizes (
n = 2–4) due to poor metabolic rate regressions (see
Section 4).
2.3. Maximum Water Velocities
Maximum water velocities (
Vf) were calculated using the formula:
where
Vf is the velocity in the culvert to be traversed (m/s),
Vs is the percentile
Ucrit value (m/s),
d is the length of the culvert, and
Evs−1 is the time increment used during the
Ucrit trials [
30]. This approach allows for setting site-specific flow criteria depending on culvert length and the level of protection sought for a given species by adjusting the percentile threshold. Additionally, this equation considers that a fish needs to swim at a speed faster than the velocity of the water to make forward progress. A range of
Ucrit (cm/s) percentiles were calculated for each species at each temperature and used to model
Vf over a range of culvert distances up to 30 m in length.
2.4. Umax Measurements
The maximal burst swimming speed (
Umax) was measured similarly to that as previously described [
31,
32]. It should be noted that there is inconsistency within the literature for terms representing a fish’s maximum swimming speed. For example, both
Umax and
Uburst have been used to describe the velocity at fatigue during constant acceleration tests [
33,
34], and
Uburst has also been used to describe the point just prior to initiating a burst-and-coast swimming mode for a minimum period (e.g., >5 s; see [
35]). Herein,
Umax will refer to the instantaneous maximum speed associated with the C-start escape response [
33,
34,
35].
Umax tests were conducted for all species under similar physiochemical conditions as the
Ucrit determination. All
Umax tests except for adult Guadalupe Bass were conducted in a cylindrical 10-gallon (38 L) white plastic tank with a diameter of ~36 cm and water depth of ~7 cm. Adult Guadalupe Bass were tested in a large green fiberglass tank with a diameter of ~172 cm and a water depth of ~38 cm. Burst swimming from an escape response was induced following a 15 min acclimation period during which the fish could move freely. A mechanical stimulus, a standard weight, was released from 1.5 m high to induce the response. The stimulus was shielded from view within a PVC pipe, and the test area was screened off to prevent visual stimulation before contacting the surface of the water. A GoPro (HERO7 Black, GoPro Inc., San Mateo, CA, USA) filming at 30 frames per second was positioned directly above the experimental tank to record the burst swimming response. Video files were uploaded and processed using Lolitrack 5 (version 5.1.0) software (Loligo Systems, Viborg, Denmark). Each video was separately calibrated for distance and area of interest masked, and a filter was created that highlighted pixels designated for fish location. Analysis was conducted on twelve frames and commenced with one frame prior to the stimulus contacting the water. This approach allowed the first 400 ms to be analyzed, which was deemed sufficient to capture the maximum speed [
32,
36]. Each specimen was recorded three times, with a 15-min interval between recordings, and each file was analyzed as described to determine the fastest response.
2.5. Cost of Transport and Optimal Swimming Speed
The cost of transport (COT) for each individual was calculated by dividing ṀO
2 by their respective swimming speeds (cm/s) at each speed increment. For the same reasons described above, the metabolic measurements of the last three intervals prior to ramp-up were averaged together. The optimal swimming speed (
Uopt) and minimum cost of transport (COT
min) were both determined by creating a second-order polynomial regression. A plot was generated, correlating the swimming speeds (cm/s) and COT for each individual fish. The x component of the vertex in the polynomial regression was solved to establish
Uopt while the y-value of the same point represented COT
min [
25]. Again, only individuals with regressions yielding
r2 values ≥ 0.7 were used for the analyses. Using the measured body lengths, measurements of
Uopt and COT
min were converted to BL/s from cm/s. Cost of transport at
Ucrit (COT
Ucrit) was calculated by dividing the MMR (mgO
2/g/s) for each fish by the corresponding
Ucrit (both BL/s and cm/s).
2.6. Statistical Analyses
Data are presented as the means ± standard error of the mean (SEM). Differences were tested for statistical significance using SigmaPlot version 12.3 (Systat Software, Inc., San Jose, CA, USA). Shapiro–Wilk normality equal variance tests were run before proceeding with one-way ANOVA. Pairwise multiple comparisons across temperature within species and life stage were conducted using the Holm–Sidak method. Data for COTmin and COTUcrit for Guadalupe Roundnose Minnow as well as the SMR for Plateau Shiner were log-transformed to satisfy the assumptions of equal variance and normality. Kruskal–Wallis one-way ANOVA on ranks was conducted following transformation. In all cases, differences were deemed significant at p < 0.05.
4. Discussion
This research primarily aimed to assess various swimming performance metrics of SGCN fishes under a range of relevant temperatures that could be used to provide recommendations of the maximum water flow rates (Vf) for stream-crossing designs that ensure the passage of these fishes. Our approach focused on measuring the maximum sustained swimming speed (Ucrit). Additional physiological endpoints were also assessed to inform broader questions related to aerobic scope, swimming efficiency, and partitioning contributions to ground speed (swimming speed minus water speed) from aerobic (Ucrit) and anaerobic swimming mechanisms (Umax).
At least for some species, it has been determined that measuring the maximum swimming capacity using swim tunnels likely underestimates the values achieved in the wild putatively, due to behavioral/motivational effects in response to confinement within the tunnel [
37,
38]. Nevertheless, studies that integrate closed swim tunnel and open flume approaches (the latter more closely mimics natural conditions) have led to various approaches to estimating the limits to culvert or fish passage flow velocities [
31,
37,
39,
40,
41,
42,
43]. Using a standard ramped-velocity
Ucrit test as a main component of this approach represents a compromise whereby time and logistical constraints associated with other methods and equipment (e.g., large custom-made flumes) are avoided, and the end results tend to be conservative in nature, given the likely inherent underestimation of the actual
Ucrit in the wild.
The approach adopted in this project, as detailed by Watson et al., offers a valuable method for estimating the passage flow velocity [
30]. This method offers fisheries managers the flexibility to select from a range of distances and
Ucrit percentile values for their modeling purposes. Furthermore, these values can be finetuned to align with specific site conditions, culvert length, and the level of protection required for a particular species by adjusting the percentile threshold. For instance, the use of the 5th, 25th, and 50th percentile
Ucrit values allows for the estimation of the maximum water velocities that facilitate the successful passage of 95%, 75%, or 50%, respectively, for a species at a given temperature and distance. This adaptable approach equips fisheries managers with a valuable tool for tailoring their assessments to the unique requirements of their conservation efforts. Additionally, in cases where multiple focal species are expected to coexist in the same habitat, it is advisable to use the swimming performance value of the species with the lowest capacity as a basis for setting flow limits. This conservative approach ensures that the habitat is suitable for the species with the most stringent requirements, thus enhancing the overall conservation efforts. The low rates of change in the calculated
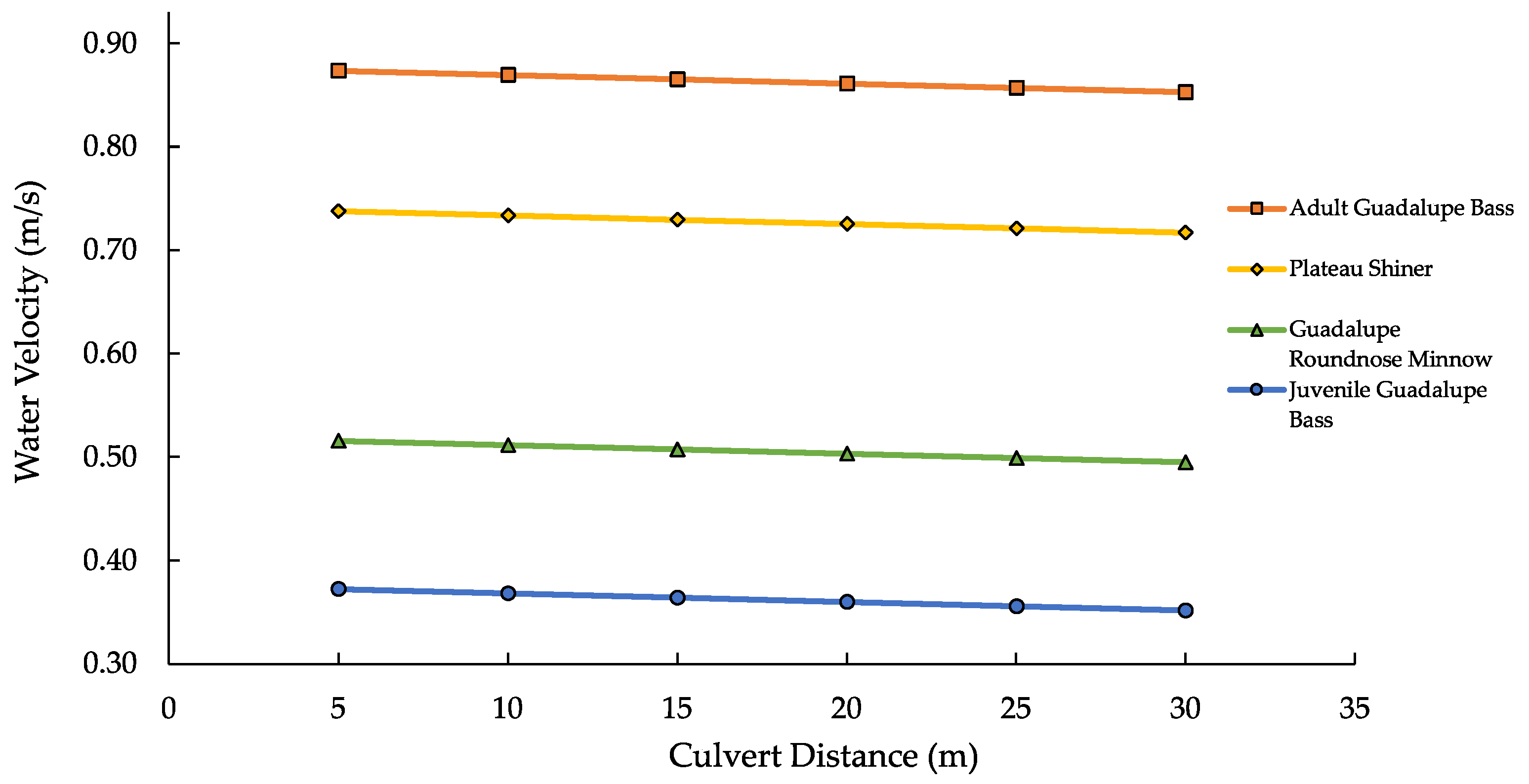
Vf’s as a function of culvert distance was due to the time increment (
Evs−1) of 20 min that was used. In the report by Watson et al., the time interval used was 5 min, but the longer time interval used in this project likely facilitates a better approximation for a wider range of culvert lengths, some of which will require a greater time to traverse.
Regarding the temperature effects,
Ucrit is generally expected to increase with temperature up to a maximum speed, beyond which the performance steadily declines as the temperature continues to rise (see for example [
44,
45]). Of the three species tested for
Ucrit, all exhibited similar trends conforming to this expectation, although statistically significant increases were only observed between 15 and 22.5 °C. These trends suggest that the temperature at which the highest
Ucrit would be expected lies either near or between the 22.5 and 30 °C temperatures. An explanation for this could be that
Ucrit (maximum aerobic performance) is limited by the capacity for oxygen transport at elevated temperatures, compressing aerobic scope [
46]. Considering the Guadalupe Bass, while these trends generally held for the adults, only the juveniles exhibited statistically significant differences, likely due to the smaller sample sizes for the adults, which were lower than anticipated (i.e.,
n = 5–6 instead of
n = 8) due to non-cooperative fish (e.g., would not swim) or technical difficulties with the video analysis for
Umax (e.g., corrupted files). It should be noted that the differences in
Ucrit for juvenile Guadalupe Bass could be at least partially attributable to the smaller sizes of fish from the 15 °C treatment, as swimming efficiency is known to scale with size, with larger fish able to swim faster than smaller fish [
47]. Metabolic rate also scales with size; however, this likely does not explain the significant difference in the MMR observed at 15 °C, given that the mass specific metabolic rate decreases with increased mass. Therefore, this difference can be safely attributed to a temperature effect. Burst swimming (
Umax) is not necessarily expected to display the same degree of temperature dependence due to the thermal compensation of fast-twitch muscles following acclimation [
48]. Indeed, there was no clear trend of a temperature effect on
Umax for any of the species tested.
As alluded to earlier, the Guadalupe Darters could not be enticed to swim within the swim tunnel and instead would resist flow by pectoral fin extension and resting on the bottom or against the back screen. Considering that these fish are found in habitats with some of the highest current velocities among the species evaluated, this suggests that small-scale refugia from currents is likely necessary for them to move upstream through high-velocity areas by burst swimming from point to point rather than a sustained effort to pass a potential barrier. Similar inabilities to measure
Ucrit due to substrate bracing have been previously reported for other benthic species [
27,
39]. Interestingly, the AS for Guadalupe Darters was significantly reduced at 30 °C compared to the lower temperatures. This collapse in AS suggests that
Ucrit would likely be severely compromised at this temperature. Nevertheless, since periodic anaerobic burst swimming is likely the preferred mode of transport for Guadalupe Darters under high flow conditions, this potential effect on
Ucrit is likely negligible from a management perspective. Thus, in light of the absence of measured
Ucrit values combined with burst swimming as the preferred mode of transport for this species,
Umax likely represents a reasonable alternative for establishing the maximum flow velocities for the safe passage of Guadalupe Darters. It is therefore recommended that water flows for this species do not exceed some fraction of
Umax (e.g., 75% or lower 95% confidence interval) to ensure that most individuals are accommodated.
It should be noted that only relatively small sample sizes for the Plateau Shiner (i.e.,
n = 2–4) were available for metabolic endpoints due to the poor
r2 values obtained using the exponential regression. This typically occurred due to the data exhibiting a bimodal distribution with an initial rise in metabolic rate followed by a steady decrease until the metabolic rate rose again to the MMR (See
Supplementary Figure S5). We reviewed video recordings to identify potential changes in swimming gait or ventilation during the swimming trials, which might help to explain these observations; however, no obvious changes were detected for either. Thus, the cause of the bimodal distribution of the metabolic data for some of these fishes remains unknown at this time.
It is expected that the SMR and MMR will increase at different rates as the temperature increases until reaching an optimum temperature where the AS is maximized [
49]. Warming past this point constrains the MMR (limiting stressor), likely due to limitations on the oxygen supply capacity (e.g., insufficient ventilation or cardiac output) while simultaneously increasing the SMR (loading stressor), resulting in the compression of AS. AS represents the total amount of energy available to an organism beyond the minimum amount required to sustain life (i.e., SMR) that can be used for activities such as foraging, migration, and reproduction, and is therefore an important ecophysiological metric. Given that only three temperatures were used, we could not determine the precise optimal temperature for aerobic scope, though some approximations can be made. AS values for the Guadalupe Roundnose Minnow were significantly higher at 30 °C compared to 15 and 22.5 °C, which would indicate that with acclimation, this species’ oxygen supply capacity is maintained at relatively high environmental temperatures. The inverse was seen for the Guadalupe Darter, where at 30 °C, the AS decreased significantly compared to 15 and 22.5 °C. This could be related to this species niche as a benthic organism, which utilizes burst swimming and therefore has likely evolved a lower dependence on aerobic performance. The Plateau Shiner and juvenile Guadalupe Bass, although not significant, displayed elevations in AS values from 15 to 22.5 °C and little change between 22.5 and 30 °C. Overall, these results indicate that the optimum temperature for the maximum aerobic capacity of juvenile Guadalupe Bass and Plateau Shiner likely lies near 22.5 °C or between 22.5 and 30 °C. These data are potentially reflective of the streams these species inhabit, where temperature variation is often mediated by spring inputs and indicate an elevated thermal vulnerability to stressors like habitat fragmentation and climate change, which could result in the exceedance of the thermal maxima.
5. Conclusions
The escalating ecological stress caused by anthropogenic activities has resulted in a significant loss of biodiversity in freshwater ecosystems worldwide. This concerning trend holds particularly true for stream-fish assemblages in the Edwards Plateau Ecoregion of Texas, where stream fragmentation, stemming from flow modifications and habitat degradation, has had a profound impact. In light of these challenges, the present study aimed to address critical knowledge gaps surrounding the swimming performance of four species of greatest conservation need native to this region: the Guadalupe Bass, Guadalupe Roundnose Minnow, Guadalupe Darter, and Plateau Shiner.
These species rely on the relative stability provided by spring complexes fed by the Edwards Aquifer, and their survival is contingent upon these specific habitats within the restricted ranges they occupy. By gathering measurements of the maximum sustained swimming speed, burst swimming speed, and metabolic traits of these fish under relevant temperature conditions, we provide valuable tools for determining site specific maximum water velocities. The data from these measurements offer valuable insights into these species’ swimming capabilities, and coupled with additional physiological endpoints such as metabolic traits, cost of transport, and optimal swimming speed, will enable managers to make informed predictions about how these species will respond to the ever-increasing challenges posed by climate change and hydrological disruptions.
These findings hold substantial practical implications as they will help shape the design and modification of stream crossings and barriers, ultimately enhancing conservation strategies. By ensuring that these structures are tailored to the swimming abilities and requirements of these vulnerable fish populations, the negative impacts of ongoing urban development and habitat fragmentation can potentially be mitigated to preserve the genetic diversity and ecological integrity of these species.