Abstract
This opinion piece presents empirical evidence to examine possible negative consequences of the use of penetrative tagging as used on the great white shark (GWS). Tagging programs currently using this method attach SPOT (Spatial Positioning Only Tags) using corrodible bolts inserted through the dorsal fin while the shark is taken out of water. Such methods can cause harm to the tagged individual. Possible adverse effects include impacts on growth, tag biofouling, wounds, heightened stress, and hemorrhaging. This method may adversely impact dorsal fin structure and the shark’s hydrodynamics. As a result, data collected may not be reflective of natural behavior. Bolted SPOT are semi-permanently affixed to the shark but can have a battery life of approximately 3.5 years. Most of these tags (69%) ceased transmitting in less than 2 years. Alternative tagging technologies exist as more humane options.
Key Contribution:
SPOT bolted into the dorsal fin are harmful to the great white shark. Bracelet tags and fin clamps are non-penetrative solutions that are viable alternative tagging methods.
1. Introduction
There are few studies that have directly investigated the effects of penetrative tagging methods on elasmobranchs, yet these tagging programs are common [1,2,3,4,5,6,7,8,9]. For example, Carcharodon carcharias (GWS, great white shark)-tagging activities have received extensive media attention, especially on “Shark week”. The primary goal for tagging GWS is to obtain shark migration data [10]. Migration data add essential information to the knowledge of GWS ecology.
This review focused on SPOT (Smart Position Only Tags), a commonly employed method [7,9]. SPOT are secured by bolting the tag into the first dorsal fin. The penetrative nature of attaching these tags damages the skin and internal structures. The paper by Jewell et al. [8] raised the concern of negative long-term consequences from these bolted tags. Penetrative tagging has caused wounds and an accumulation of biofouling on GWS dorsal fins and tags [7,8]. Additionally, this tagging method may affect fin growth in juvenile GWS [8]. These effects have the capacity to compromise the hydrodynamic role of the dorsal fin and alter natural behavior [11].
This paper also reports evidence indicating that hemorrhaging occurs when a GWS is removed from the water and placed on a flat platform for tagging. Typical practice in tagging programs involves removing GWS from the water so that the research team has complete access to the shark for collecting samples “https://www.ocearch.org/tracker/ (accessed on 7 May 2024)”. GWS, like other elasmobranchs, are cartilaginous and lack a solid, non-flexible rib cage to protect vital organs from damage [12]. Due to this skeletal flexibility, excessive pressure is placed on the internal organs when the weight of the shark is not properly supported when GWS is out of water. This phenomenon also has occurred in beached whales, which have a stronger, bony skeleton [13]. This trauma leads to dark hemorrhagic bands forming ventrally along the snout, jaw area, underbelly, below the gills, and caudal peduncle. Other studies have used less harmful methods, whereby the shark is in a harness or restrained via a tail rope in water while being handled [14]
Tag longevity was also examined in GWS equipped with a dorsal-fin-mounted SPOT. The purpose was to quantify tag longevity and evaluate whether tags were lasting for the total transmission duration as programmed by researchers.
2. Materials and Methods
SPOT longevity data were obtained from the Ocearch tagging program website “https://www.ocearch.org/tracker/ (accessed on 7 May 2024)”. These data are summarized in Table S1. Longevity was determined by the number of days the tag was displayed as transmitting. The data were then compiled into a histogram with bin lengths of one year. Tag longevity data for other shark species were obtained from the literature and are summarized in Table S1. Images of GWS with likely hemorrhaging are published on the Ocearch website “https://www.ocearch.org/tracker/ (accessed on 7 May 2024)”. These data are recorded in Table S2.
3. Results: SPOT Longevity
One commonly used tag model, SPOT-257, should transmit for approximately 3.5 years according to the manufacturer, Wildlife Computers. SPOT-257 was a commonly used tag by Ocearch (Wildlife Computers, pers comm October 2023). The majority of SPOT (69%) used by Ocearch ceased transmitting in less than 2 years (Figure 1). SPOT longevity data for Prionace glauca, Sphyrna mokarran, and Sphyrna lewini showed durations of less than a year (Table 1) [15,16,17], although SPOT-257 was not used in these studies (Stevens et al. [15]: SPOT-4 and -5; Heim et al. [16]: SPOT-364; Drymon and Wells [17]: SPOT-6). All tags were manufactured by Wildlife Computers. Programmed tag battery lifetimes are listed in Table 1. The study on S. mokarran by [17] was short term and is not included in Table 1.
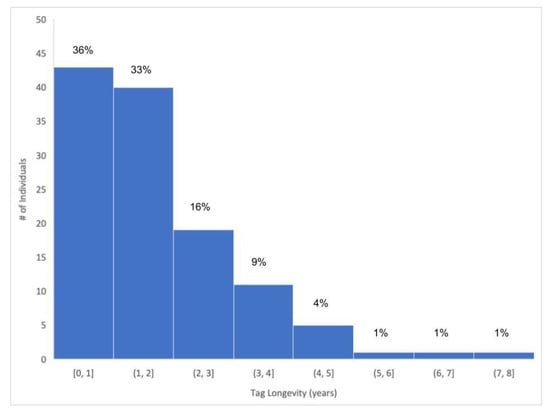
Figure 1.
Tag longevity of 121 bolted SPOT applied to GWS tagged by Ocearch. Sixty-nine percent (69%) of these tags ceased transmitting in under 2 years.

Table 1.
Longevity data from other shark species also tagged with a bolted dorsal fin SPOT.
4. Discussion:
SPOT use the Argos satellite system to approximate a tagged individual’s position “https://wildlifecomputers.com/ (accessed on 7 May 2024)”. Depending on the make and model of the tag, accuracy and resolution vary (see “https://wildlifecomputers.com/ (accessed on 7 May 2024)” for specifics). SPOT rely on the tag’s antenna breaking the surface [7]. As a result, gaps in data have arisen when these tags are used on species that remain submerged for extended periods [18]. To apply the tag, some studies remove the shark from the water [18,19]. The tag is then secured with nuts and corrodible bolts through a hole drilled into the fin. This attachment method is meant to be permanent. Possible reasons for reduced longevity in satellite tags are: (1) technical failure, (2) the tag battery running out, and (3) mortality [15,17,20].
There are six possible adverse effects resulting from the use of bolted SPOT: (1) growth, (2) tag biofouling, (3) wounds, (4) stress, (5) hemorrhaging and (6) drag on swimming hydrodynamics.
4.1. Growth
Studies investigating the effects of penetrative tagging methods on growth have indicated that effects are species specific and depend on food availability, injuries sustained during the tagging process, and irritation caused by the tags [1]. A literature review [1] summarized that growth was affected in the following: Ginglymostoma cirratum, Squalus acanthias, Sphyrna tiburo, Negaprion brevirostris, Mustelus lenticulatus, Carcharhinus tilstoni, and Carcharhinus sorrah. The growth rate of Negaprion brevirostris was not greatly affected by tagging when food was not a limiting factor [21]. Tagging did not affect the growth rate of Galeocerdo cuvier [22]. Changes to body size caused by tagging were deemed non-detrimental in Hemiscyllium ocellatum [23]. Effects on growth due to various tagging methods may be associated with body size at the time of tagging. For example, juvenile N. brevirostris experienced a significantly decreased growth rate when tagged with dart tags [24]. Also, bolted SPOT have been implicated in affecting the growth of sub-adult GWS dorsal fins, resulting in deformation of a single shark’s fin, when the tag did not release within the 12–24-month window recommended for deployment by Jewell et al. [8]. The authors concluded that these tags should not be used on juvenile GWS for this reason [8].
4.2. Tag Biofouling
Antifouling paints have been used to prevent tag biofouling [7,8]. Biofouling involves the colonization of algae and other micro and macro-organisms when a biofilm forms on surfaces exposed to seawater [25]. The first published study to report biofouling on SPOT using photographic evidence was Jewell et al. [8]. Fouling occurred in one shark’s fin ~5 months after applying antifouling paint to the tag [8] (Figure 2). Hammerschlag et al. [7] noted that tagging may facilitate parasite recruitment. Wounds and infections surrounding the area of insertion were also identified as risks [7]. Tissue degradation and infection caused by SPOT biofouling has been documented in dolphins [26].
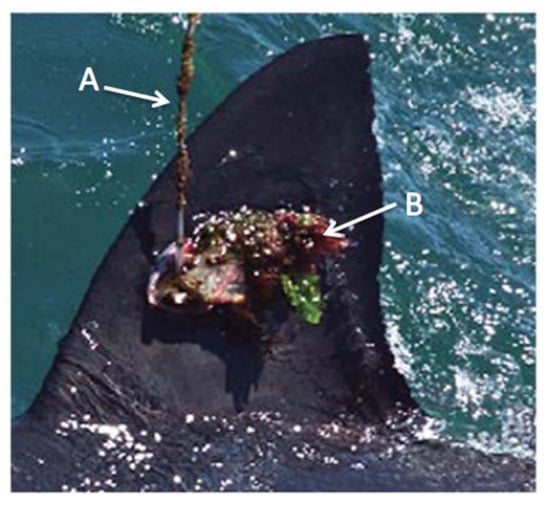
Figure 2.
A SPOT on the dorsal fin of a GWS after 172 days. This tag had been coated with antifouling paint. Algae and other attached organisms can be seen on the satellite antenna (A) and the tag body (B). Modified from Jewell et al. [8].
4.3. Wounds
Jewell et al. [8] found that bolted SPOT become biofouled and cause hyperpigmentation and structural damage to the dorsal fin (Figure 3). Of the images of 15 tagged GWS, 8 had signs of hyperpigmentation and 2 had fouling on their tags. One individual had both fouling and a dorsal fin that leaned to the left. The leaning of the dorsal fin was thought to be due to (1) the weight of the algal growth on the tag, (2) the weight of the tag, or (3) the combined weight of both the algae and the tag [8].
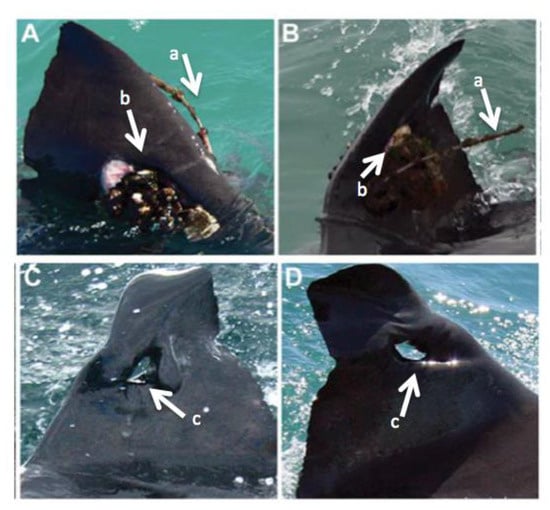
Figure 3.
Images of GWS dorsal fins equipped with SPOT for over 24 months. Images (A,B) show fouling on the tag and a left-leaning fin (left-leaning was cited in [8]). The antenna is the “a” arrow and the biofouled tag is the “b” arrow. Images (C,D) show fin degradation around a puncture wound where a tag was previously affixed. The “c” arrow points to the open wound caused by the detached tag. Modified from Jewell et al. [8].
4.4. Stress
Stress is defined as “a physiological cascade of events that occurs when the organism is attempting to resist death or reestablish homeostatic norms in the face of insult” [27]. Factors that have led to changes in homeostatic state in elasmobranchs during tagging are: time out of the water, cortisol and lactate levels due to time fighting on hook and line, and muscular fatigue [28]. Stress responses have been observed and measured in field studies that deploy penetrative tags [28]. However, studies on physiological stress responses in GWS being tagged with dorsal fin-mounted SPOT have not yet been conducted. Additionally, stress responses in elasmobranchs have been found to be species specific [29].
Stress responses can also be triggered by pain. As described in Lacap [30], elasmobranchs possess nociceptors in the snout and on the top of the head. Nociceptors are a prerequisite for pain perception. Lacap [30] concluded that a shark’s capacity to feel pain should not be ruled out.
4.5. Hemorrhaging
Elasmobranchs appeared to hemorrhage in response to induced trauma due to handling while on tagging platforms. Crushing of internal organs is also a recognized issue and has been mitigated using supports such as harnesses [14]. Hemorrhagic bands have formed ventrally along the snout, abdomen, and caudal peduncle of GWS (Figure 4). Hemorrhaging has been characterized by red banding on tissues [31].

Figure 4.
SPOT-tagged GWS on a tagging deck. The jaw area, snout, and caudal peduncle are reddened and indicate hemorrhaging (white arrows). Modified from the Ocearch website “https://www.ocearch.org/tracker/ (accessed on 7 May 2024)”.
4.6. Impact on Fin Hydrodynamics
The dorsal fin of GWS is a dynamic stabilizer and penetrative tagging may inhibit its function in swimming. GWS are thunniform swimmers [11,32]. Thunniform swimmers rely on the caudal hydrofoil to maintain thrust when swimming at high speeds [32,33]. The thunniform mode of swimming is primarily driven by caudal fin oscillations [34,35]. A hydrodynamic consequence stemming from this swimming mode is the potential recoil brought about via lateral oscillations of the head. The dorsal fin of GWS acts as a dynamic stabilizer during swimming [11]. The dorsal fin acts as a center of mass point of the shark. Additionally, lamnid sharks lack a ventrally flattened head. A ventrally flattened head reduces roll [11]. To compensate, the lamnid dorsal fin acts as an additional dynamic stabilizer to reduce roll [11,36]. Thus, the location and mass of the dorsal fin act as a dynamic stabilizer while the shark is actively swimming.
Other than the location of the dorsal fin itself, the shark’s ability to transfer tensile strength between the perimysial connective tissues (PCTs) and inner-musculature tissues of the body to those located within the dorsal fin also contributes to the dorsal fin’s role as a dynamic stabilizer [11]. PCTs extend approximately one-quarter of the way into the dorsal fin and are attached to cartilaginous radials via dense cartilaginous tissue sheaths that wrap around the radials [11]. The PCTs also attach to the dermis and dorsal septum [11]. The radials themselves are attached via connective tissue to stiff pterygiophores located at the base of the dorsal fin and extend approximately halfway through the fin [11]. The next layer of tissues, the stratum compactum, is composed of scattered, unstacked bundles of ceratotrichia coupled with groups of fiber bundles [11]. The fiber bundles are stacked to promote tensile strength while not preventing fiber movement [11]. The ceratotrichia are filamentous dermal fibers that extend throughout the length of the dorsal fin and act as structural support [37]. The ceratotrichia are flexible and, when perimysial muscle fibers contract within the body cavity, the contraction places tension on the ceratotrichia [11]. This stiffens the dorsal fin in the direction contralateral to the contracting fibers and increases hydrostatic pressure within the body. The opposite occurs when the fibers are relaxed, and hydrostatic pressure is reduced [38]. Thus, changes in hydrostatic pressure initiate tension or relaxation of the dorsal fin. Lingham-Soliar [11] describe this mechanism as something akin to the rigging of a ship’s mast. Thus, the shark’s dorsal fin morphological function is being impacted by drilling holes in it. The shark’s dorsal fin is an integral part of the body and covered by skin and placoid denticles [12]. It is not like a teleost’s fin, which can be torn and will regrow. However, elasmobranchs have recovered from partial removal of the dorsal fin [39,40]. However, this may impair natural behaviors such as predation [39]. Also, securing a SPOT on the dorsal fin may also affect dorsal fin hydrodynamics due to added drag and an uneven weight distribution across the dorsal fin.
4.7. Alternative Tagging Methods and Future Directions
The great white shark (GWS) is listed as “vulnerable” by the International Union for Conservation of Nature (IUCN) [41]. Concern around using SPOT bolted to the fin stems from how researchers only obtaining a relatively short-term dataset from individual tags that (1) are meant to be semi-permanently affixed to the dorsal fin, which may have harmful effects on tagged individuals, and (2) that such harmful methods are imposed upon a vulnerable species. However, individual tag data can be compiled into a larger dataset [42,43].
Other tagging methods, such as dart and implanted tags, have also been used on elasmobranchs [1]. Dart tags are conventionally deployed using a harpoon-like device to stab the tag into the upper epaxial musculature while the shark is in the water or on a boat [44]. Dart tags eventually accumulate biofouling organisms that add drag to the tag. Acoustic tags have been surgically implanted into the abdominal cavity [1]. Surgical implantation has taken place both with the shark in the water and on the deck of a boat [1]. In addition to the invasiveness of the surgical procedure, changes in natural behavior have occurred post-implantation. For example, tagged sharks have been observed leaving their aggregation areas after the procedure [Lobel pers. obs]. Both of these tags have associated stress due to handling, as well as direct physical trauma with resulting wounds. Dart tags have been used with satellite transmitters but obviously these do not work with a surgically implanted tag.
There are two examples of alternative methods that either reduce or eliminate adverse effects of bolting tags: bracelet tagging and fin clamping. A bracelet tag is a non-penetrative method that is temporary [45,46]. It has been used with acoustic tags that are attached to a degradable bracelet that encircles the caudal peduncle. These are attached to a shark that is in water and has been rendered into tonic immobility as the shark is brought alongside a boat [45,46]. Bracelet tags have not yet been paired with SPOT, but alterations are possible that could allow an antenna to transmit, such as taping the satellite antenna to the upper caudal fin. Fin clamps are also non-penetrative and temporary [6,47]. Tags are slid onto a clamp attached to the end of a tagging pole and are secured to the dorsal fin after the trigger on the pole is activated [6,47]. The tag is applied to free-swimming sharks at the surface [6,47]. Detachment time can be pre-programmed [6,47]. Fin clamps have caused necrosis in the dorsal fin 5 weeks post-application [47]. This is one reason fin clamps are currently deployed for short time intervals. Fin clamp design needs to be further innovated to eventually be attached long term without harm.
5. Conclusions
Due to the structural damage to the dorsal fin, as well as possible health consequences and data concerns, it is recommended that alternatives to penetrative tagging methods be developed. No other species, especially “vulnerable” ones, are subject to such invasive tagging methods. Non-penetrative methods, such as bracelet tags and fin clamps, should be further engineered to improve elasmobranch welfare and obtain data that are reflective of natural behavior and not a shark’s response to invasive wounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9060231/s1.
Author Contributions
Conceptualization, P.S.L. and G.L.H.; methodology, G.L.H. and P.S.L.; writing—original draft preparation, G.L.H.; writing—review and editing, P.S.L. and G.L.H. All authors have read and agreed to the published version of the manuscript.
Funding
The Boston University Marine Program provided funding for G.L.H. to present this research at the 2024 annual meeting of the Joint Meeting of Ichthyologists and Herpetologists.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are included in this article/Supplementary Materials.
Acknowledgments
We thank Emily Turner for discussions about shark fin anatomy, which was the topic of her Masters thesis. We thank the anonymous reviewers for their constructive and helpful comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kohler, N.E.; Turner, P.A. Shark tagging: A review of conventional methods and studies. Environ. Biol. Fishes 2001, 60, 191–223. [Google Scholar] [CrossRef]
- Moyes, C.D.; Fragoso, N.; Musyl, M.K.; Brill, R.W. Predicting postrelease survival in large pelagic fish. Trans. Am. Fish. Soc. 2006, 135, 1389–1397. [Google Scholar] [CrossRef]
- Wilson, S.G.; Stewart, B.S.; Polovina, J.J.; Meekan, M.G.; Stevens, J.D.; Galuardi, B. Accuracy and precision of archival tag data: A multiple-tagging study conducted on a whale shark (Rhincodon typus) in the Indian Ocean. Fish. Oceanogr. 2007, 16, 547–554. [Google Scholar] [CrossRef]
- Lobel, P.S. Diver Eco-Tourism and the Behavior of Reef Sharks and Rays—An Overview. In Diving for Science 2008, Proceedings of the American Academy of Underwater Sciences 27th Scientific Symposium, La Jolla, CA, USA, 14–15 March 2008; Brueggeman, P., Pollock, N.W., Eds.; American Academy of Underwater Sciences: Dauphin Island, AL, USA, 2008; pp. 103–113. [Google Scholar]
- Campana, S.; Joyce, W.; Manning, M. Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags. Mar. Ecol. Progress. Ser. 2009, 387, 241–253. [Google Scholar] [CrossRef]
- Gleiss, A.C.; Norman, B.; Liebsch, N.; Francis, C.; Wilson, R.P. A new prospect for tagging large free-swimming sharks with motion-sensitive data-loggers. Fish. Res. 2009, 97, 11–16. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Gallagher, A.J.; Lazarre, D.M. A review of Shark Satellite Tagging Studies. J. Exp. Mar. Biol. Ecol. 2011, 398, 1–8. [Google Scholar] [CrossRef]
- Jewell, O.J.; Wcisel, M.A.; Gennari, E.; Towner, A.V.; Bester, M.N.; Johnson, R.L.; Singh, S. Effects of smart position only (spot) tag deployment on White Sharks Carcharodon carcharias in South Africa. PLoS ONE 2011, 6, e27242. [Google Scholar] [CrossRef]
- Turner, E.N. Observations on the Use of Bolting in Shark Tagging. Master’s Thesis, Boston University, Boston, MA, USA, 2023. [Google Scholar]
- Ziegler, M.; Quinlan, M.; Strassberg-Phillips, Z.; Shah, M.; Vreeken, L.; Jones, C.; Goodfellow, K.; Lefcout, J.; Anderson, R.; Heimerl, K. “How’s shelby the turtle today?” strengths and weaknesses of interactive animal-tracking maps for environmental communication. In Proceedings of the COMPASS ‘21: ACM SIGCAS Conference on Computing and Sustainable Societies, Virtual Event Australia, 28 June–2 July 2021; pp. 346–349. [Google Scholar]
- Lingham-Soliar, T. Dorsal fin in the white shark, Carcharodon carcharias: A dynamic stabilizer for fast swimming. J. Morphol. 2005, 263, 1–11. [Google Scholar] [CrossRef]
- Klimley, A.P.; Oerding, S. The Biology of Sharks and Rays; University of Chicago Press: Chicago, IL, USA, 2014. [Google Scholar]
- Christie Douglas, E. The Literature of Nature and the Quest for the Sacred. Way 1994, 81, 4–14. [Google Scholar]
- Butcher, P.A.; Lee, K.A.; Brand, C.P.; Gallen, C.R.; Green, M.; Smoothey, A.F.; Peddemors, V.M. Capture Response and Long-Term Fate of White Sharks (Carcharodon carcharias) after Release from SMART Drumlines. Biology 2023, 12, 1329. [Google Scholar] [CrossRef]
- Stevens, J.D.; Bradford, R.W.; West, G.J. Satellite tagging of blue sharks (Prionace glauca) and other pelagic sharks off eastern Australia: Depth behaviour, temperature experience and movements. Mar. Biol. 2010, 157, 575–591. [Google Scholar] [CrossRef]
- Heim, V.; Lüscher, D.; Hottinger, J.; Ebert, D. Development and validation of a drill attachment for faster and safer deployments of fin-mounted geolocators in large-bodied sharks. Anim. Biotelemetry 2022, 10, 33. [Google Scholar] [CrossRef]
- Drymon, J.M.; Wells, R.D. Double tagging clarifies post-release fate of great hammerheads (Sphyrna mokarran). Anim. Biotelemetry 2017, 5, 28. [Google Scholar] [CrossRef]
- Weng, K.C.; Castilho, P.C.; Morrissette, J.M.; Landeira-Fernandez, A.M.; Holts, D.B.; Schallert, R.J.; Goldman, K.J.; Block, B.A. Satellite tagging and cardiac physiology reveal niche expansion in Salmon Sharks. Science 2005, 310, 104–106. [Google Scholar] [CrossRef]
- Bonfil, R.; Meÿer, M.; Scholl, M.C.; Johnson, R.; O’Brien, S.; Oosthuizen, H.; Swanson, S.; Kotze, D.; Paterson, M. Transoceanic migration, spatial dynamics, and population linkages of White Sharks. Science 2005, 310, 100–103. [Google Scholar] [CrossRef]
- Hays, G.C.; Bradshaw, C.J.A.; James, M.C.; Lovell, P.; Sims, D.W. Why do Argos satellite tags deployed on marine animals stop transmitting? J. Exp. Mar. Biol. Ecol. 2007, 349, 52–60. [Google Scholar] [CrossRef]
- Samuel, H. Biological materials for the study of age and growth in a tropical marine elasmobranch, the lemon shark, Negaprion brevirostris. In Workshop on Age Determination of Oceanic Pelagic Fishes: Tunas, Billfishes, and Sharks; National Oceanic and Atmospheric Administration: Miami, FL, USA, 1983. [Google Scholar]
- Natanson, L.J.; Casey, J.G.; Kohler, N.E.; Colket, T. Growth of the tiger shark, Galeocerdo cuvier, in the western North Atlantic based on tag returns and length frequencies; and a note on the effects of tagging. Fish. Bull. 1999, 97, 944–953. [Google Scholar]
- Heupel, M.R.; Bennett, M.B. Histology of dart tag insertion sites in the epaulette shark. J. Fish Biol. 1997, 50, 1034–1041. [Google Scholar] [CrossRef]
- Manire, C.A.; Gruber, S.H. Effect of M-type dart tags on field growth of juvenile lemon sharks. Trans. Am. Fish. Soc. 1991, 120, 776–780. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Balmer, B.C.; Schwacke, L.H.; Wells, R.S. Linking Dive Behavior to Satellite-Linked Tag Condition for a Bottlenose Dolphin (Tursiops truncatus) along Florida’s Northern Gulf of Mexico Coast. Aquat. Mamm. 2010, 36, 1–8. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. General. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Skomal, G.; Lobel, P.S.; Marshall, G. The use of animal-borne imaging to assess post-release behavior as it relates to capture stress in Grey Reef Sharks, Carcharhinus amblyrhynchos. Mar. Technol. Soc. J. 2007, 41, 44–48. [Google Scholar] [CrossRef]
- Manire, C.; Hueter, R.; Hull, E.; Spieler, R. Serological changes associated with gill-net capture and restraint in three species of sharks. Trans. Am. Fish. Soc. 2001, 130, 1038–1048. [Google Scholar] [CrossRef]
- Lacap, R.M. A New Approach to Determine the Presence of Nociception in Elasmobranchs. Master’s Thesis, California State University Northridge, Los Angeles, CA, USA, 2022. [Google Scholar]
- Poynton, S.L.; Campbell, T.W.; Palm, H.W. Skin lesions in captive lemon sharks Negaprion brevirostris (Carcharhinidae) associated with the monogenean Neodermophthirius harkemai (Microbothriidae). Dis. Aquat. Org. 1997, 31, 29–33. [Google Scholar] [CrossRef]
- Lighthill, M.J. Hydromechanics of Aquatic Animal Propulsion. Annu. Rev. Fluid. Mech. 1969, 1, 413–446. [Google Scholar] [CrossRef]
- Fish, F.E. Comparative kinematics and hydrodynamics of odontocete cetaceans: Morphological and ecological correlates with swimming performance. J. Exp. Biol. 1998, 201, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Donley, J.M.; Sepulveda, C.A.; Konstantinidis, P.; Gemballa, S.; Shadwick, R.E. Convergent evolution in mechanical design of Lamnid Sharks and Tunas. Nature 2004, 429, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Gemballa, S.; Konstantinidis, P.; Donley, J.M.; Sepulveda, C.; Shadwick, R.E. Evolution of high-performance swimming in sharks: Transformations of the musculotendinous system from subcarangiform to thunniform swimmers. J. Morphol. 2006, 267, 477–493. [Google Scholar] [CrossRef]
- Thomson, K.S.; Simanek, D.E. Body form and locomotion in Sharks. Am. Zool. 1977, 17, 343–354. [Google Scholar] [CrossRef]
- Goodrich, E.S. On the dermal fin-rays of fishes–living and extinct. J. Cell Sci. 1904, S2-47, 465–522. [Google Scholar] [CrossRef]
- Wainwright, S.A.; Vosburgh, F.; Hebrank, J.H. Shark skin: Function in Locomotion. Science 1978, 202, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Mumby, P.J. Survival of a grey reef shark Carcharhinus amblyrhynchos without a dorsal fin. J. Fish. Biol. 2019, 94, 820–822. [Google Scholar] [CrossRef]
- Towner, A.; Smale, M.J.; Jewell, O. Boat strike wound healing in Carcharodon carcharias. Glob. Perspect. Biol. Life Hist. White Shark 2012, 3, 77–84. [Google Scholar]
- Rigby, C.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Jabado, R.W.; Liu, K.M.; Marshall, A.; Pacoureau, N. White shark (Carcharodon carcharias). IUCN Red List. Available online: https://www.iucnredlist.org/species/3855/212629880 (accessed on 18 April 2024).
- Kock, A.A.; Lombard, A.T.; Daly, R.; Goodall, V.; Meÿer, M.; Johnson, R.; Fischer, C.; Koen, P.; Irion, D.; Gennari, E.; et al. Sex and size influence the spatiotemporal distribution of white sharks, with implications for interactions with fisheries and spatial management in the southwest Indian Ocean. Front. Mar. Sci. 2022, 9, 443. [Google Scholar] [CrossRef]
- Queiroz, N.; Humphries, N.E.; Couto, A.; Vedor, M.; da Costa, I.; Sequeira, A.M.; Mucientes, G.; Santos, A.M.; Abascal, F.J.; Abercrombie, D.L. Global spatial risk assessment of sharks under the footprint of fisheries. Nature 2019, 572, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kohler, N.E.; Turner, P.A. Distributions and Movements of Atlantic Shark Species: A 52-Year Retrospective Atlas of Mark and Recapture Data. Mar. Fish. Rev. 2018, 81, 1–93. [Google Scholar] [CrossRef]
- Lobel, P.S. Tagging Sharks with Acoustic Bracelets: A Less Invasive Method. In Proceedings of the 2007 Animal-Borne Imaging Symposium, Washington, DC, USA, 10–13 October 2007; National Geographic Society: Washington, DC, USA, 2007; pp. 127–131. [Google Scholar]
- Lobel, P.S. Tracking Grey Reef Sharks Using a Novel Method for Acoustic Tag Attachment and the “Crittercam”. In Diving for Science 2012, Proceedings of the 31th American Academy of Underwater Sciences Symposium, Monterey, CA, USA, 24–29 September 2012; American Academy of Underwater Sciences: Dauphin Island, AL, USA, 2012; pp. 135–143. [Google Scholar]
- Chapple, T.K.; Gleiss, A.C.; Jewell, O.J.; Wikelski, M.; Block, B.A. Tracking Sharks without teeth: A non-invasive rigid tag attachment for large predatory sharks. Anim. Biotelemetry 2015, 3, 14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).