Light Intensity of Phosphorescent-Netting Pots and Determining Their Visibility to Snow Crab (Chionoecetes opilio) Using Visual Modeling Techniques †
Abstract
1. Introduction
2. Materials and Methods
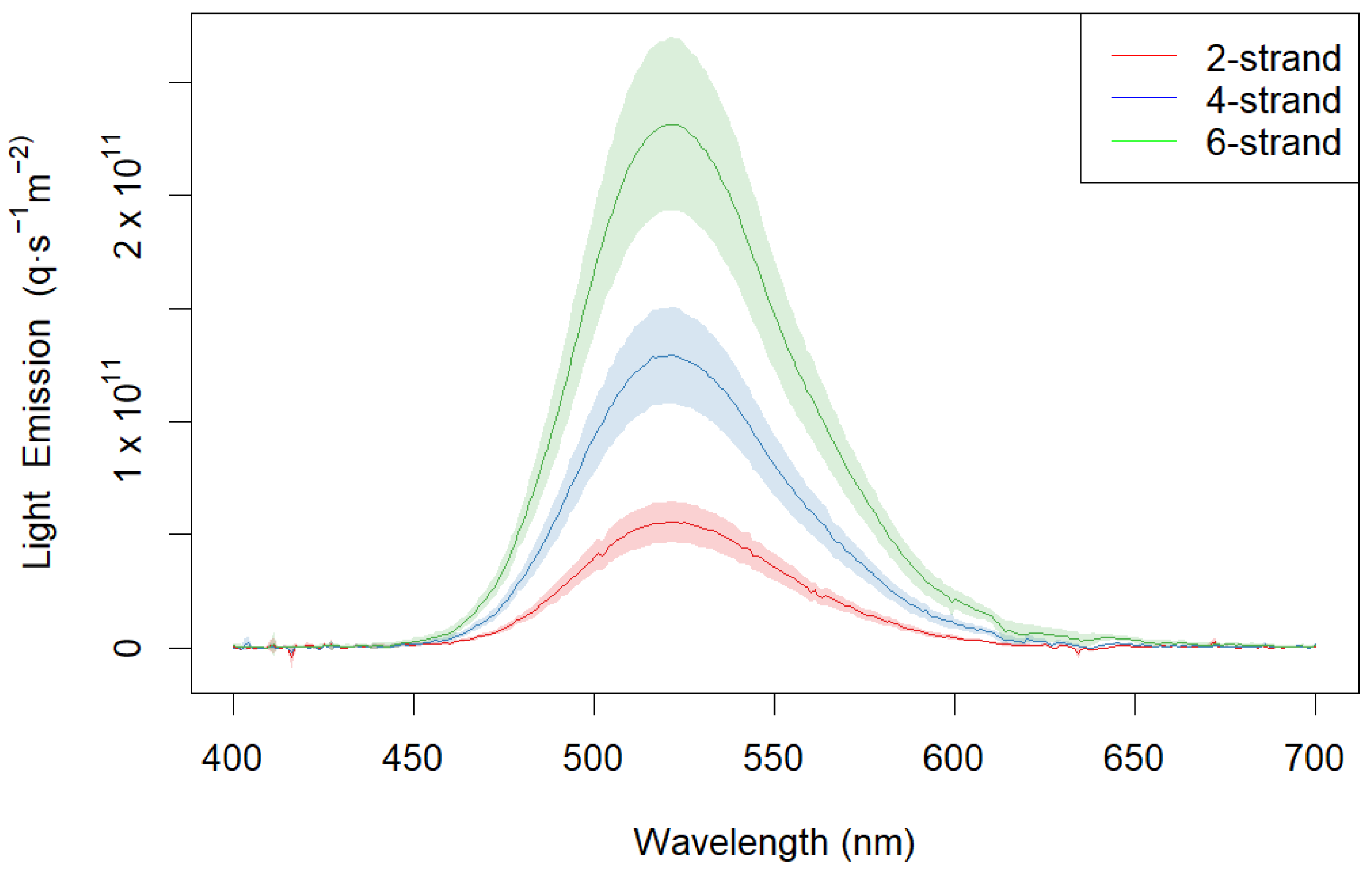
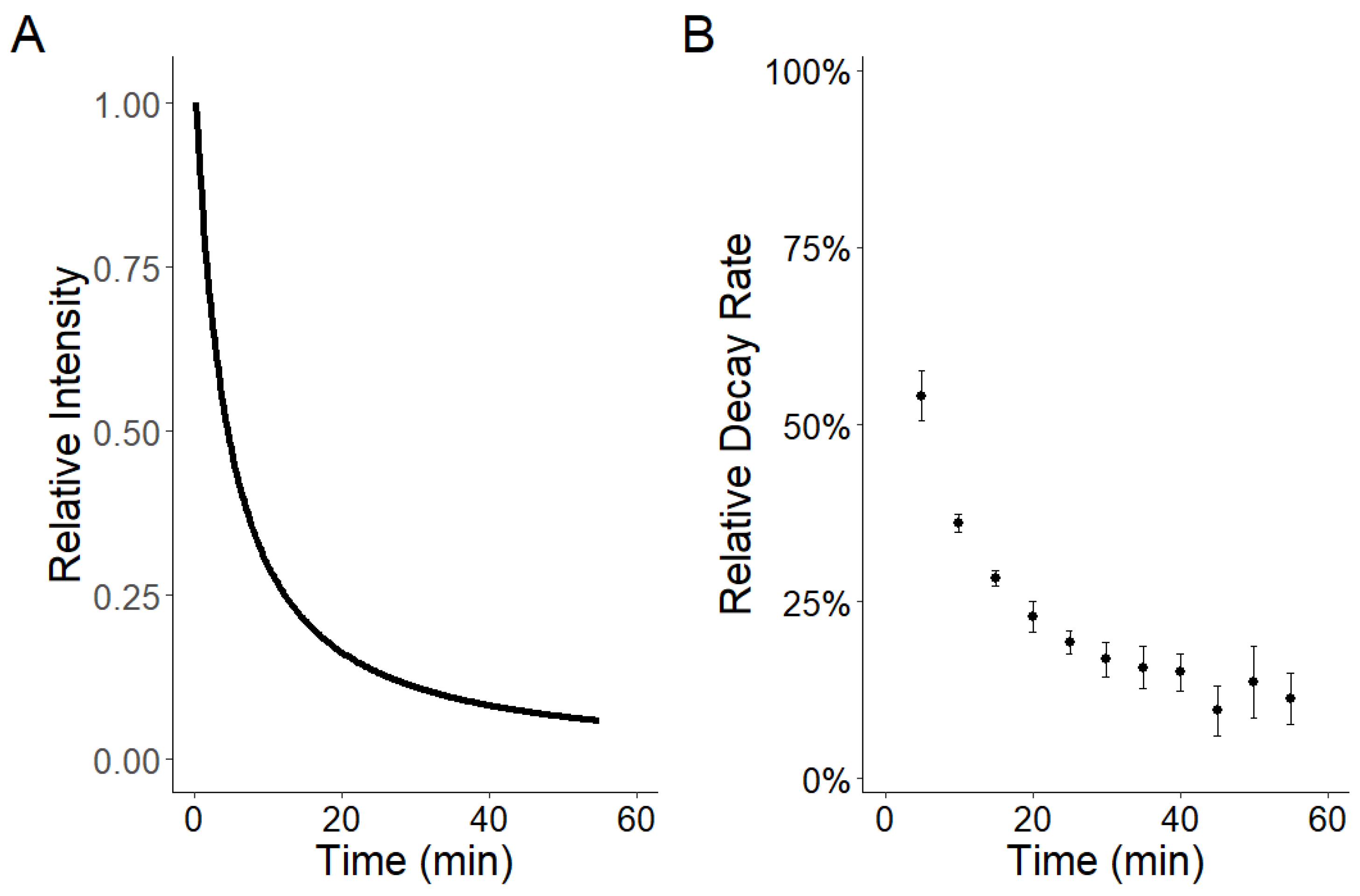
2.1. Light Characteristics of Phosphorescent Twine
2.2. Snow Crab Visual Characteristics
2.2.1. Visual Acuity
2.2.2. Spectral Sensitivity
2.2.3. Contrast Sensitivity (Michelson Contrast)
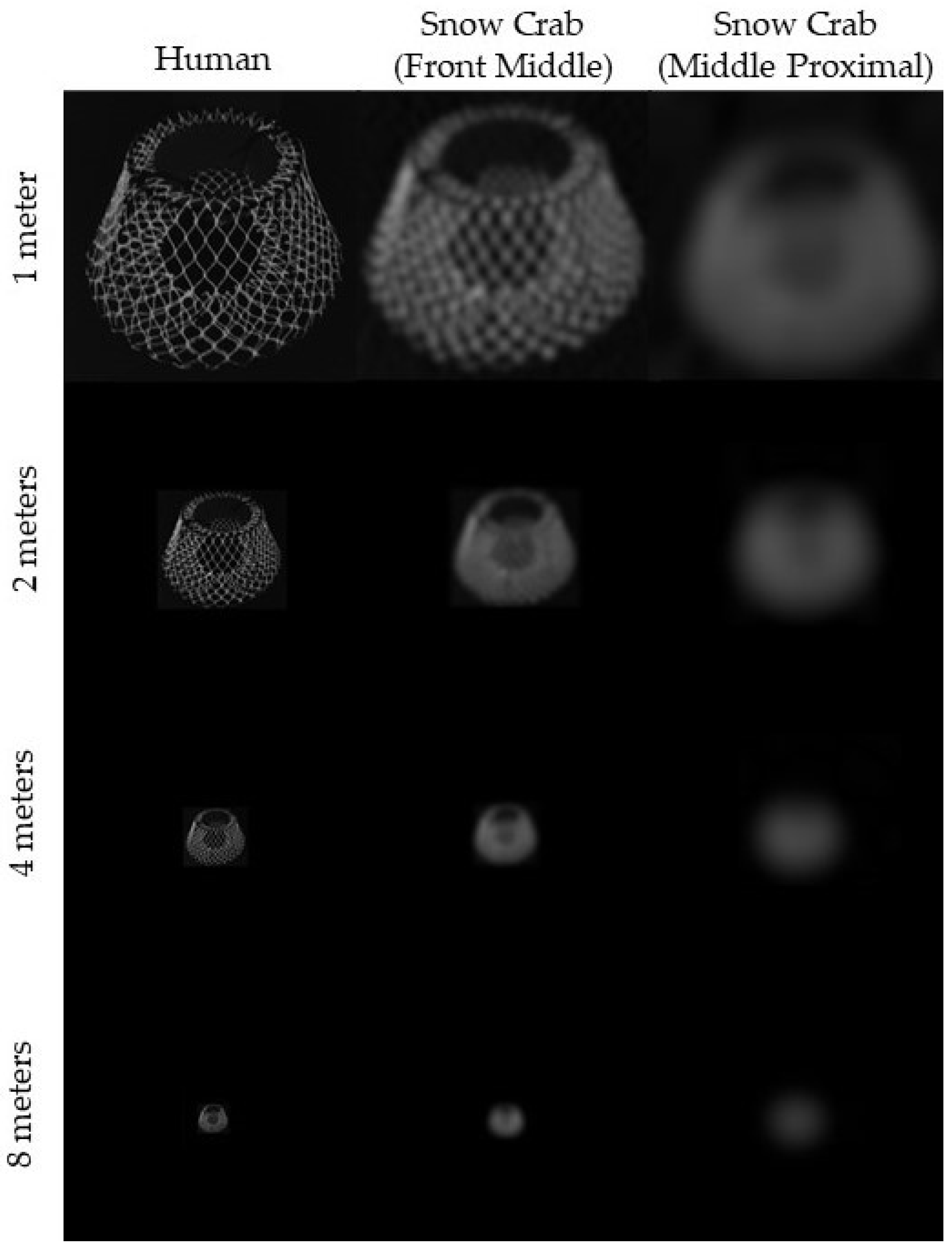
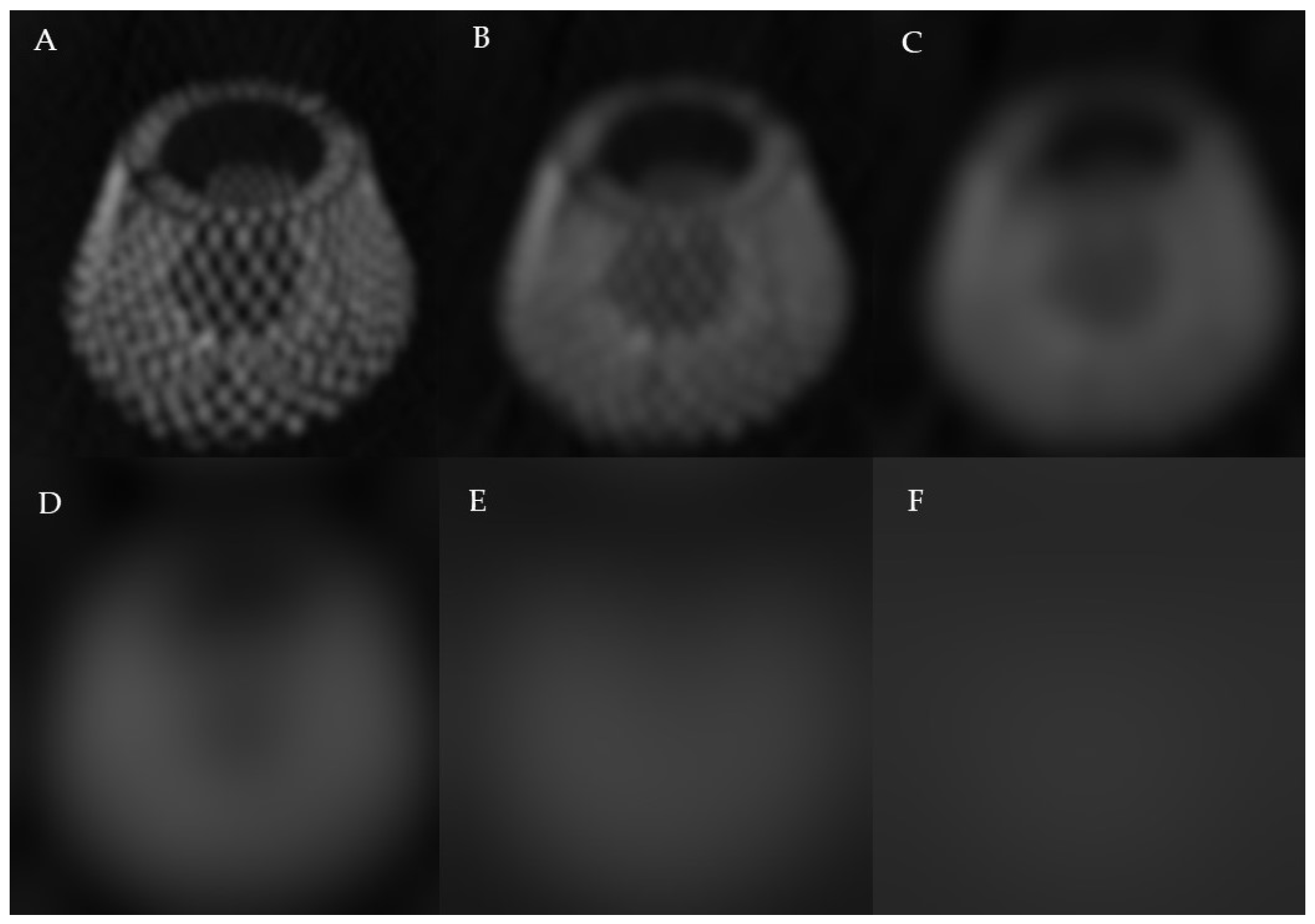
2.3. Pot Viewed at Depth by Snow Crab
2.3.1. Light from the Pot Area
2.3.2. Solid Angle
2.4. Environmental Conditions Affecting Viewing
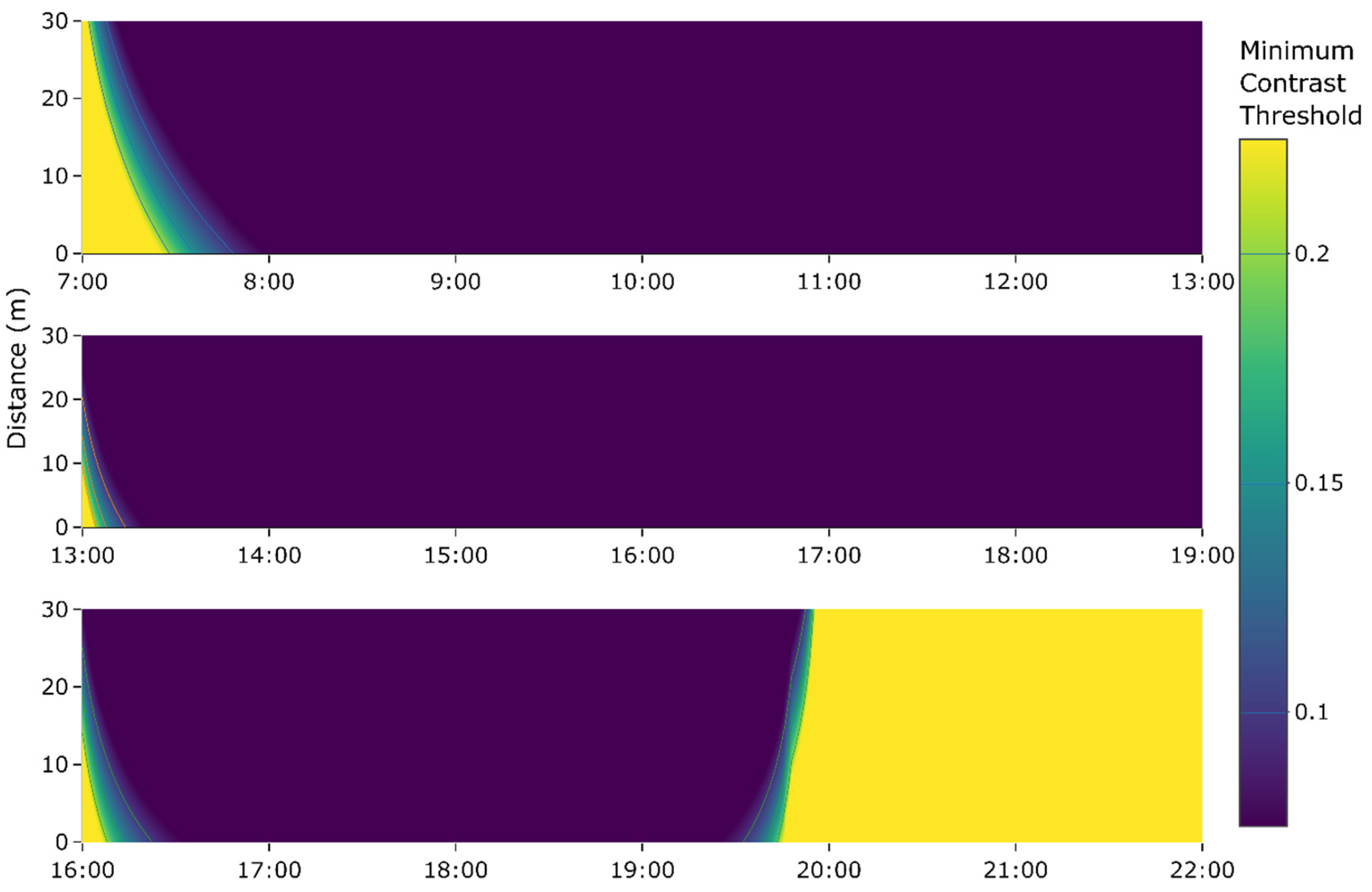
2.4.1. Ambient Light Intensity and the Spectral Curve
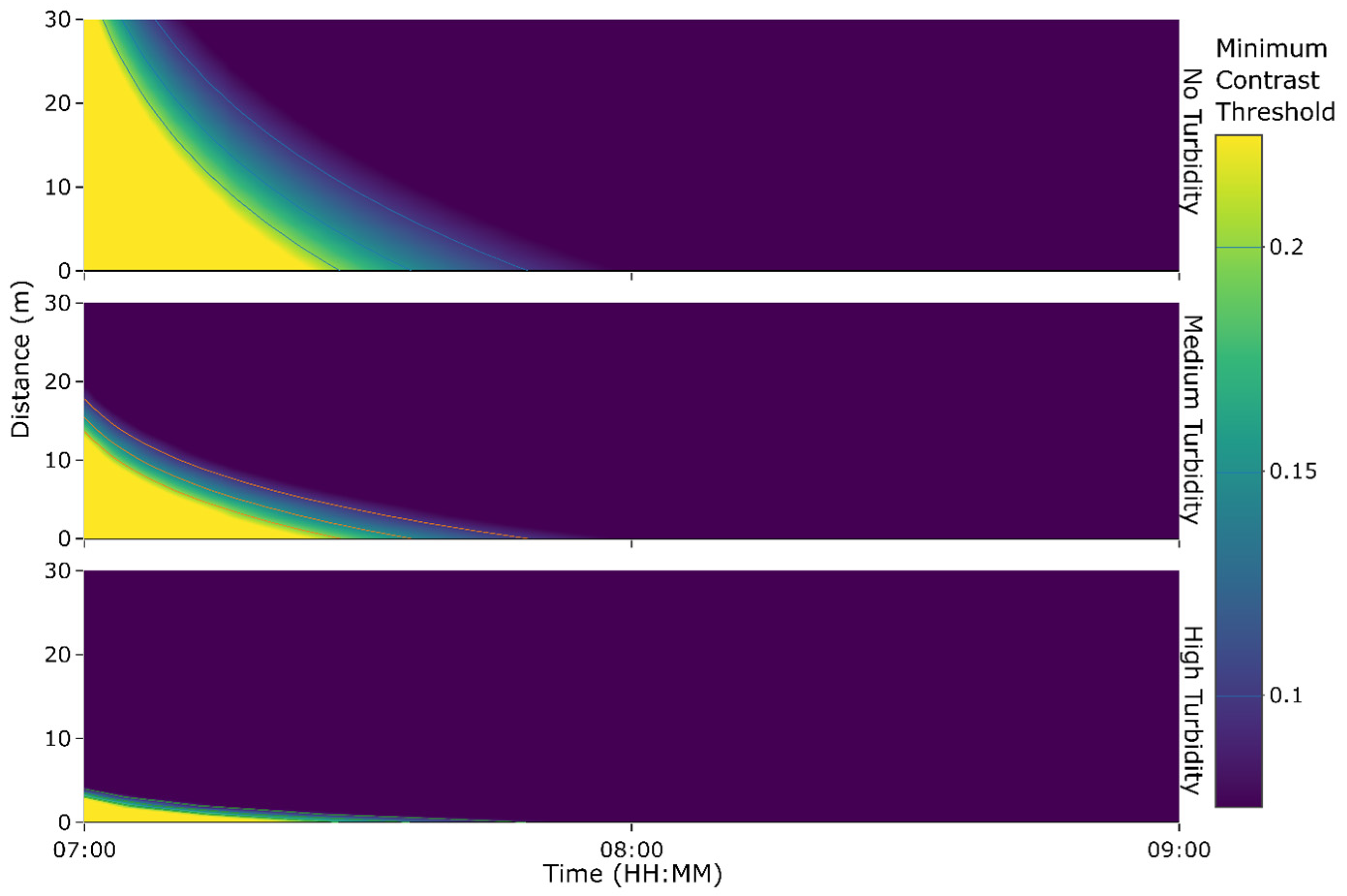
2.4.2. Turbidity in the Benthic Boundary Layer
2.5. Photon Flux and Michelson Contrast
3. Results
3.1. Phosphorescent Twine Characteristics
3.2. Snow Crab Visual Acuity Measurements
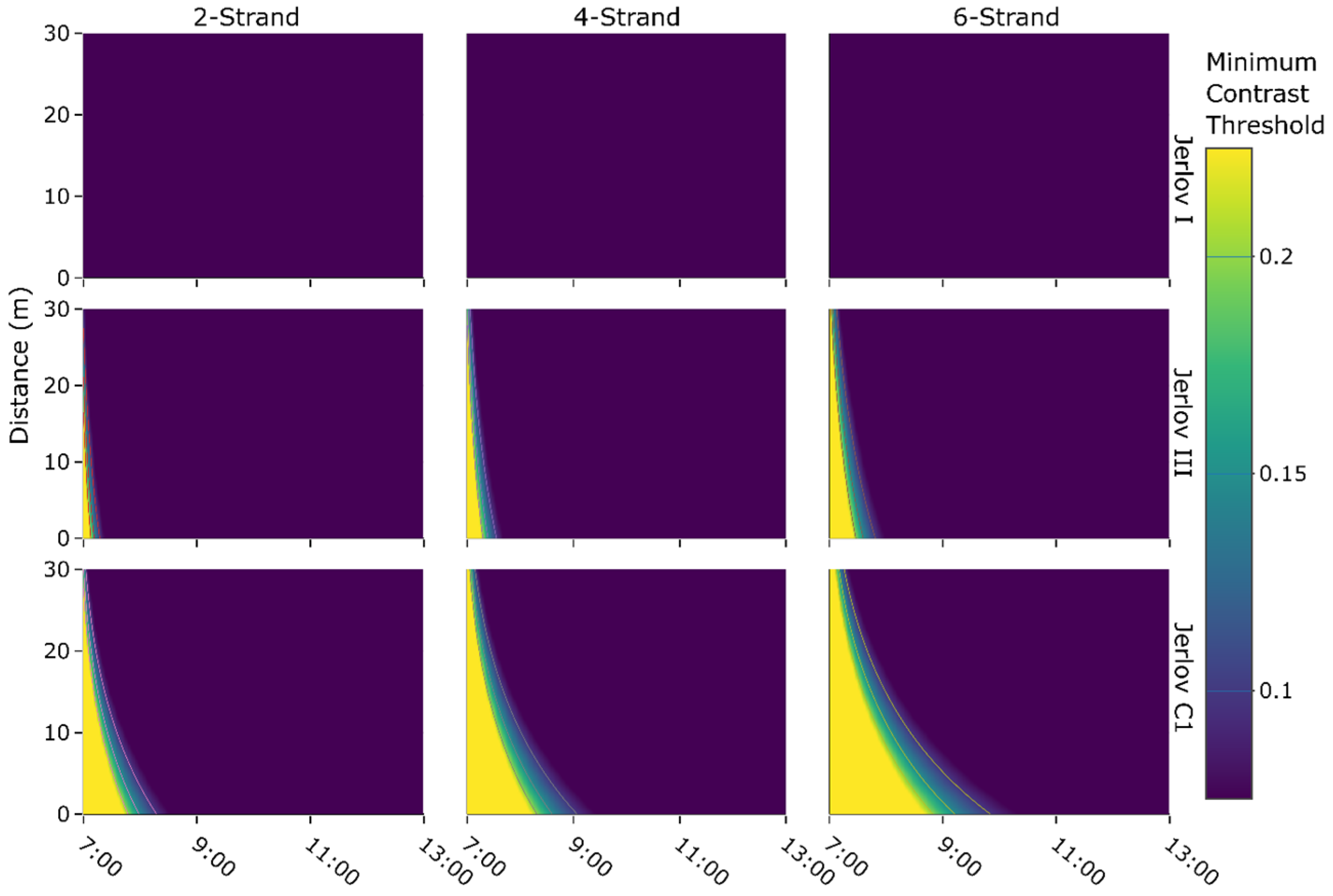
3.3. Contrast of Phosphorescent Light in Different Water Types
4. Discussion
4.1. Main Factors Influencing the Visibility of Light from Phosphorescent-Netting
4.2. Strontium Aluminate Properties
4.3. Visibility of Phosphorescent Netting in Different Conditions
4.4. Snow Crab Visual Characteristics and Their Environment
4.5. Reduced Light Sensitivity in Damaged Eyes
4.6. Knowledge Gaps
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- 1.
- SACALC 3.14 software settings.
- 2.
- SMARTS v2.9.5 code settings
References
- FFA. Seafood Industry Year in Review 2022. Newfoundland and Labrador Canada. Fisheries, Forestry, and Agriculture. 2023. Available online: https://www.gov.nl.ca/ffa/files/Seafood-Industry-Year-in-Review-2022.pdf (accessed on 10 May 2024).
- Mullowney, D.R.J.; Baker, K.D.; Pantin, J.R. Hard to manage? Dynamics of soft-shell crab in the Newfoundland and Labrador snow crab fishery. Front. Mar. Sci. 2021, 8, 591496. [Google Scholar] [CrossRef]
- DFO. Assessment of Newfoundland and Labrador (Divisions 2HJ3KLKNOP4R) Snow Crab. Canadian Science Advisory Secretariat, Science Advisory Report 2022/012. 2022. Available online: https://publications.gc.ca/collections/collection_2022/mpo-dfo/fs70-6/Fs70-6-2022-012-eng.pdf (accessed on 10 May 2024).
- Nguyen, K.Q.; Winger, P.D. Artificial light in commercial industrialized fishing applications: A review. Rev. Fish. Sci. Aquac. 2019, 27, 106–126. [Google Scholar] [CrossRef]
- Mullowney, D.R.J.; Baker, K.D.; Pedersen, E.J. Harvesting strategies during a forecasted decline in the Newfoundland and Labrador snow crab fishery. Fish. Res. 2020, 232, 105707. [Google Scholar] [CrossRef]
- Olsen, L.; Herrmann, B.; Sistiaga, M.; Grimaldo, E. Effect of gear soak time on size selection in the snow crab pot fishery. Fish. Res. 2019, 214, 157–165. [Google Scholar] [CrossRef]
- Nguyen, K.Q.; Winger, P.D.; Morris, C.; Grant, S.M. Artificial lights improve the catchability of snow crab (Chionoecetes opilio) traps. Aquac. Fish. 2017, 2, 124–133. [Google Scholar] [CrossRef]
- Olsen, L.; Herrmann, B.; Grimaldo, E.; Sistiaga, M. Effect of pot design on the catch efficiency of snow crabs (Chionoecetes opilio) in the Barents Sea fishery. PLoS ONE 2019, 14, e0219858. [Google Scholar] [CrossRef]
- Cerbule, K.; Herrmann, B.; Grimaldo, E.; Sistiaga, M.; Brinkhof, J.; Vollstad, J. Understanding and predicting the effect of entrance cone diameters on the catch efficiency of snow crabs (Chionoecetes opilio) in conical pots. Reg. Stud. Mar. Sci. 2022, 52, 102237. [Google Scholar] [CrossRef]
- Winger, P.D.; Walsh, P.J. Selectivity, efficiency, and underwater observations of modified trap designs for the snow crab (Chionoecetes opilio) fishery in Newfoundland and Labrador. Fish. Res. 2011, 109, 107–113. [Google Scholar] [CrossRef]
- Anders, N.; Ingólfsson, Ó.A.; Jørgensen, T.; Løkkeborg, S.; Humborstad, O.-B. Investigating the potential of escape openings and reduced mesh size to optimize snow crab (Chionoecetes opilio) pot catches in the Barents Sea. Fish. Res. 2023, 258, 106517. [Google Scholar] [CrossRef]
- Weissburg, M.J.; Zimmer-Faust, R.K. Odor plumes and how blue crabs use them in finding prey. J. Exp. Biol. 1994, 197, 349–375. [Google Scholar] [CrossRef]
- Zimmer-Faust, R.K.; Finelli, C.M.; Pentcheff, N.D.; Wethey, D.S. Odor Plumes and Animal Navigation in Turbulent Water Flow: A Field Study. Bio Bull. 1995, 188, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.Q.; Winger, P.D.; Wood, J.; Donovan, M.; Humborstad, O.-B.; Lokkeborg, S.; Bayse, S.M. Application of luminescent netting in traps to improve the catchability of the snow crab Chionoecetes opilio. Mar. Coast. Fish. Dyn. Manag. Ecosyst. Sci. 2019, 11, 295–304. [Google Scholar] [CrossRef]
- Anesh, M.P.; Gulrez, S.K.H.; Anis, A.; Shaikh, H.; Ali Mohsin, M.E.; AL-Zahrani, S.M. Developments in Eu+2-doped strontium aluminate and polymer/strontium aluminate composite. Adv. Polym. Technol. 2014, 33, 21436. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4: Eu2 +, Dy3 +. J. Electrochem. Soc. 1996, 143, 2670. [Google Scholar] [CrossRef]
- Fouzar, S.; Eftimov, T.; Kostova, I.; Benmounah, A.; Lakhssassi, A. Effects of temperature on the time responses of strontium aluminates. Opt. Mater. 2021, 122, 111619. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Rubio-Marcos, F.; Rodriguez, M.A.; Fernandez, J.F. Long lasting phosphors: SrAl2O4:Eu, Dy as the most studied material. Renew. Sustain. Energy Rev. 2018, 81, 2759–2770. [Google Scholar] [CrossRef]
- Aas, E.; Højerslev, N.K.; Høkedal, J.; Sørensen, K. Optical water types of the Nordic Seas and adjacent areas. Oceanologia 2013, 55, 471–482. [Google Scholar] [CrossRef]
- Cronin, T.W.; Johnsen, S.; Marshall, N.J.; Warrant, E.J. Visual Ecology; Princeton University Press: Princeton, NJ, USA, 2014; Available online: http://www.jstor.org/stable/j.ctt6wq1c9 (accessed on 10 May 2024).
- Jerlov, N.G. Irradiance optical classification. In Optical Oceanography; American Elsevier Publishing Co.: New York, NY, USA, 1968; pp. 118–120. [Google Scholar] [CrossRef]
- Caves, E.M.; Frank, T.M.; Johnsen, S. Spectral sensitivity, spatial resolution and temporal resolution and their implications for conspecific signalling in cleaner shrimp. J. Exp. Biol. 2016, 219, 597–608. [Google Scholar] [CrossRef]
- Cronin, T.W.; Forward, R.B. The visual pigments of crabs. J. Comp. Physiol. A 1988, 162, 463–478. [Google Scholar] [CrossRef]
- Meyers, T.R.; Morris, R.; Jackson, T.M.; Dissen, J.N.; Slater, L.M.; Groner, M.L. Black eye syndrome and a systemic rickettsia-like organism in Alaskan Chionoecetes spp. crabs, including normal eyestalk microanatomy. Dis. Aquat. Org. 2022, 150, 103–124. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 May 2024).
- Maia, R.; Gruson, H.; Endler, J.A.; White, T.E. pavo 2: New tools for the spectral and spatial analysis of colour in R. Methods Ecol. Evol. 2019, 10, 1097–1107. [Google Scholar] [CrossRef]
- Johnsen, S. The Optics of Life: A Biologist’s Guide to Light in Nature; Princeton University Press: Princeton, NJ, USA, 2012; Available online: http://www.jstor.org/stable/j.ctt7s4q4 (accessed on 10 May 2024).
- Whitcher, R. SACALC3; OECD. Nuclear Energy Agency Data Bank: Issy-Les Moulineaux, France, 2012. [Google Scholar]
- Eftimov, T.; Kostova, I.; Arapova, A.; Patronov, G. Rise and decay time responses of Sr aluminate phosphorescent materials. J. Lumin. 2021, 235, 117985. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Baldwin-Fergus, J.L.; Johnsen, S.; Osborn, K.J. A unique apposition compound eye in the mesopelagic hyperiid amphipod Paraphronima gracilis. Curr. Biol. 2015, 25, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Land, M.F.; Nilsson, D.-E. Animal Eyes, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar] [CrossRef]
- Feller, K.D.; Sharkey, C.R.; McDuffee-Altekruse, A.; Bracken-Grissom, H.D.; Lord, N.P.; Porter, M.L.; Schweikert, L.E. Surf and turf vision: Patterns and predictors of visual acuity in compound eye evolution. Arthropod Struct. Dev. 2021, 60, 101002. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, D.-E.; Ro, A.-I. Did neural pooling for night vision lead to the evolution of neural superposition eyes? J. Comp. Physiol. A 1994, 175, 289–302. [Google Scholar] [CrossRef]
- Caves, E.M.; Johnsen, S. AcuityView: An r package for portraying the effects of visual acuity on scenes observed by an animal. Methods Ecol. Evol. 2018, 9, 793–797. [Google Scholar] [CrossRef]
- Marshall, J.; Kent, J.; Cronin, T. Visual adaptations in crustaceans: Spectral sensitivity in diverse habitats. In Adaptive Mechanisms in the Ecology of Vision; Archer, S.N., Djamgoz, M.B.A., Loew, E.R., Partridge, J.C., Vallerga, S., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 285–327. [Google Scholar] [CrossRef]
- Marshall, J.N.; Cronin, T.W.; Frank, T.M. Visual adaptations in crustaceans: Chromatic, developmental, and temporal aspects. In Sensory Processing in Aquatic Environments; Collin, S.P., Marshall, N.J., Eds.; Springer: New York, NY, USA, 2003; pp. 343–372. [Google Scholar] [CrossRef]
- Dawis, S.M. Polynomial expressions of pigment nomograms. Vis. Res. 1981, 21, 1427–1430. [Google Scholar] [CrossRef]
- Dartnall, H.J.A. The interpretation of spectral sensitivity curves. Br. Med. Bull. 1953, 9, 24–30. [Google Scholar] [CrossRef]
- Drerup, C.; How, M.J. Polarization contrasts and their effect on the gaze stabilization of crustaceans. J. Exp. Biol. 2021, 224, jeb229898. [Google Scholar] [CrossRef]
- Douglas, R.H.; Hawryshyn, C.W. Behavioural studies of fish vision: An analysis of visual capabilities. In The Visual System of Fish; Douglas, R.H., Djamgoz, M.B.A., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 373–418. [Google Scholar]
- Solonenko, M.G.; Mobley, C.D. Inherent optical properties of Jerlov water types. Appl. Opt. 2015, 54, 5392–5401. [Google Scholar] [CrossRef] [PubMed]
- Partridge, J.C. The colour sensitivity and vision of fishes. In Light and Life in the Sea; Herring, P.J., Campbell, A.K., Whitfield, M., Maddock, L., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 167–184. [Google Scholar]
- Santon, M.; Bitton, P.-P.; Dehm, J.; Fritsch, R.; Harant, U.K.; Anthes, N.; Michiels, N.K. Redirection of ambient light improves predator detection in a diurnal fish. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192292. [Google Scholar] [CrossRef] [PubMed]
- Pepin, P.; Maillet, G.; Fraser, S.; Doyle, G.; Robar, A.; Shears, T.; Redmond, G. Optical, Chemical, and Biological Oceanographic Conditions on the Newfoundland and Labrador Shelf during 2014–2015. Fisheries and Oceans Canada, Science Branch. Research Document: 2017/009. 2017. Available online: https://waves-vagues.dfo-mpo.gc.ca/library-bibliotheque/40601845.pdf (accessed on 10 May 2024).
- Nilsson, D.-E.; Warrant, E.; Johnsen, S. Computational visual ecology in the pelagic realm. Philos. Trans. R. Soc. B 2014, 369, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.; Widder, E.A.; Mobley, C.D. Propagation and perception of bioluminescence: Factors affecting counterillumination as a cryptic strategy. Bio. Bull. 2004, 207, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gueymard, C.A. The SMARTS spectral irradiance model after 25 years: New developments and validation of reference spectra. Sol. Energy 2019, 187, 233–253. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, T. The light field in the ocean: Its modification and exploitation by the pelagic biota. In Light and Life in the Sea; Herring, P.J., Campbell, A.K., Whitfield, M., Maddock, L., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 3–18. [Google Scholar]
- Loew, E.R.; McFarland, W.N. The underwater visual environment. In The Visual System of Fish; Douglas, R.H., Djamgoz, M.B.A., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 1–43. [Google Scholar]
- Nicol, J.A.C. The photoenvironment. In The Eyes of Fishes; Oxford University Press: Oxford, UK, 1989; pp. 4–16. [Google Scholar]
- Doujak, F.E. Can a shore crab see a star? J. Exp. Bio. 1985, 116, 385–393. [Google Scholar] [CrossRef]
- Mishra, S.B.; Mishra, A.K.; Revaprasadu, N.; Hillie, K.T.; Steyn, W.J.v.; Coetsee, E.; Swart, H.C. Strontium aluminate/polymer composites: Morphology, luminescent properties, and durability. J. Appl. Polym. Sci. 2009, 112, 3347–3354. [Google Scholar] [CrossRef]
- Ge, M.; Guo, X.; Yan, Y. Preparation and study on the structure and properties of rare-earth luminescent fiber. Text. Res. J. 2012, 82, 677–684. [Google Scholar] [CrossRef]
- Frank, C.C.H.; Bayse, S.M. The effect of variable light intensity in luminescent-netting pots on the catch of snow crab (Chionoecetes opilio). Aquac. Fish. 2023; in press. [Google Scholar] [CrossRef]
- Burrows, M.; Horridge, G.A. Eyecup withdrawal in the crab, Carcinus, and its interaction with the optokinetic response. J. Exp. Biol. 1968, 49, 285–297. [Google Scholar] [CrossRef]
- Su, T.L.; Lim, S.L. To flee or not to flee: Characterising differentiated anti-predatory responses of two mangrove crabs. Ethol. Ecol. Evol. 2015, 29, 181–192. [Google Scholar] [CrossRef]
- Conan, G.Y.; Starr, M.; Comeau, M.; Therriault, J.-C.; Hernandez, F.X.M.; Robichaud, G. Life history strategies, recruitment fluctuations, and management of the Bonne bay fjord Atlantic snow crab (Chionoecetes opilio). In High Latitude Crabs: Biology, Management, and Economics; Alaska Sea Grant: Anchorage, AK, USA, 1996; pp. 59–97. [Google Scholar]
- Ottmar, M.L.; Ryer, C.H.; Hurst, T.P. Comparative predator-mediated habitat use in early juvenile southern Tanner crab (Chionoecetes bairdi), snow crab (Chionoecetes opilio), and red king crab (Paralithodes camtschaticus). J. Exp. Mar. Biol. Ecol. 2022, 555, 151792. [Google Scholar] [CrossRef]
- Hiller-Adams, P.; Case, J.F. Eye size of pelagic crustaceans as a function of habitat depth and possession of photophores. Vis. Res. 1988, 28, 667–680. [Google Scholar] [CrossRef]
- Nalbach, H.-O. Multisensory control of eyestalk orientation in decapod crustaceans: An ecological approach. J. Crustac. Biol. 1990, 10, 382–399. [Google Scholar] [CrossRef]
- Smolka, J.; Hemmi, J.M. Topography of vision and behaviour. J. Exp. Biol. 2009, 212, 3522–3532. [Google Scholar] [CrossRef] [PubMed]
- Mullowney, D.R.J.; Dawe, E.; Colbourne, E.B.; Rose, G.A. A review of factors contributing to the decline of Newfoundland and Labrador snow crab (Chionoecetes opilio). Rev. Fish Biol. Fish. 2014, 24, 639–657. [Google Scholar] [CrossRef]
- Tuten, W.S.; Harmening, W.M. Foveal vision. Curr. Biol. 2021, 31, R701–R703. [Google Scholar] [CrossRef] [PubMed]
- Zeil, J.; Hemmi, J.M. The visual ecology of fiddler crabs. J. Comp. Physiol. A 2006, 192, 1–25. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. The crustacean eye: Dark/light adaptation, polarization sensitivity, flicker fusion frequency, and photoreceptor damage. Zool. Sci. 2001, 18, 1175–1197. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Meyer-Rochow, V.B.; Nishimura, K.; Eguchi, E. Fatty acid composition and ultrastructure of photoreceptive membranes in the crayfish Procambarus clarkii under conditions of thermal and photic stress. J. Comp. Physiol. B 1997, 167, 1–8. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Light-induced damage to the photoreceptors of spiny lobsters and other crustaceans. Crustaceana 1994, 67, 95–109. [Google Scholar] [CrossRef]
- Gaten, E. Optics and phylogeny: Is there insight? The evolution of superposition eyes in Decapoda (Crustacea). Contrib. Zool. 1998, 67, 223–235. [Google Scholar] [CrossRef]
- Herring, P.J.; Campbell, A.K.; Whitfield, M.; Maddock, L. Light and Life in the Sea; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Frank, T.M.; Johnsen, S.; Cronin, T.W. Light and vision in the deep-sea benthos: II. Vision in deep-sea crustaceans. J. Exp. Biol. 2012, 215, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Schweikert, L.E.; Thomas, K.N.; Moreno, V.M.; Casaubon, A.; Golightly, C.; Bracken-Grissom, H.D. Ecological predictors and functional implications of eye size in deep-sea shrimps. Front. Ecol. Evol. 2022, 10, 787315. [Google Scholar] [CrossRef]
- Bagheri, Z.M.; Jessop, A.-L.; Partridge, J.C.; Osborn, K.J.; Hemmi, J.M. A new computational model illuminates the extraordinary eyes of Phronima. PLoS Comput. Biol. 2022, 18, e1010545. [Google Scholar] [CrossRef] [PubMed]
- Schweikert, L.E.; Davis, A.L.; Johnsen, S.; Bracken-Grissom, H.D. Visual perception of light organ patterns in deep-sea shrimps and implications for conspecific recognition. Ecol. Evol. 2020, 10, 9503–9513. [Google Scholar] [CrossRef] [PubMed]
- Warrant, E.J. Seeing better at night: Life style, eye design and the optimum strategy of spatial and temporal summation. Vis. Res. 1999, 39, 1611–1630. [Google Scholar] [CrossRef]
- Cote, D.; Nicolas, J.-M.; Whoriskey, F.; Cook, A.M.; Broome, J.; Regular, P.M.; Baker, D. Characterizing snow crab (Chionoecetes opilio) movements in the Sydney Bight (Nova Scotia, Canada): A collaborative approach using multiscale acoustic telemetry. Can. J. Fish. Aquat. Sci. 2018, 76, 334–346. [Google Scholar] [CrossRef]
- Florko, K.R.N.; Davidson, E.R.; Lees, K.J.; Hammer, L.J.; Lavoie, M.-F.; Lennox, R.J.; Simard, E.; Archambault, P.; Auger-Methe, M.; McKindsey, C.W.; et al. Tracking movements of decapod crustaceans: A review of a half-century of telemetry-based studies. Mar. Ecol. Prog. Ser. 2021, 679, 219–239. [Google Scholar] [CrossRef]
- Maynard, D.R.; Robichaud, D.A. Short Term Movements of Snow Crabs (Chionecetes opilio) in Bay of Islands, Newfoundland, as Monitored by Ultrasonic Trackings; Research Document: 86/50; Department of Fisheries and Oceans, Canadian Atlantic Fisheries Scientific Advisory Committee: Dartmouth, NS, Canada, 1986.
- Shettle, E.P.; Fenn, R.W. Models for the Aerosols of the Lower Atmosphere and the Effects of Humidity Variations on Their Optical Properties. Optical Physics Division, Air Force Geophysics Laboratory, Environmental Research Papers No. 676. 1979. Available online: https://apps.dtic.mil/sti/citations/ADA085951 (accessed on 10 May 2024).

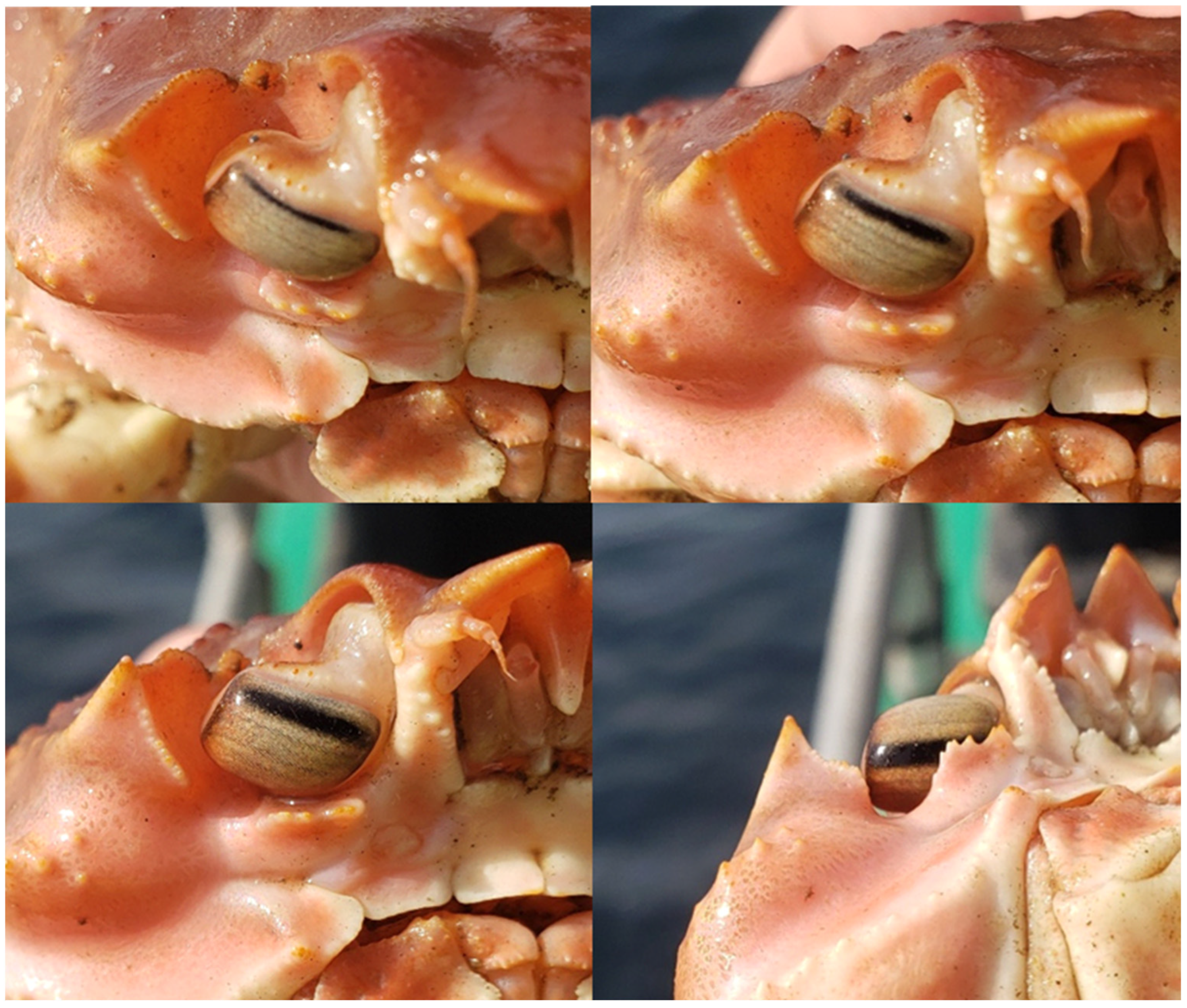
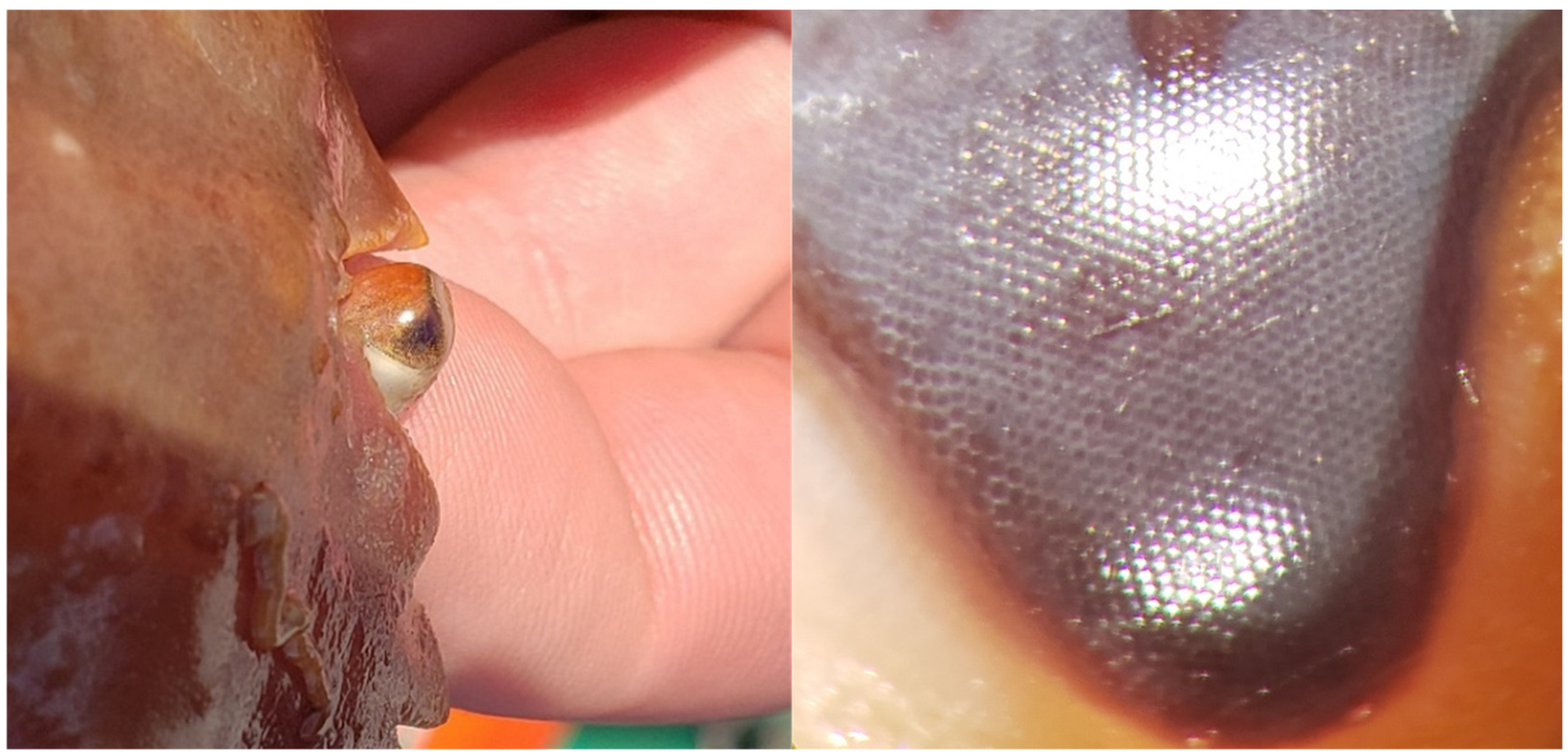
| Measurement | Front D. | Front M. | Front P. | Mid D. | Mid M. | Mid P. | Rear D. | Rear M. |
|---|---|---|---|---|---|---|---|---|
| Mean (mm) | 0.057 | 0.061 | 0.060 | 0.067 | 0.071 | 0.059 | 0.076 | 0.076 |
| SD (mm) | 0.004 | 0.003 | 0.003 | 0.003 | 0.003 | 0.004 | 0.004 | 0.005 |
| R (mm) | 1.2 | 3.8 | 2.2 | 1.5 | 3.7 | 1.1 | 1.2 | 2.8 |
| Δø (rad) | 0.048 | 0.016 | 0.027 | 0.045 | 0.019 | 0.054 | 0.063 | 0.027 |
| U (m) | 9.2 | 27.4 | 16.2 | 9.9 | 23.1 | 8.2 | 7.0 | 16.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frank, C.; Bayse, S.; Steiner, R.; Bitton, P.-P. Light Intensity of Phosphorescent-Netting Pots and Determining Their Visibility to Snow Crab (Chionoecetes opilio) Using Visual Modeling Techniques. Fishes 2024, 9, 185. https://doi.org/10.3390/fishes9050185
Frank C, Bayse S, Steiner R, Bitton P-P. Light Intensity of Phosphorescent-Netting Pots and Determining Their Visibility to Snow Crab (Chionoecetes opilio) Using Visual Modeling Techniques. Fishes. 2024; 9(5):185. https://doi.org/10.3390/fishes9050185
Chicago/Turabian StyleFrank, Colin, Shannon Bayse, Rioghnach Steiner, and Pierre-Paul Bitton. 2024. "Light Intensity of Phosphorescent-Netting Pots and Determining Their Visibility to Snow Crab (Chionoecetes opilio) Using Visual Modeling Techniques" Fishes 9, no. 5: 185. https://doi.org/10.3390/fishes9050185
APA StyleFrank, C., Bayse, S., Steiner, R., & Bitton, P.-P. (2024). Light Intensity of Phosphorescent-Netting Pots and Determining Their Visibility to Snow Crab (Chionoecetes opilio) Using Visual Modeling Techniques. Fishes, 9(5), 185. https://doi.org/10.3390/fishes9050185







