Dynamic Changes of Environment and Gut Microbial Community of Litopenaeus vannamei in Greenhouse Farming and Potential Mechanism of Gut Microbial Community Construction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Determination of Environmental Factors
2.3. DNA Extraction
2.4. 16S rRNA Sequencing and Bioinformatic Analysis
3. Results and Discussion
3.1. Environmental Factors
3.2. Basic Information of Bacterial Communities
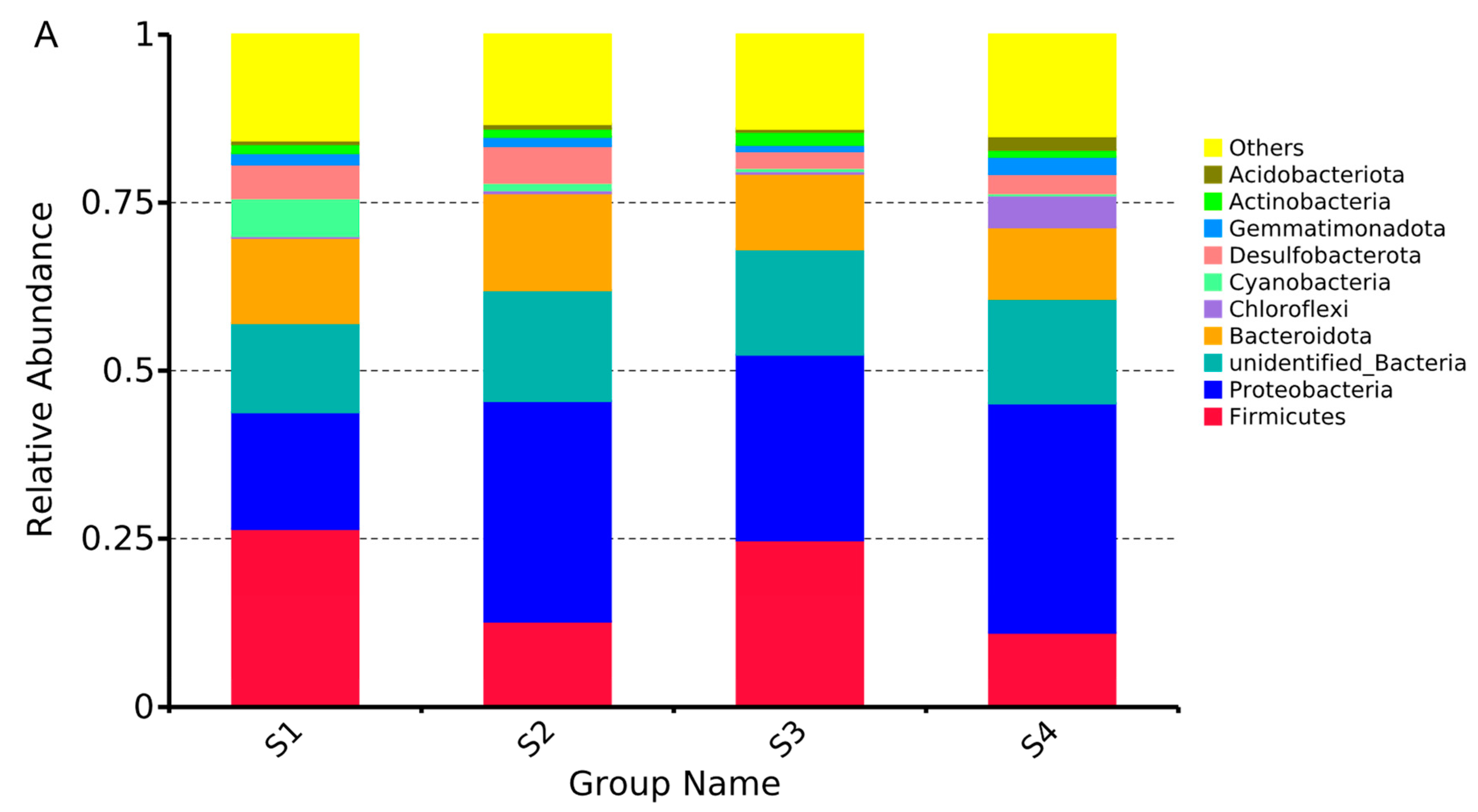
3.3. Temporal Dynamics of Microorganisms in Water
3.4. Temporal Dynamics of Microorganisms in Sediment
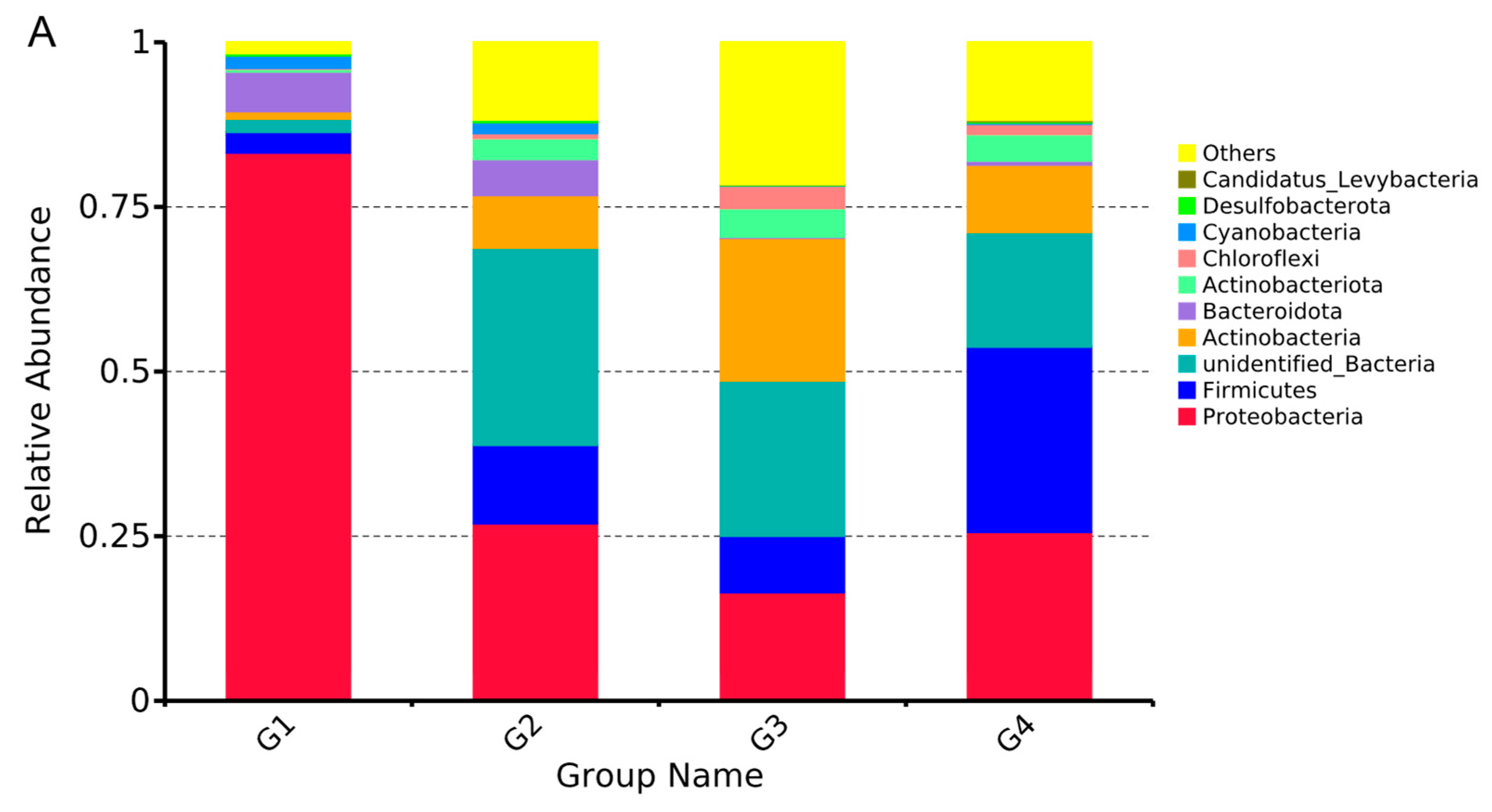
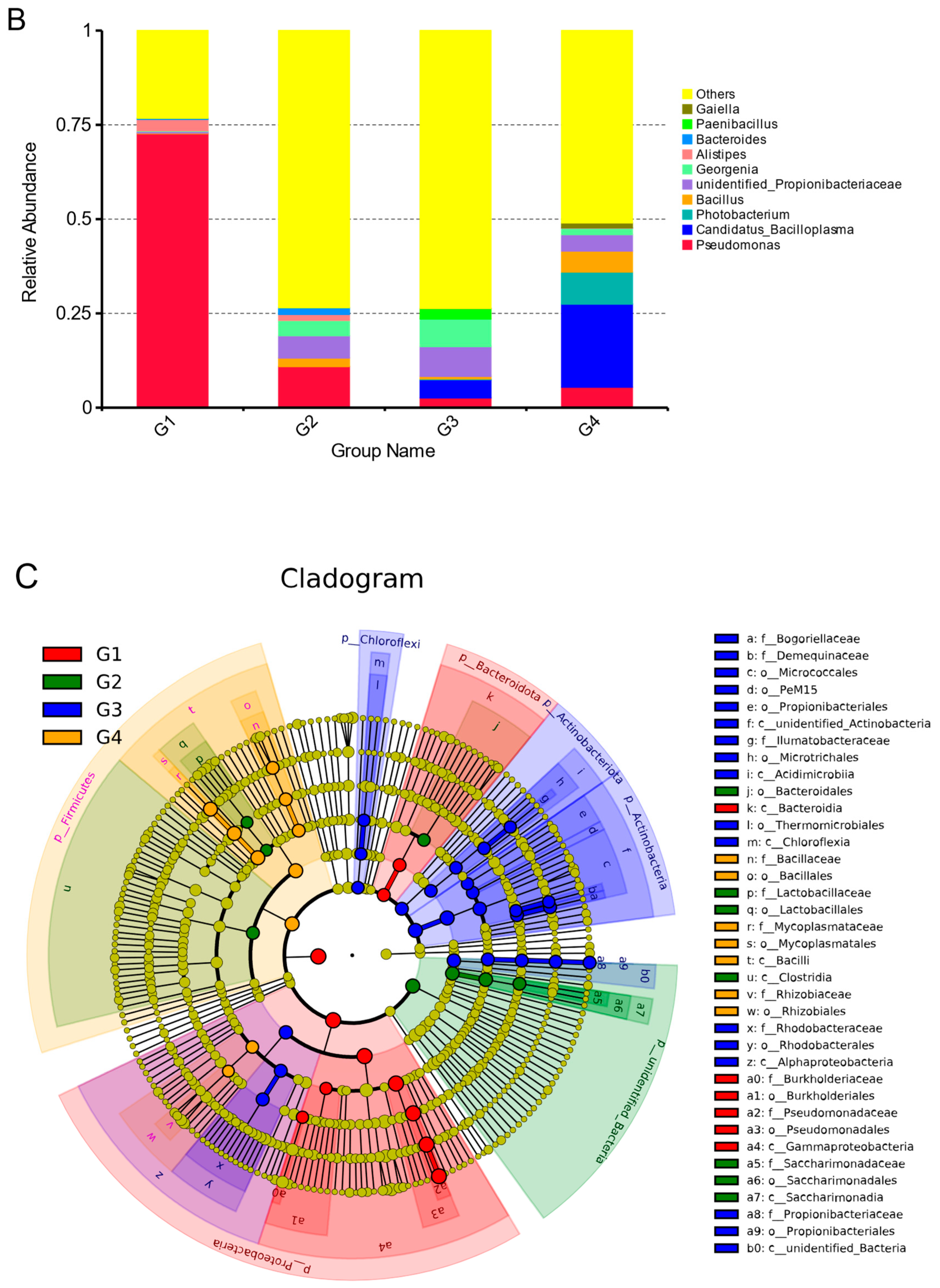
3.5. Temporal Dynamics of Microorganisms in Gut
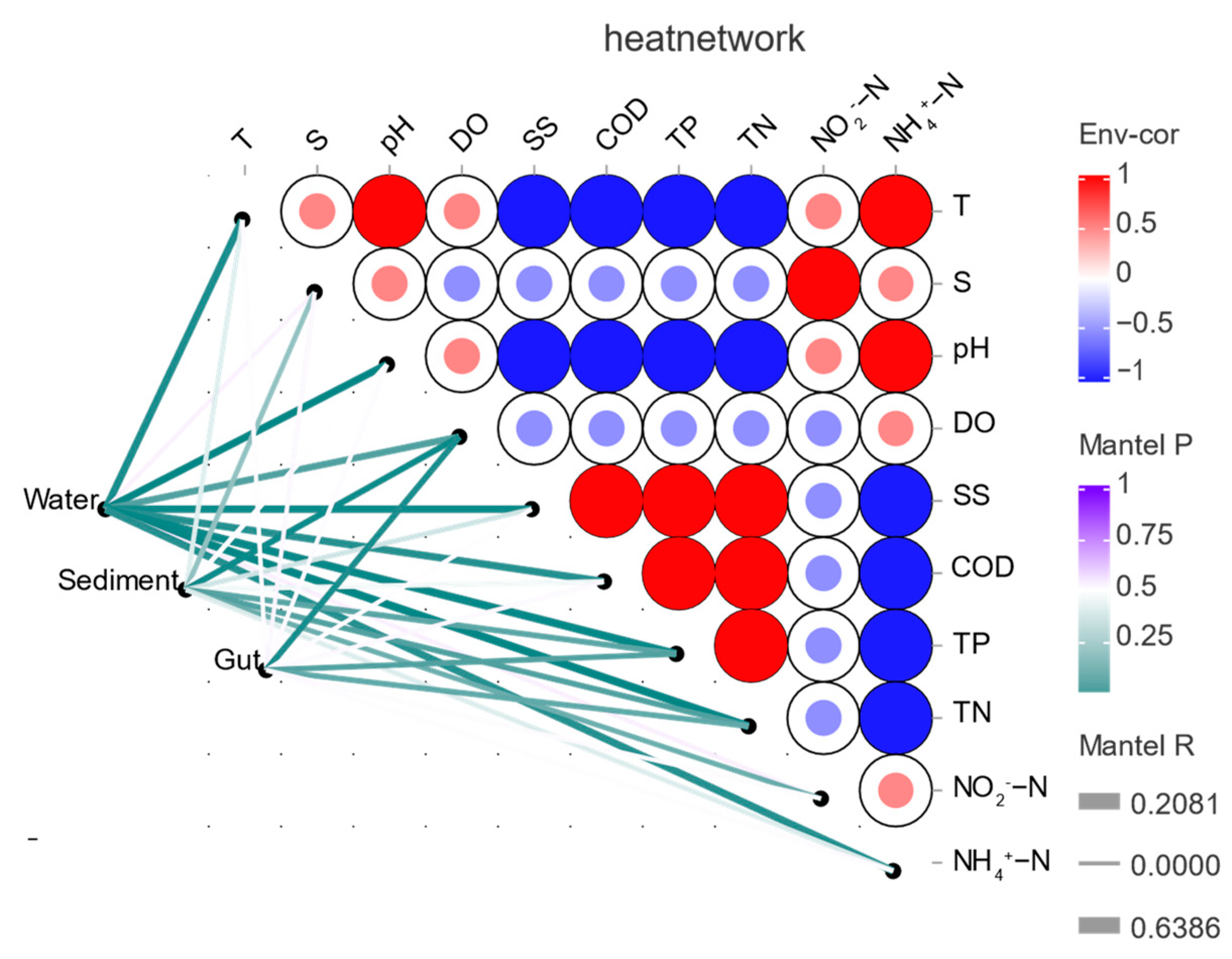
3.6. Correlations between Bacterial Communities and Environmental Factors
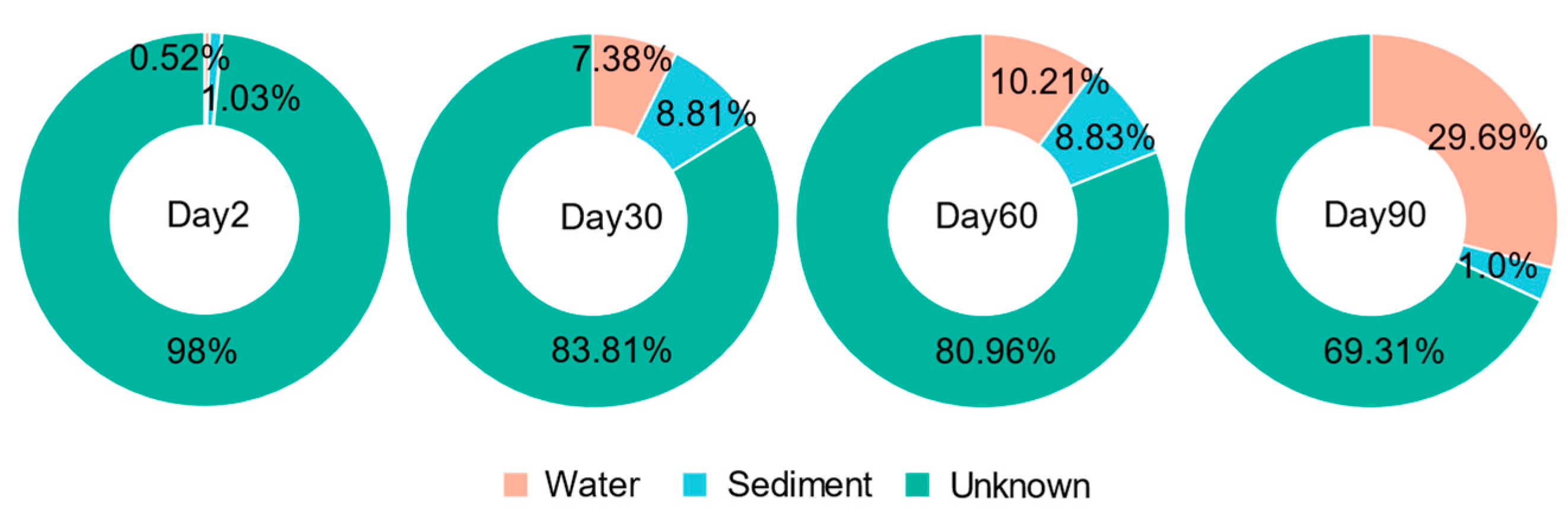
3.7. Source Tracker Analysis
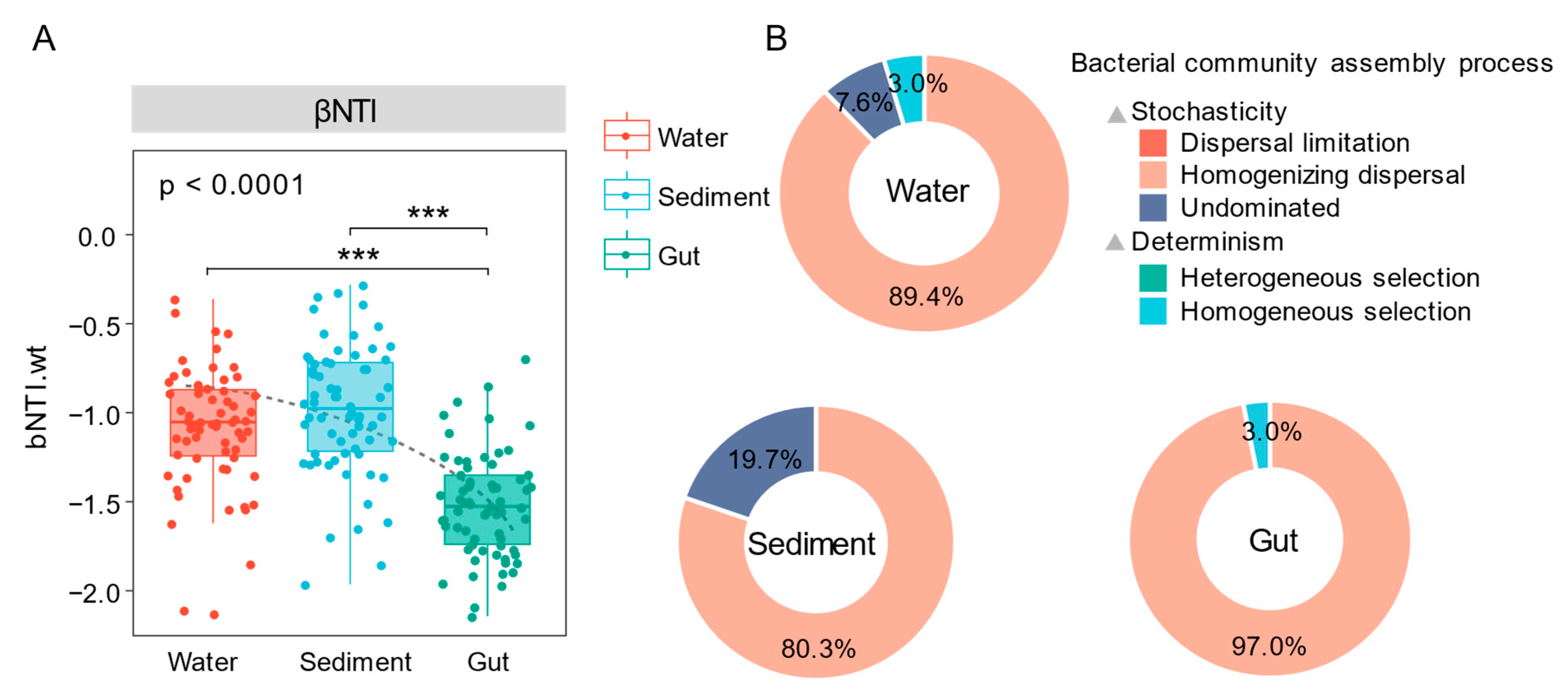
3.8. Analysis of Community Construction Mechanism
4. Discussion
4.1. Analysis of Dynamic Changes of Gut Microbial Community
4.2. Analysis of the Influence Factors of the Gut Microorganisms
4.3. Potential Mechanisms of Community Construction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, P.; Wei, P.; Zhang, B.; Zhao, Y.; Li, Q.; Chen, X.; Zeng, D.; Peng, M.; Yang, C.; Peng, J. Identification of microRNAs involved in cold adaptation of Litopenaeus vannamei by high-throughput sequencing. Gene 2018, 677, 24–31. [Google Scholar] [CrossRef]
- Liu, H.Y.; Chen, G.F.; Li, L.X.; Lin, Z.X.; Tan, B.P.; Dong, X.H.; Yang, Q.H.; Chi, S.Y.; Zhang, S.; Zhou, X.Q. Supplementing artemisinin positively influences growth, antioxidant capacity, immune response, gut health and disease resistance against Vibrio parahaemolyticus in Litopenaeus vannamei fed cottonseed protein concentrate meal diets. Fish Shellfish Immunol. 2022, 131, 105–118. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Ma, Z.; Wan, R.; Song, X.; Gao, L. The effect of three culture methods on intensive culture system of pacific white shrimp (Litopenaeus vannamei). J. Ocean Univ. China 2013, 12, 434–440. [Google Scholar] [CrossRef]
- Chang, Z.-Q.; Neori, A.; He, Y.-Y.; Li, J.-T.; Qiao, L.; Preston, S.I.; Liu, P.; Li, J. Development and current state of seawater shrimp farming, with an emphasis on integrated multi-trophic pond aquaculture farms, in China—A review. Rev. Aquac. 2020, 12, 2544–2558. [Google Scholar] [CrossRef]
- Dou, Y.; Wen, M.; Shen, H.; Zhang, S.; Jiang, G.; Qiao, Y.; Cheng, J.; Cao, X.; Wan, X.; Sun, X. Intestinal microbiota differences in Litopenaeus vannamei shrimp between greenhouse and aquaponic rearing. Life 2023, 13, 525. [Google Scholar] [CrossRef]
- Xu, W.; Cao, Y.; Fan, P.; Hu, X.; Xu, Y.; Su, H.; Yu, W.; Wen, G.; Guangdong, S. Current Situation and Development Direction of Shrimp Engineering Culture in China. Hans J. Agric. Sci. 2019, 9, 938–944. [Google Scholar]
- Zhou, L.; Qu, Y.; Qin, J.G.; Chen, L.; Han, F.; Li, E. Deep insight into bacterial community characterization and relationship in the pond water, sediment and the gut of shrimp (Penaeus japonicus). Aquaculture 2021, 539. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Wang, C.; Zeng, Y.; Lai, Z. Proportions of Pacific white shrimp, Litopenaeus vannamei, gut microbiota from ambient microbiota increased with aquaculture process. J. World Aquac. Soc. 2022, 54, 982–993. [Google Scholar] [CrossRef]
- Wu, J.Y.; Yan, M.C.; Sang, Y.; Li, F.; Luo, K.; Hu, L.H. Correlation between intestinal microbiota and growth of white shrimp (Litopenaeus vannamei). Appl. Ecol. Environ. Res. 2021, 19, 4993–5005. [Google Scholar] [CrossRef]
- Huang, F.; Pan, L.; Song, M.; Tian, C.; Gao, S. Microbiota assemblages of water, sediment, and intestine and their associations with environmental factors and shrimp physiological health. Appl. Microbiol. Biotechnol. 2018, 102, 8585–8598. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, L.; Huang, F.; Gao, S.; Su, C.; Zhang, M.; He, Z. Metagenomic analysis of composition, function and cycling processes of microbial community in water, sediment and effluent of Litopenaeus vannamei farming environments under different culture modes. Aquaculture 2019, 506, 280–293. [Google Scholar] [CrossRef]
- Van Der Gast, C.J.; Ager, D.; Lilley, A.K. Temporal scaling of bacterial taxa is influenced by both stochastic and deterministic ecological factors. Environ. Microbiol. 2008, 10, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.R.; Stephens, W.Z.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J. 2016, 10, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.C.; van der Giezen, M.; Daniels, C.L.; Stentiford, G.D.; Bass, D. Spatial and temporal axes impact ecology of the gut microbiome in juvenile European lobster (Homarus gammarus). ISME J. 2020, 14, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zheng, C.; Zheng, Z.; Wei, Y.; Lu, K.; Zhu, J. Nutrient enrichment during shrimp cultivation alters bacterioplankton assemblies and destroys community stability. Ecotoxicol. Environ. Saf. 2018, 156, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, H.; Wang, L.-B.; Wan, X.; Shi, W.-J.; Yang, Z.-Y.; Jiang, Q.; Shen, H.; Hu, R.-H.; Guan, X.-P. Study on bacterial community structure in rearing water in small green-house of Litopenaeus vannamei. South China Fish. Sci. 2023, 19, 29–41. [Google Scholar]
- Wang, C.; Zhou, Y.; Lv, D.; Ge, Y.; Li, H.; You, Y. Change in the intestinal bacterial community structure associated with environmental microorganisms during the growth of Eriocheir sinensis. MicrobiologyOpen 2019, 8, e00727. [Google Scholar] [CrossRef] [PubMed]
- Angthong, P.; Chaiyapechara, S.; Rungrassamee, W. Shrimp microbiome and immune development in the early life stages. Dev. Comp. Immunol. 2023, 147, 104765. [Google Scholar] [CrossRef]
- Kaur, H.; Setia, H.; Bhatia, R. Manoeuvering amid nanoparticle overload: A microbial perspective. Mater. Today Proc. 2023, 78, 804–808. [Google Scholar] [CrossRef]
- Rajta, A.; Setia, H.; Shukla, S.; Bhatia, R. Heterotrophic aerobic denitrification by novel bacterium Georgenia daeguensis ARB2 for treatment of nitrate contaminated waters. Int. J. Environ. Sci. Technol. 2022, 19, 3133–3144. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Zhang, T.; Wang, S.; Hao, J.; Wu, Z.; Li, A. Comparative Analysis of the Symbiotic Microbiota in the Chinese Mitten Crab (Eriocheir sinensis): Microbial Structure, Co-Occurrence Patterns, and Predictive Functions. Microorganisms 2023, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Foysal, M.J.; Fotedar, R.; Tay, C.-Y.; Gupta, S.K. Dietary supplementation of black soldier fly (Hermetica illucens) meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ 2019, 7, e6891. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Ng, T.H.; Wu, J.-H.; Chen, J.-W.; Wang, H.-C. Microbiome dynamics in a shrimp grow-out pond with possible outbreak of acute hepatopancreatic necrosis disease. Sci. Rep. 2017, 7, 9395. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wan, X.; Xie, G.; Dong, X.; Wang, X.; Huang, J. Insights into the histopathology and microbiome of Pacific white shrimp, Penaeus vannamei, suffering from white feces syndrome. Aquaculture 2020, 527, 735447. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Zhai, Q.; Chang, Z.; Li, J. Nitrogen cycling process and application in different prawn culture modes. Rev. Aquac. 2024; early view. [Google Scholar] [CrossRef]
- Padeniya, U.; Davis, D.A.; Wells, D.E.; Bruce, T.J. Microbial interactions, growth, and health of aquatic species in biofloc systems. Water 2022, 14, 4019. [Google Scholar] [CrossRef]
- Tang, L.; Zeng, Z.; Zhou, Y.; Wang, B.; Zou, P.; Wang, Q.; Ying, J.; Wang, F.; Li, X.; Xu, S. Bacillus amyloliquefaciens SC06 Induced AKT–FOXO signaling pathway-mediated autophagy to alleviate oxidative stress in IPEC-J2 Cells. Antioxidants 2021, 10, 1545. [Google Scholar] [CrossRef]
- Tao, X.; He, J.; Lu, J.; Chen, Z.; Jin, M.; Jiao, L.; Masagounder, K.; Liu, W.; Zhou, Q. Effects of Bacillus subtilis DSM 32315 (Gutcare®) on the growth performance, antioxidant status, immune ability and intestinal function for juvenile Litopenaeus vannamei fed with high/low-fishmeal diets. Aquac. Rep. 2022, 26, 101282. [Google Scholar] [CrossRef]
- Goh, J.X.H.; Tan, L.T.-H.; Law, J.W.-F.; Khaw, K.-Y.; Zengin, G.; Chan, K.G.; Letchumanan, V.; Lee, L.-H.; Goh, B.-H. Probiotics: Comprehensive Exploration of the Growth Promotion Mechanisms in Shrimps. Prog. Microbes Mol. Biol. 2023, 6. [Google Scholar] [CrossRef]
- Amiin, M.K.; Lahay, A.F.; Putriani, R.B.; Reza, M.; Putri, S.M.E.; Sumon, M.A.A.; Jamal, M.T.; Santanumurti, M.B. The role of probiotics in vannamei shrimp aquaculture performance—A review. Vet. World 2023, 16, 638. [Google Scholar] [CrossRef]
- Su, J.; Ji, D.; Lin, M.; Chen, Y.; Sun, Y.; Huo, S.; Zhu, J.; Xi, B. Developing surface water quality standards in China. Resour. Conserv. Recycl. 2017, 117, 294–303. [Google Scholar] [CrossRef]
- Sabilu, K.; Supriyono, E.; Nirmala, K.; Jusadi, D.; Widanarni, W. Sedimentary waste nutrients, water quality and production profiles of intensive Penaeus vannamei culture reared in low salinities. Aquac. Aquar. Conserv. Legis. 2021, 14, 683–694. [Google Scholar]
- Junda, M. Development of intensive shrimp farming, Litopenaeus vannamei in land-based ponds: Production and management. J. Phys. Conf. Ser. 2018, 1028, 012020. [Google Scholar] [CrossRef]
- Han, S.; Wang, B.; Wang, M.; Liu, Q.; Zhao, W.; Wang, L. Effects of ammonia and nitrite accumulation on the survival and growth performance of white shrimp Litopenaeus vannamei. Invertebr. Surviv. J. 2017, 14, 221–232. [Google Scholar]
- Wei, X.; Shi, F.; Chen, Z.; Feng, J.; Zhu, L. Response of bacterial communities (Marivita, Marinobacter, and Oceanicaulis) in the phycosphere to the growth of Phaeodactylum tricornutum in different inorganic nitrogen sources. Front. Mar. Sci. 2023, 10, 1086166. [Google Scholar] [CrossRef]
- Lin, G.; Sun, F.; Wang, C.; Zhang, L.; Zhang, X. Assessment of the effect of Enteromorpha prolifera on bacterial community structures in aquaculture environment. PLoS ONE 2017, 12, e0179792. [Google Scholar] [CrossRef]
- Zheng, Q.; Lu, J.; Wang, Y.; Jiao, N. Genomic reconstructions and potential metabolic strategies of generalist and specialist heterotrophic bacteria associated with an estuary Synechococcus culture. FEMS Microbiol. Ecol. 2019, 95, fiz017. [Google Scholar] [CrossRef]
- He, T.; Li, Z.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef]
- Ruan, Y.; Taherzadeh, M.J.; Kong, D.; Lu, H.; Zhao, H.; Xu, X.; Liu, Y.; Cai, L. Nitrogen removal performance and metabolic pathways analysis of a novel aerobic denitrifying halotolerant Pseudomonas balearica strain RAD-17. Microorganisms 2020, 8, 72. [Google Scholar] [CrossRef]
- Zhang, M.; Li, A.; Yao, Q.; Wu, Q.; Zhu, H. Nitrogen removal characteristics of a versatile heterotrophic nitrifying-aerobic denitrifying bacterium, Pseudomonas bauzanensis DN13-1, isolated from deep-sea sediment. Bioresour. Technol. 2020, 305, 122626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, Y.; Zeng, Y.; Sun, X.; Tao, R.; Mei, Y.; Qu, M. Simultaneous nitrification and denitrification by Pseudomonas sp. Y-5 in a high nitrogen environment. Environ. Sci. Pollut. Res. 2022, 29, 69491–69501. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, X.; Peijnenburg, W.; Zhang, M.; Sun, L.; Zhai, Y.; Yu, Q.; Wu, J.; Lu, T.; Qian, H. Alteration of dominant cyanobacteria in different bloom periods caused by abiotic factors and species interactions. J. Environ. Sci. 2021, 99, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Awal, S.; Shaika, N.A.; Khan, S. Cyanobacterial blooms in earthen aquaculture ponds and their impact on fisheries and human health in Bangladesh. Aquac. Res. 2022, 53, 5129–5141. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baures, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Dai, W.; Li, C. Advances, challenges, and directions in shrimp disease control: The guidelines from an ecological perspective. Appl. Microbiol. Biotechnol. 2016, 100, 6947–6954. [Google Scholar] [CrossRef]
- Yan, Q.; Stegen, J.C.; Yu, Y.; Deng, Y.; Li, X.; Wu, S.; Dai, L.; Zhang, X.; Li, J.; Wang, C.; et al. Nearly a decade-long repeatable seasonal diversity patterns of bacterioplankton communities in the eutrophic Lake Donghu (Wuhan, China). Mol. Ecol. 2017, 26, 3839–3850. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Y.; Yu, Z.; Shen, G.; Cheng, H.; Tao, S. Effects of environmental factors on the distribution of microbial communities across soils and lake sediments in the Hoh Xil Nature Reserve of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2022, 838, 156148. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Zhang, P.; Xue, K.; Liang, Y.; Van Nostrand, J.D.; Yang, Y.; He, Z.; Wu, L.; Stahl, D.A. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc. Natl. Acad. Sci. USA 2014, 111, E836–E845. [Google Scholar] [CrossRef] [PubMed]
- De Schryver, P.; Defoirdt, T.; Sorgeloos, P. Early mortality syndrome outbreaks: A microbial management issue in shrimp farming? PLoS Pathog. 2014, 10, e1003919. [Google Scholar] [CrossRef]
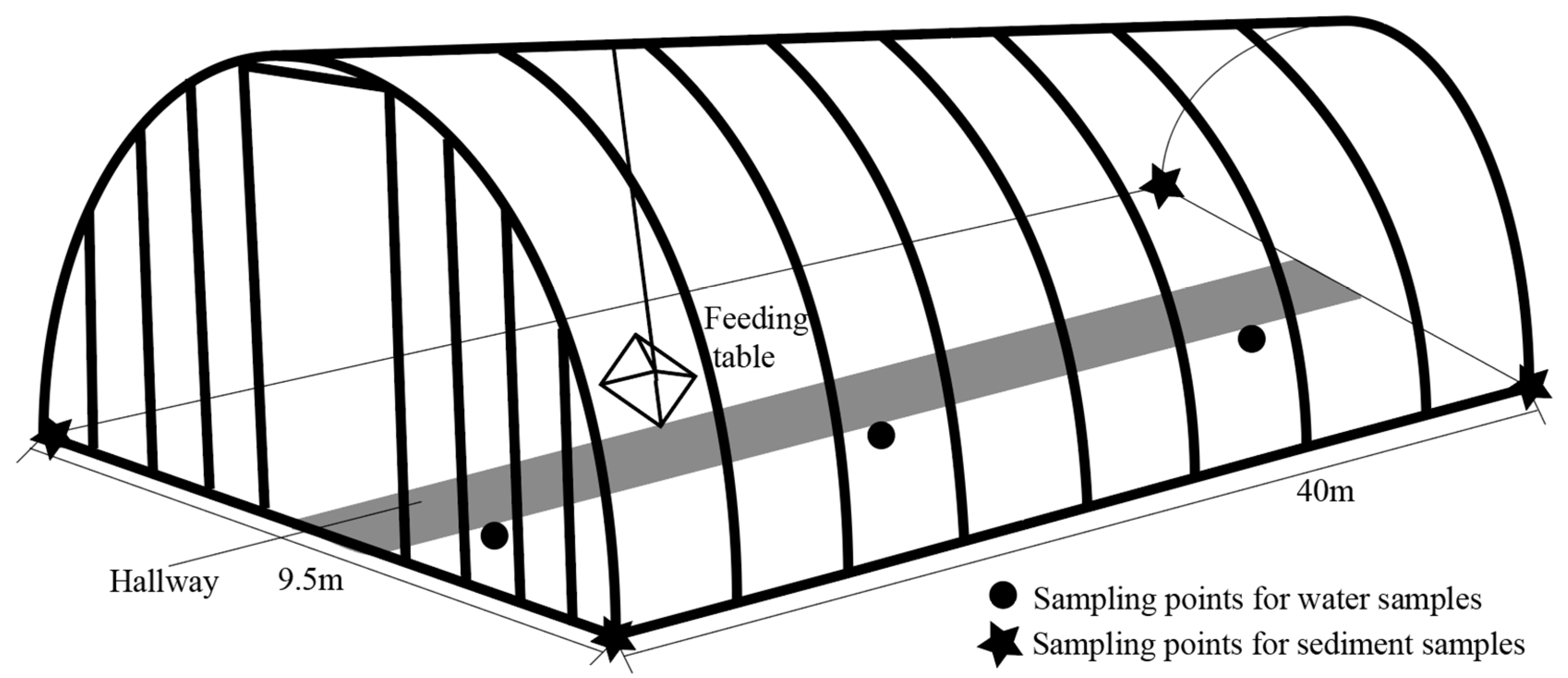
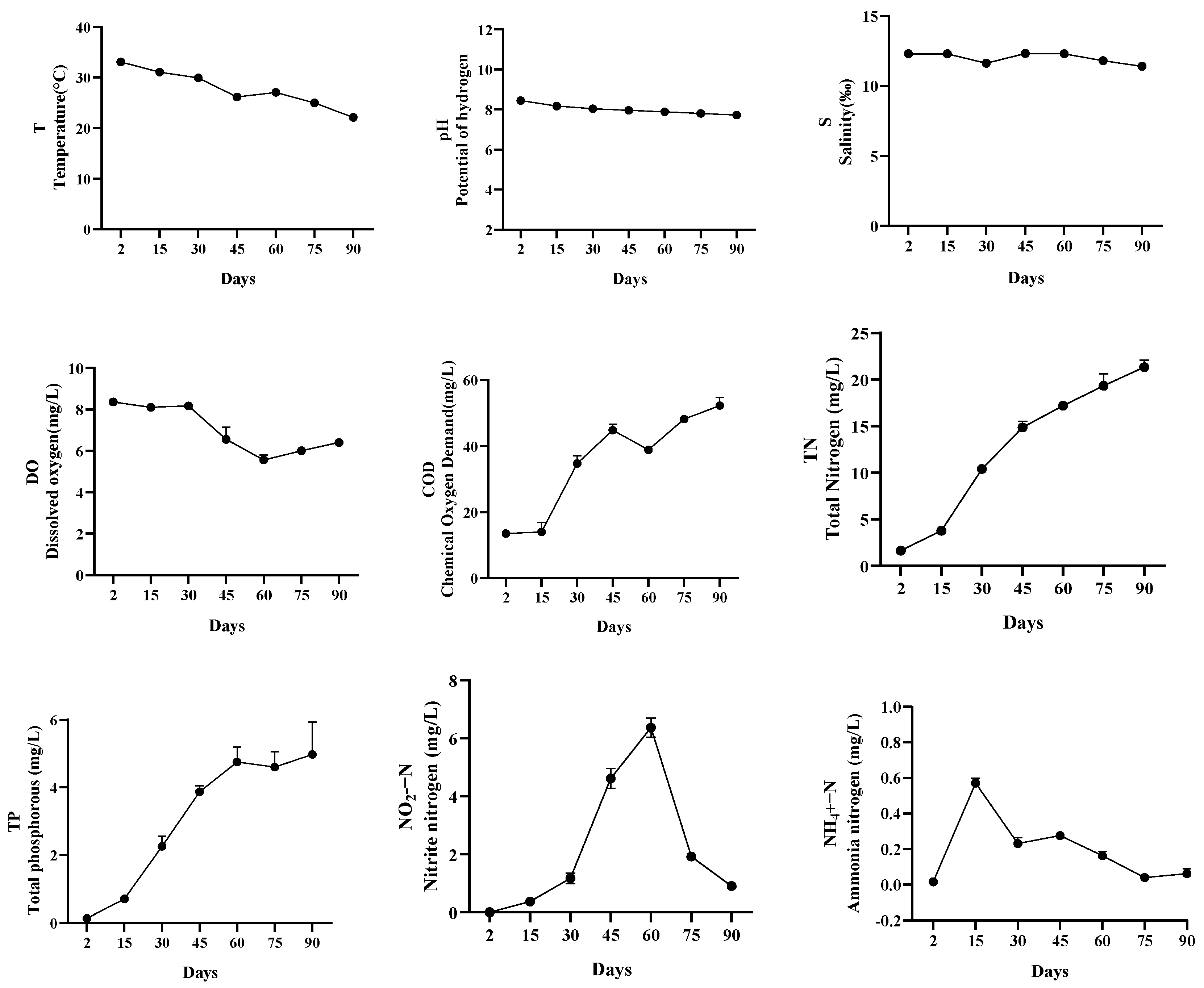
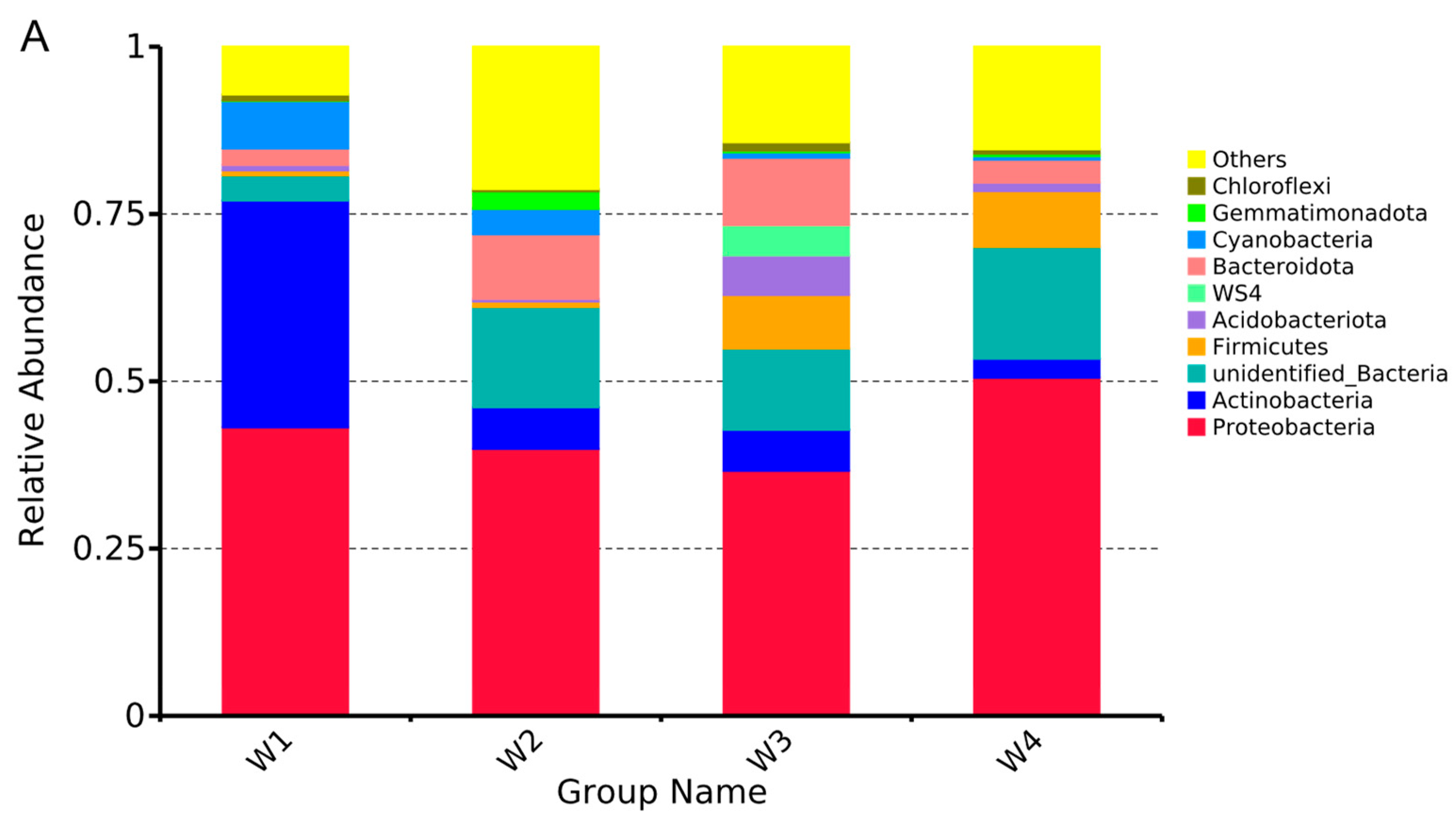
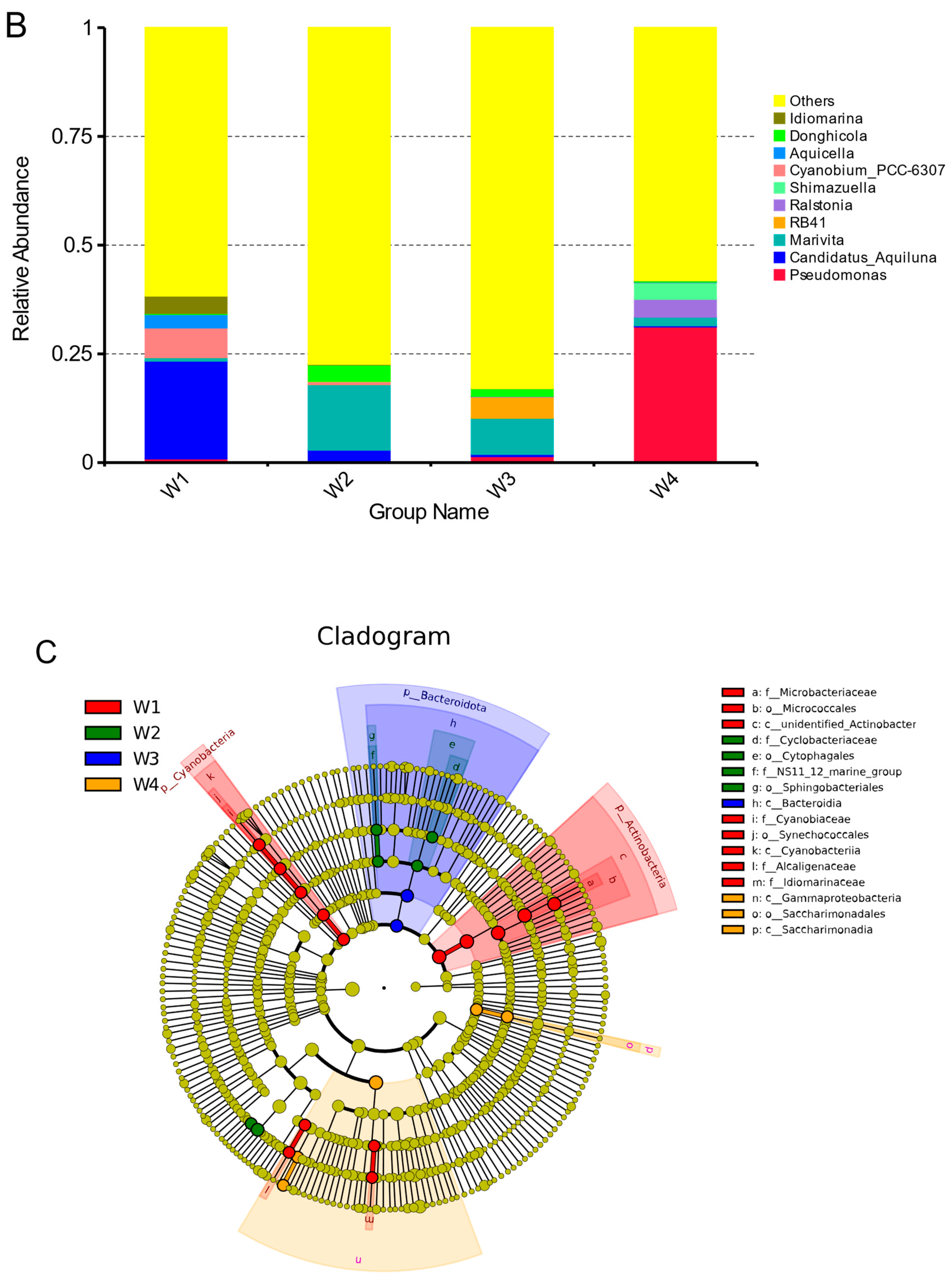

| Sample Number | Sample Properties | Sampling Time |
|---|---|---|
| W1-1, W1-2, W1-3 | Water | Day 2 |
| W2-1, W2-2, W2-3 | Water | Day 30 |
| W3-1, W3-2, W3-3 | Water | Day 60 |
| W4-1, W4-2, W4-3 | Water | Day 90 |
| S1-1, S1-2, S1-3 | Sediment | Day 2 |
| S2-1, S2-2, S2-3 | Sediment | Day 30 |
| S3-1, S3-2, S3-3 | Sediment | Day 60 |
| S4-1, S4-2, S4-3 | Sediment | Day 90 |
| G1-1, G1-2, G1-3 | Gut | Day 2 |
| G2-1, G2-2, G2-3 | Gut | Day 30 |
| G3-1, G3-2, G3-3 | Gut | Day 60 |
| G4-1, G4-2, G4-3 | Gut | Day 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Gu, S.; Wang, L.; Shi, W.; Jiang, Q.; Wan, X. Dynamic Changes of Environment and Gut Microbial Community of Litopenaeus vannamei in Greenhouse Farming and Potential Mechanism of Gut Microbial Community Construction. Fishes 2024, 9, 155. https://doi.org/10.3390/fishes9050155
Li H, Gu S, Wang L, Shi W, Jiang Q, Wan X. Dynamic Changes of Environment and Gut Microbial Community of Litopenaeus vannamei in Greenhouse Farming and Potential Mechanism of Gut Microbial Community Construction. Fishes. 2024; 9(5):155. https://doi.org/10.3390/fishes9050155
Chicago/Turabian StyleLi, Hui, Shuwen Gu, Libao Wang, Wenjun Shi, Qi Jiang, and Xihe Wan. 2024. "Dynamic Changes of Environment and Gut Microbial Community of Litopenaeus vannamei in Greenhouse Farming and Potential Mechanism of Gut Microbial Community Construction" Fishes 9, no. 5: 155. https://doi.org/10.3390/fishes9050155
APA StyleLi, H., Gu, S., Wang, L., Shi, W., Jiang, Q., & Wan, X. (2024). Dynamic Changes of Environment and Gut Microbial Community of Litopenaeus vannamei in Greenhouse Farming and Potential Mechanism of Gut Microbial Community Construction. Fishes, 9(5), 155. https://doi.org/10.3390/fishes9050155





