The Effects of Dietary Fermented Soybean Residue on the Growth, Antioxidant Capacity, Digestive Enzyme Activities, and Microbial Compositions of the Intestine in Furong Crucian Carp (Furong Carp♀ × Red Crucian Carp♂)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Design
2.3. Fish and Experimental Conditions
2.4. Collecting Samples
2.5. Analyzing the Composition of Diets and Fish
2.6. Serum Biochemical and Antioxidant Parameters
2.7. Intestinal Digestive Enzymes
2.8. Intestinal Histomorphology
2.9. The Intestinal Microbial Composition
2.10. Statistical Analysis
3. Results
3.1. Growth Performance and Physical Parameters
3.2. Whole Body Composition
3.3. Serum Biochemical Parameters
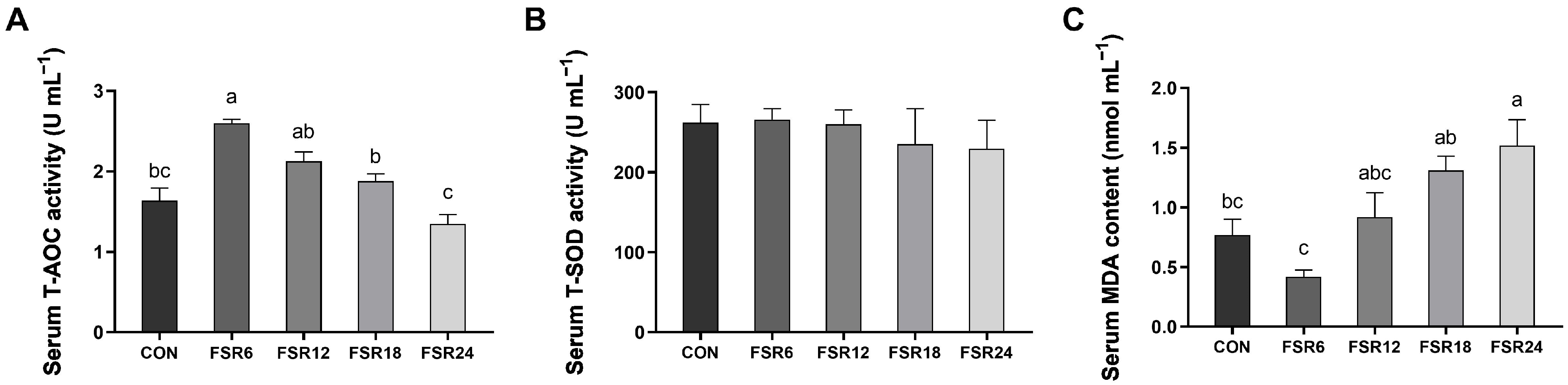
3.4. Serum Antioxidant Parameters
3.5. Intestinal Digestive Enzyme Activities
3.6. Intestinal Histomorphology
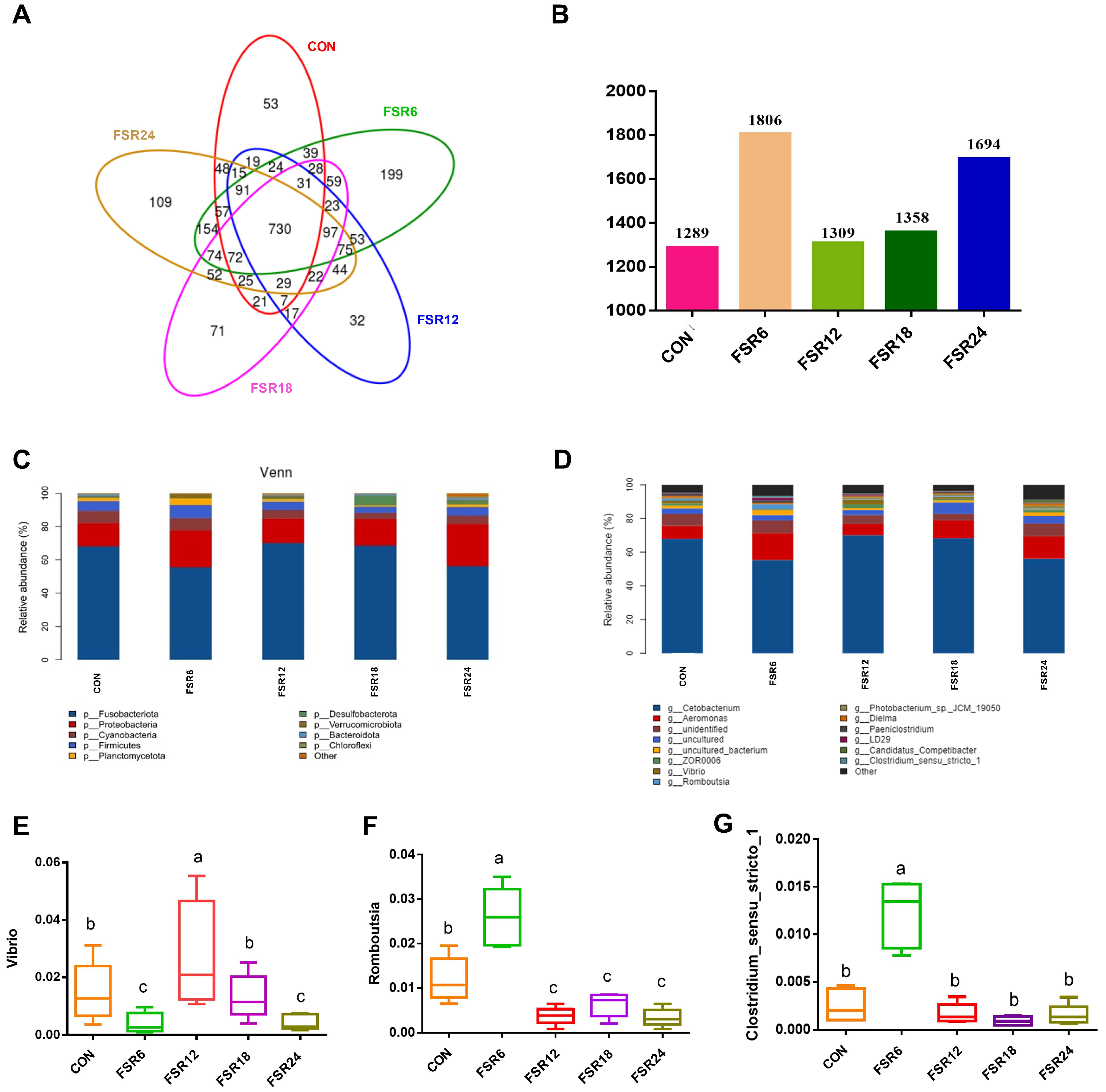
3.7. Intestinal Microbiota Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Zhu, D.; Li, K.; Yang, Y.; Lei, Z.; Zhang, Z. Soybean curd residue: Composition, utilization, and related limiting factors. Int. Sch. Res. Not. 2013, 2013, 423590. [Google Scholar] [CrossRef]
- Mok, W.K.; Tan, Y.X.; Lee, J.; Kim, J.; Chen, W.N. A metabolomic approach to understand the solid-state fermentation of okara using Bacillus subtilis WX-17 for enhanced nutritional profile. AMB Express 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.; Nishino, N. Bacterial and fungal microbiota associated with the ensiling of wet soybean curd residue under prompt and delayed sealing conditions. Microorganisms 2020, 8, 1334. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.; Zhang, N.; Bak, K.H.; Soladoye, O.P.; Aluko, R.E.; Fu, Y.; Zhang, Y. Insights into formation, detection and removal of the beany flavor in soybean protein. Trends Food Sci. Technol. 2021, 112, 336–347. [Google Scholar] [CrossRef]
- Choi, I.S.; Kim, Y.G.; Jung, J.K.; Bae, H.-J. Soybean waste (okara) as a valorization biomass for the bioethanol production. Energy 2015, 93, 1742–1747. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S. Changes in volatile profile of soybean residue (okara) upon solid-state fermentation by yeasts. J. Sci. Food Agric. 2017, 97, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Tang, B.; Wang, Q.; Xu, Z.; Sun, L.; Ma, J.; Li, S.; Xu, H.; Lei, P. The bio-processing of soybean dregs by solid state fermentation using a poly γ-glutamic acid producing strain and its effect as feed additive. Bioresour. Technol. 2019, 291, 121841. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Li, K.; Lei, Z.; Zhang, Z. Characterization of physicochemical properties of fermented soybean curd residue by Morchella esculenta. Int. Biodeterior. Biodegrad. 2016, 109, 113–118. [Google Scholar] [CrossRef]
- Vong, W.C.; Yang, K.L.C.A.; Liu, S.-Q. Okara (soybean residue) biotransformation by yeast Yarrowia lipolytica. Int. J. Food Microbiol. 2016, 235, 1–9. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, P.-F.; Lin, S.-M.; Tang, R.-J.; Chen, Y.-J.; Luo, L. Partial substitution of soybean meal with fermented soybean residue in diets for juvenile largemouth bass, Micropterus salmoides. Aquac. Nutr. 2018, 24, 1213–1222. [Google Scholar] [CrossRef]
- Kari, Z.A.; Kabir, M.A.; Mat, K.; Rusli, N.D.; Razab, M.K.A.A.; Ariff, N.S.N.A.; Edinur, H.A.; Rahim, M.Z.A.; Pati, S.; Dawood, M.A.; et al. The possibility of replacing fish meal with fermented soy pulp on the growth performance, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus). Aquac. Rep. 2021, 21, 100815. [Google Scholar] [CrossRef]
- Zulhisyam, A.K.; Kabir, M.A.; Munir, M.B.; Wei, L.S. Using of fermented soy pulp as an edible coating material on fish feed pellet in African catfish (Clarias gariepinus) production. Aquac. Aquar. Conserv. Legis. 2020, 13, 296–308. [Google Scholar]
- Wang, J.L.; Wu, Y.A.; Li, C.W. Conventional freshwater-fish new hybrids, Furong crucian carp. Sci. Fish Farming 2010, 5, 42. [Google Scholar]
- Negawoldes, T.Y. Review on nutritional limitations and opportunities of using rapeseed meal and other rape seed by-products in animal feeding. J. Nutr. Health Food Eng. 2018, 8, 43–48. [Google Scholar]
- AOAC. Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Vâlsănescu, T.; Mateescu, M.A.; Schell, H.D.; Enache, E.; BenŢia, T.; Scânteie, L.; Zarchievici, V.; Rotaru, C. All-reagent test tablets and method for rapid and selective α-amylase iodometric determination. Anal. Biochem. 1985, 146, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.S.; Li, W.; Zhang, D.D.; Xie, M.F.; Yang, B.; Zhang, R.X.; Li, H.; Lu, Z.M.; Xu, Z.H.; Shi, J.S. Biochemical characterization of an arginine-specific alkaline trypsin from Bacillus licheniformis. Int. J. Mol. Sci. 2015, 16, 30061–30074. [Google Scholar] [CrossRef] [PubMed]
- Lessinger, J.M.; Dourson, J.L.; Férard, G. Importance of standardization of lipase assays by using appropriate calibrators. Clin. Chem. 1996, 42, 1979–1983. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, P.F.; Chen, Y.J.; Luo, Q.; Li, H.; Lin, S.M. Effects of fermented soybean residue on growth performance, plasma biochemical indexes and antioxidant capacity of Jian carp. J. Anim. Nutr. 2018, 30, 1387–1395. [Google Scholar]
- Kari, Z.A.; Kabir, M.A.; Dawood, M.A.; Razab, M.K.A.A.; Ariff, N.S.N.A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Mat, K.; Ismail, T.A.; et al. Effect of fish meal substitution with fermented soy pulp on growth performance, digestive enzyme, amino acid profile, and immune-related gene expression of African catfish (Clarias gariepinus). Aquaculture 2022, 546, 737418. [Google Scholar] [CrossRef]
- Austin, B. Vibrios as causal agents of zoonoses. Vet. Microbiol. 2010, 140, 310–317. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Jalbert, S.M.; Adlercreutz, H.; Goldin, B.R.; Rasmussen, H.; Schaefer, E.J.; Ausman, L.M. Lipoprotein response to diets high in soy or animal protein with and without isoflavones in moderately hypercholesterolemic subjects. Arter. Thromb. Vasc. Biol. 2002, 22, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Watanabe, Y.; Yokoyama, S.-I. Okara, soybean residue, prevents obesity in a diet-induced murine obesity model. Biosci. Biotechnol. Biochem. 2007, 71, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.; Yokoyama, W.; Hong, Y.; Barttley, G.; Rupérez, P. Effect of high-fat diets supplemented with okara soybean by-product on lipid profiles of plasma, liver and faeces in Syrian hamsters. Food Chem. 2011, 124, 72–79. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.; Guan, D.; Wang, Y.; Zhang, Z. Evaluation of solid-state fermentation by Ganoderma lucidum using soybean curd residue. Food Bioprocess Technol. 2013, 6, 1856–1867. [Google Scholar] [CrossRef]
- Azarm, H.M.; Lee, S.-M. Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, amino acid and biochemical parameters of juvenile black sea bream Acanthopagrus schlegeli. Aquac. Res. 2014, 45, 994–1003. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Mui, J.-J. Comparison of dietary inclusion of commercial and fermented soybean meal on oxidative status and non-specific immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2017, 63, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Javanmardi, F.; Jazayeri, S.M.H.M.; Jabbari, M.; Rahmani, J.; Barati, F.; Nickho, H.; Davoodi, S.H.; Roshanravan, N.; Khaneghah, A.M. Techniques, perspectives, and challenges of bioactive peptide generation: A comprehensive systematic review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1488–1520. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Vij, S. In vitro stability of bioactive peptides derived from fermented soy milk against heat treatment, pH and gastrointestinal enzymes. LWT 2018, 91, 303–307. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, B.; Sun, C.; Zhou, Q.; Zheng, X.; Liu, M.; Xu, G.; Jin, W.; Tian, H.; Hu, H. Promotion of improved intestinal barrier health by soybean-derived bioactive peptides in Chinese mitten crab (Eriocheir sinensis) fed a low fishmeal diet. Br. J. Nutr. 2023, 131, 974–986. [Google Scholar] [CrossRef]
- Refstie, S.; Sahlström, S.; Bråthen, E.; Baeverfjord, G.; Krogedal, P. Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture 2005, 246, 331–345. [Google Scholar] [CrossRef]
- Iwashita, Y.; Suzuki, N.; Yamamoto, T.; Shibata, J.-I.; Isokawa, K.; Soon, A.H.; Ikehata, Y.; Furuita, H.; Sugita, T.; Goto, T. Supplemental effect of cholyltaurine and soybean lecithin to a soybean meal-based fish meal-free diet on hepatic and intestinal morphology of rainbow trout Oncorhynchus mykiss. Fish. Sci. 2008, 74, 1083–1095. [Google Scholar] [CrossRef]
- Knudsen, D.; Urán, P.; Arnous, A.; Koppe, W.; Frøkiær, H. Saponin-containing subfractions of soybean molasses induce enteritis in the distal intestine of Atlantic salmon. J. Agric. Food Chem. 2007, 55, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, X.; Xu, Z.R.; Wang, Y.Z.; Liu, J.X. Effects of fermented soybean meal on digestive enzyme activities and intestinal morphology in broilers. Poult. Sci. 2007, 86, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.S.d.L.; Tellez, G.; Farnell, M.B.; Balog, J.M.; Anthony, N.B.; Pavlidis, H.O.; Donoghue, A.M. Hypobaric hypoxia in ascites resistant and susceptible broiler genetic lines influences gut morphology. Poult. Sci. 2005, 84, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Bereded, N.K.; Abebe, G.B.; Fanta, S.W.; Curto, M.; Waidbacher, H.; Meimberg, H.; Domig, K.J. The impact of sampling season and catching site (wild and aquaculture) on gut microbiota composition and diversity of Nile tilapia (Oreochromis niloticus). Biology 2021, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Mangifesta, M.; Mancabelli, L.; Milani, C.; Gaiani, F.; De’angelis, N.; De’angelis, G.L.; van Sinderen, D.; Ventura, M.; Turroni, F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018, 8, 13974. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Y.; Yan, S.; Song, B.; Liu, Y.; Gao, M.; Tang, D.; Guo, Y. Soya saponin improves egg-laying performance and immune function of laying hens. J. Anim. Sci. Biotechnol. 2021, 12, 126. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Tang, J.; Li, W.; Zhou, Q.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Zhuo, Y.; Jiang, X.; Zhao, H.; et al. Effect of heating, microbial fermentation, and enzymatic hydrolysis of soybean meal on growth performance, nutrient digestibility, and intestinal microbiota of weaned piglets. J. Anim. Sci. 2023, 101, skad384. [Google Scholar] [CrossRef] [PubMed]
- Egidius, E. Vibriosis: Pathogenicity and pathology. A review. Aquaculture 1987, 67, 15–28. [Google Scholar] [CrossRef]


| Ingredients | CON | FSR6 | FSR12 | FSR18 | FSR24 |
|---|---|---|---|---|---|
| Fish meal a | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Soybean meal b | 26.00 | 26.00 | 26.00 | 26.00 | 26.00 |
| Rapeseed meal c | 22.00 | 20.15 | 18.27 | 16.40 | 14.50 |
| Meat meal | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Rice bran | 8.00 | 6.00 | 4.00 | 2.00 | 0 |
| Flour | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| Soybean oil | 0.80 | 0.65 | 0.53 | 0.40 | 0.30 |
| Fermented soybean residue d | 0 | 6.00 | 12.00 | 18.00 | 24.00 |
| Wheat bran | 10.00 | 8.00 | 6.00 | 4.00 | 2.00 |
| Ca(H2PO4)2 | 1.30 | 1.40 | 1.60 | 1.80 | 1.90 |
| Bentonite | 1.90 | 1.80 | 1.60 | 1.40 | 1.30 |
| Premix e | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition | |||||
| Moisture | 9.88 | 10.04 | 10.15 | 10.23 | 10.21 |
| Crude protein | 33.86 | 33.49 | 34.11 | 33.56 | 32.70 |
| Crude fat | 5.10 | 5.08 | 5.05 | 5.05 | 5.02 |
| Ash | 10.30 | 9.96 | 9.59 | 9.69 | 9.83 |
| Gross energy (KJ/g) | 14.43 | 14.46 | 14.50 | 14.47 | 14.44 |
| Parameters | CON | FSR6 | FSR12 | FSR18 | FSR24 | p-Value |
|---|---|---|---|---|---|---|
| IBW (g) | 24.99 ± 0.01 | 24.99 ± 0.01 | 25.04 ± 0.04 | 24.99 ± 0.01 | 25.01 ± 0.02 | 0.333 |
| FBW (g) | 104.35 ± 1.58 ab | 108.34 ± 1.36 a | 105.23 ± 1.18 ab | 100.42 ± 1.38 b | 91.79 ± 1.23 c | <0.001 |
| WGR (%) | 317.52 ± 6.28 ab | 333.49 ± 5.51 a | 320.16 ± 5.17 ab | 301.88 ± 5.50 b | 267.07 ± 4.75 c | <0.001 |
| SGR (%/day) | 2.51 ± 0.03 ab | 2.57 ± 0.02 a | 2.52 ± 0.02 ab | 2.44 ± 0.02 b | 2.28 ± 0.02 c | <0.001 |
| FCR (%) | 1.62 ± 0.03 ab | 1.54 ± 0.03 c | 1.60 ± 0.02 ab | 1.74 ± 0.05 b | 1.93 ± 0.04 a | <0.001 |
| FI (g/fish/d) | 1.06 ± 0.01 bc | 1.03 ± 0.01 c | 1.06 ± 0.01 bc | 1.10 ± 0.01 b | 1.18 ± 0.01 a | <0.001 |
| SR (%) | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 98.66 ± 0.43 | 100 ± 0.00 | 0.062 |
| CF (g/cm3) | 2.85±0.04 | 2.84±0.05 | 2.76±0.05 | 2.78±0.06 | 2.85±0.07 | 0.631 |
| VSI (%) | 11.12 ± 0.36 a | 10.36 ± 0.21 ab | 10.04 ± 0.34 b | 10.02 ± 0.19 b | 9.98 ± 0.21 b | 0.018 |
| HSI (%) | 2.24 ± 0.15 | 1.95 ± 0.13 | 2.12 ± 0.16 | 1.92 ± 0.08 | 1.81 ± 0.14 | 0.186 |
| Parameters | CON | FSR6 | FSR12 | FSR18 | FSR24 | p-Value |
|---|---|---|---|---|---|---|
| Moisture | 73.58 ± 0.23 | 74.87 ± 0.32 | 74.47 ± 0.29 | 74.78 ± 0.51 | 74.05 ± 1.14 | 0.543 |
| Crude protein | 16.91 ± 0.05 | 17.00 ± 0.29 | 16.88 ± 0.23 | 16.95 ± 0.12 | 17.78 ± 0.50 | 0.167 |
| Crude lipid | 6.83 ± 0.20 a | 5.65 ± 0.27 b | 5.74 ± 0.21 ab | 4.83 ± 0.25 b | 5.02 ± 0.34 b | <0.001 |
| Parameters | CON | FSR6 | FSR12 | FSR18 | FSR24 | p-Value |
|---|---|---|---|---|---|---|
| AST (U/L) | 230.14 ± 65.45 | 214.11 ± 35.92 | 148.53 ± 33.08 | 173.99 ± 29.71 | 150.26 ± 21.91 | 0.504 |
| ALT (U/L) | 31.44 ± 2.99 | 27.84 ± 3.31 | 26.96 ± 2.64 | 26.30 ± 4.47 | 23.13 ± 3.16 | 0.547 |
| TP (g/L) | 27.88 ± 0.10 | 27.95 ± 0.73 | 27.01 ± 0.65 | 25.37 ± 1.31 | 27.27 ± 1.15 | 0.283 |
| ALB (g/L) | 13.61 ± 0.47 | 13.08 ± 0.79 | 12.35 ± 0.44 | 11.86 ± 0.51 | 12.45 ± 0.69 | 0.300 |
| GLB (g/L) | 14.20 ± 0.49 | 14.60 ± 0.75 | 14.40 ± 0.24 | 13.60 ± 0.98 | 15.00 ± 0.63 | 0.665 |
| GLU (mg/dL) | 5.63 ± 0.22 a | 4.99 ± 0.41 ab | 4.88 ± 0.45 ab | 4.90 ± 0.37 ab | 3.80 ± 0.44 b | 0.041 |
| TC (mmol/L) | 4.34 ± 0.10 a | 4.10 ± 0.16 ab | 4.09 ± 0.13 ab | 3.51 ± 0.27 b | 4.12 ± 0.21 ab | 0.049 |
| TG (mmol/L) | 3.35 ± 0.20 a | 2.89 ± 0.22 ab | 2.80 ± 0.04 ab | 2.43 ± 0.15 b | 3.27 ± 0.21 a | 0.008 |
| Parameters | CON | FSR6 | FSR12 | FSR18 | FSR24 | p-Value |
|---|---|---|---|---|---|---|
| Amylase (U/mg) | 1.19 ± 0.15 b | 1.93 ± 0.32 a | 1.69 ± 0.06 a | 1.23 ± 0.18 b | 1.02 ± 0.07 b | 0.027 |
| Trypsin (U/mg) | 7.50 ± 2.20 | 8.10 ± 3.18 | 7.75 ± 4.76 | 5.41 ± 1.23 | 7.52 ± 3.02 | 0.973 |
| Lipase (U/g) | 9.14 ± 0.90 | 6.63 ± 1.84 | 6.30 ± 1.12 | 8.91 ± 1.53 | 7.05 ± 1.42 | 0.502 |
| Items | CON | FSR6 | FSR12 | FSR18 | FSR24 | p-Value |
|---|---|---|---|---|---|---|
| Observed species | 556.00 ± 54.61 | 600.16 ± 51.44 | 782.20 ± 144.62 | 817.58 ± 112.84 | 573.34 ± 95.68 | 0.315 |
| PD whole tree | 41.93 ± 2.78 | 47.04 ± 4.51 | 54.03 ± 7.74 | 57.88 ± 5.72 | 43.52 ± 5.56 | 0.339 |
| Shannon | 2.31 ± 0.43 b | 2.25 ± 0.18 b | 3.27 ± 0.37 ab | 3.94 ± 0.50 a | 2.10 ± 0.31 b | 0.009 |
| Simpson | 0.51 ± 0.08 b | 0.49 ± 0.04 b | 0.62 ± 0.05 ab | 0.76 ± 0.06 a | 0.43 ± 0.05 b | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, Z.; Li, F.; Hu, L.; Xiao, T.; Zhao, Y.; Yang, M. The Effects of Dietary Fermented Soybean Residue on the Growth, Antioxidant Capacity, Digestive Enzyme Activities, and Microbial Compositions of the Intestine in Furong Crucian Carp (Furong Carp♀ × Red Crucian Carp♂). Fishes 2024, 9, 138. https://doi.org/10.3390/fishes9040138
Wang H, Zhang Z, Li F, Hu L, Xiao T, Zhao Y, Yang M. The Effects of Dietary Fermented Soybean Residue on the Growth, Antioxidant Capacity, Digestive Enzyme Activities, and Microbial Compositions of the Intestine in Furong Crucian Carp (Furong Carp♀ × Red Crucian Carp♂). Fishes. 2024; 9(4):138. https://doi.org/10.3390/fishes9040138
Chicago/Turabian StyleWang, Hongquan, Zheming Zhang, Feilong Li, Liang Hu, Tiaoyi Xiao, Yurong Zhao, and Mengxi Yang. 2024. "The Effects of Dietary Fermented Soybean Residue on the Growth, Antioxidant Capacity, Digestive Enzyme Activities, and Microbial Compositions of the Intestine in Furong Crucian Carp (Furong Carp♀ × Red Crucian Carp♂)" Fishes 9, no. 4: 138. https://doi.org/10.3390/fishes9040138
APA StyleWang, H., Zhang, Z., Li, F., Hu, L., Xiao, T., Zhao, Y., & Yang, M. (2024). The Effects of Dietary Fermented Soybean Residue on the Growth, Antioxidant Capacity, Digestive Enzyme Activities, and Microbial Compositions of the Intestine in Furong Crucian Carp (Furong Carp♀ × Red Crucian Carp♂). Fishes, 9(4), 138. https://doi.org/10.3390/fishes9040138






