Combining Ability of Female Channel Catfish, Ictalurus punctatus, and Male Blue Catfish, I. furcatus, for Early Growth Performance of Their Progeny †
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Matings
2.3. Spawning Procedures
2.4. Fish Husbandry
2.5. Data Gathering and Analyses
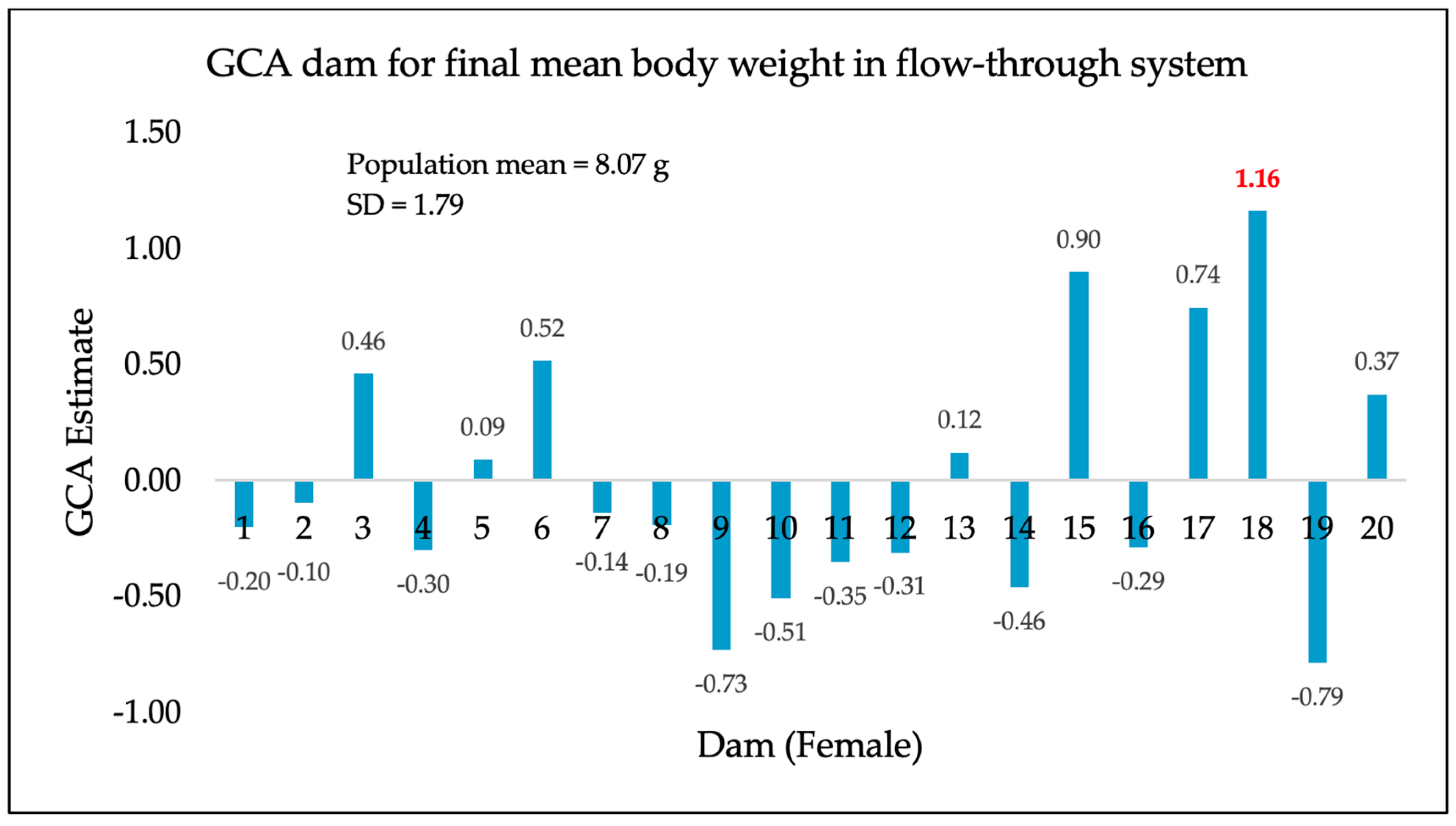
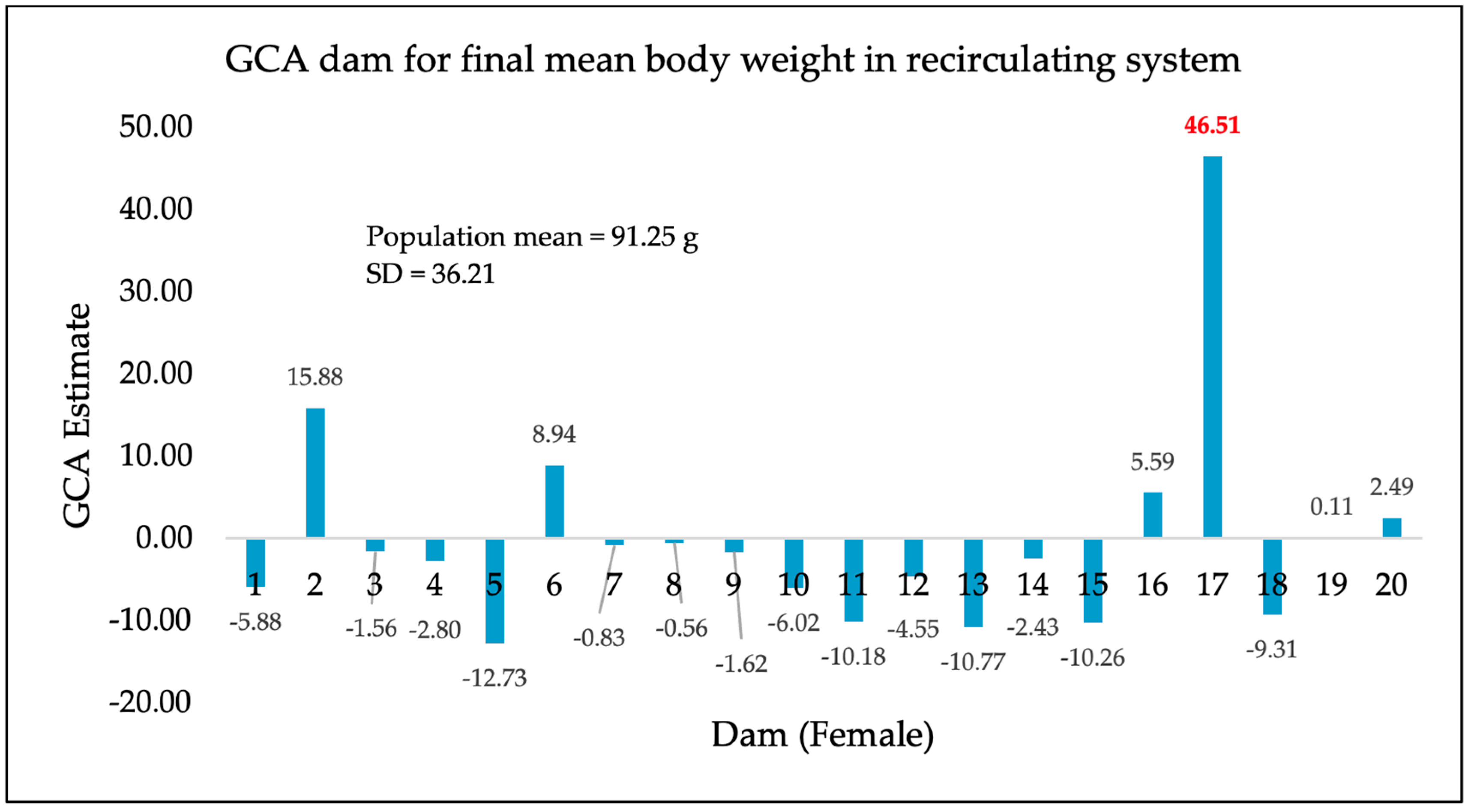
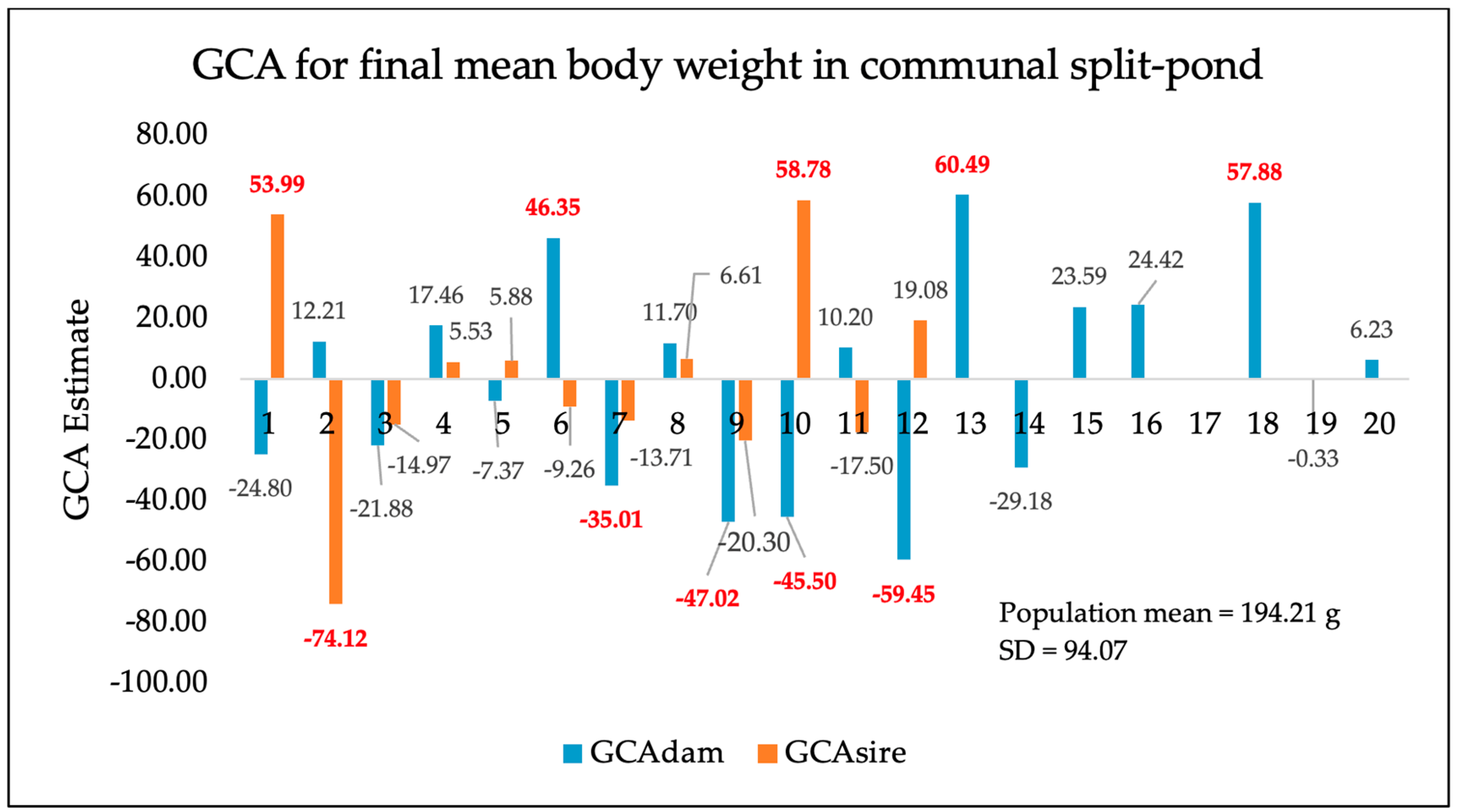
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA, National Agricultural Statistic Service. Catfish Production Report. 2023. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/bg257f046/6w925t15n/6395xq11j/cfpd0723.pdf (accessed on 5 January 2024).
- Giudice, J.J. Growth of Blue × Channel catfish hybrid as compared to its parent species. Progress. Fish-Cult. 1966, 28, 142–145. [Google Scholar] [CrossRef]
- Yant, D.R.; Smitherman, R.O.; Green, O.L. Production of hybrid (Blue × Channel) catfish and Channel Catfish in ponds. Proc. Southeast Assoc. Fish Game Comm. 1976, 29, 82–86. [Google Scholar]
- Dunham, R.A.; Smitherman, R.O.; Goodman, R.K. Comparison of mass selection, crossbreeding, and hybridization for improving growth of Channel Catfish. Progress. Fish-Cult. 1987, 49, 293–296. [Google Scholar] [CrossRef]
- Dunham, R.A.; Brummett, R.E.; Ella, M.O.; Smitherman, R.O. Genotype-environment interactions for growth of Blue, Channel, and hybrid catfish in ponds and cages at varying densities. Aquaculture 1990, 85, 143–151. [Google Scholar] [CrossRef]
- Dunham, R.A.; Umali, G.M.; Beam, R.; Kristanto, A.H.; Trask, M. Comparison of production traits of NWAC103 Channel Catfish, NWAC103 Channel Catfish × Blue Catfish hybrids, Kansas select 21 Channel Catfish, and Blue Catfish grown at commercial densities and exposed to natural bacterial epizootics. N. Am. J. Aquac. 2008, 70, 98–106. [Google Scholar] [CrossRef]
- Dunham, R.A.; Brummett, R.E. Response of two generations of selection to increased body weight in Channel Catfish, Ictalurus punctatus compared to hybridization with Blue Catfish, I. furcatus, Males. J. Appl. Aquac. 1999, 3, 211–222. [Google Scholar] [CrossRef]
- Argue, B.J.; Liu, Z.; Dunham, R.A. Dress-out and fillet yields of Channel Catfish, Ictalurus punctatus, Blue Catfish, I. furcatus, and their F1, F2 and backcross hybrids. Aquaculture 2003, 228, 81–90. [Google Scholar] [CrossRef]
- Li, M.H.; Robinson, E.H.; Manning, B.B.; Yant, D.R.; Chatakondi, N.G.; Bosworth, B.G.; Wolters, W.R. Comparison of the Channel Catfish, Ictalurus punctatus, (NWAC103 strain) and the Channel × Blue catfish, I. punctatus × I. furcatus, F1 hybrid for growth, feed efficiency, processing yield, and body composition. J. Appl. Aquac. 2004, 15, 63–71. [Google Scholar] [CrossRef]
- Brown, T.W.; Chappell, J.A.; Boyd, C.E. A commercial-scale, in pond raceway system for ictalurid catfish production. Aquac. Eng. 2011, 44, 72–79. [Google Scholar] [CrossRef]
- Dunham, R.A.; Smitherman, R.O.; Webber, C. Relative tolerance of Channel × Blue hybrid and Channel catfish to low oxygen concentrations. Progress. Fish-Cult. 1983, 45, 55–56. [Google Scholar] [CrossRef]
- Ella, M.O. Genotype-Environment Interactions for Growth Rate of Blue, Channel, and Hybrid Catfish Grown at Varying Stocking Densities. Master’s Thesis, Auburn University, Auburn, AL, USA, 1984. [Google Scholar]
- Wolters, W.R.; Wise, D.J.; Klesius, P.H. Survival and antibody response of Channel Catfish, Blue Catfish and Channel Catfish female × Blue Catfish male hybrids after exposure to Edwardsiella ictaluri. J. Aquat. Anim. Health 1996, 8, 249–254. [Google Scholar] [CrossRef]
- Arias, C.R.; Cai, W.; Peatman, E.J.; Bullard, S.A. Catfish hybrid Ictalurus punctatus × I. furcatus exhibits higher resistance to columnaris disease than the parental species. Dis. Aquat. Org. 2012, 100, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Dunham, R.A.; RSmitherman, O.; Brooks, M.J.; Benchakan, M.; Chappell, J.A. Paternal predominance in reciprocal Channel-Blue hybrid catfish. Aquaculture 1982, 29, 389–396. [Google Scholar] [CrossRef]
- Brooks, M.J.; Smitherman, R.O.; Chappell, J.A.; Dunham, R.A. Sex-weight relations in Blue, Channel, and White catfishes: Implications for brood stock selection. Progress. Fish-Cult. 1982, 44, 105–106. [Google Scholar] [CrossRef]
- Brooks, M.J.; Smitherman, R.O.; Chappell, J.A.; Williams, J.C.; Dunham, R.A. Length variation in species and hybrid populations of Blue, Channel and White catfishes. Proc. Southeast. Assoc. Fish Game Comm. 1982, 36, 190–195. [Google Scholar]
- Yant, D. Production of Hybrid Blue Ictalurus furcatus (Lesueur) Male, Channel I. punctatus (Rafinesque) Female Catfish and Channel Catfish in Earthen Ponds. Master’s Thesis, Auburn University, Auburn, AL, USA, 1975. [Google Scholar]
- Chappell, J.A. An Evaluation of Twelve Genetic Groups of Catfish for Suitability in Commercial Production. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 1979. [Google Scholar]
- Huang, Y.W.; Lowell, R.T.; Dunham, R.A. Carcass characteristics of Channel and hybrid catfish, and quality changes during refrigerated storage. J. Food Sci. 1994, 59, 64–66. [Google Scholar] [CrossRef]
- Bosworth, B.G.; Wolters, W.R.; Silva, J.L.; Chamul, R.S.; Park, S. Comparison of production, meat yield, and meat quality traits of NWAC 103 line channel catfish (Ictalurus punctatus), Norris line channel catfish, and channel catfish female × blue catfish male (I. furcatus) F1 hybrids. N. Am. J. Aquac. 2004, 66, 177–183. [Google Scholar] [CrossRef]
- Bosworth, B.G. Effects of winter feeding on growth, body composition, and processing traits of co-cultured blue catfish (Ictalurus furcatus), channel catfish (I. punctatus) and channel catfish × blue catfish hybrids. N. Am. J. Aquac. 2012, 74, 553–559. [Google Scholar] [CrossRef]
- Dunham, R.A.; Argue, B.J. Seinability of Channel Catfish, Blue Catfish, and F1, F2, F3 and backcross hybrids in earthen ponds. Progress. Fish-Cult. 1998, 60, 214–220. [Google Scholar] [CrossRef]
- Tave, D.; McGinty, A.; Chappell, J.A.; Smitherman, R.O. Relative harvestability by angling of Blue Catfish, Channel Catfish, and their reciprocal hybrids. N. Am. J. Aquac. 1981, 1, 73–76. [Google Scholar] [CrossRef]
- Dunham, R.A.; Smitherman, R.O.; Goodman, R.K.; Kemp, P. Comparison of strains, crossbreeds, and hybrids of Channel Catfish for vulnerability to angling. Aquaculture 1986, 57, 193–201. [Google Scholar] [CrossRef]
- Bosworth, B.G.; Waldbieser, G. General and specific combining ability of male blue catfish (Ictalurus furcatus) and female channel catfish (Ictalurus punctatus) for growth and carcass yield of their F1 hybrid progeny. Aquaculture 2014, 420–421, 147–153. [Google Scholar] [CrossRef]
- Dunham, R.A.; Ramboux, A.C.R.; Perera, D.A. Effect of strain on the growth, survival and sexual dimorphism of channel × blue catfish hybrids grown in earthen ponds. Aquaculture 2014, 420–421, S20–S24. [Google Scholar] [CrossRef]
- Dunham, R.A.; Ramboux, A.C.R.; Perera, D.A. Effect of strain on tolerance of low dissolved oxygen of channel × blue catfish hybrids. Aquaculture 2014, 420–421, S25–S28. [Google Scholar] [CrossRef]
- Bosworth, B.G.; Wolters, W.R.; Wise, D.J.; Li, M.H. Growth, feed conversion, fillet proximate composition and resistance to Edwardsiella ictaluri of channel catfish, Ictalurus punctatus (Rafinesque), blue catfish, Ictalurus furcatus (Lesueur), and their reciprocal Fl hybrids fed 25% and 45% protein diets. Aquac. Res. 1998, 29, 251–257. [Google Scholar] [CrossRef]
- Salem, A.H.; Ali, M.A. Combining ability for sunflower yield contributing characters and oil content over different water supply environments. J. Am. Sci. 2012, 8, 227–233. [Google Scholar]
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing system. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Wolters, W.R.; Johnson, M.R. Analysis of a diallel cross to estimate effects of crossing on resistance to enteric septicemia in channel catfish, Ictalurus punctatus. Aquaculture 1995, 137, 263–269. [Google Scholar] [CrossRef]
- Vandeputte, M.K.; Mauger, S.; Dupont-Nivet, M.; De Guerry, D.; Rodina, M.; Gela, D.; Vallod, D.; Chevassus, B.; Linhart, O. Heritability estimates for growth-related traits using microsatellite parentage assignment in juvenile common carp (Cyprinus carpio L.). Aquaculture 2004, 235, 223–236. [Google Scholar] [CrossRef]
- Saillant, E.; Dupont-Nivet, M.; Haffray, P.; Chatain, B. Estimates of heritability and genotype–environment interactions for bodyweight in sea bass (Dicentrarchus labrax L.) raised under communal rearing conditions. Aquaculture 2006, 254, 139–147. [Google Scholar] [CrossRef]
- Wang, X.; Ross, K.E.; Saillant, E.; Gatlin, D.M., III; Gold, J.R. Quantitative genetics and heritability of growth-related traits in hybrid striped bass (Morone chrysops × Morone saxatilis). Aquaculture 2006, 261, 535–545. [Google Scholar] [CrossRef]
- Lutz, C.G.; Armas-Rosales, A.M.; Saxton, A.M. Genetic effect influencing in six varieties of tilapia (Oreochromis) and their reciprocal crosses. Aquac. Res. 2010, 41, e770–e780. [Google Scholar] [CrossRef]
- Su, S.; Xu, P.; Yuan, X. Estimates of combining ability and heterosis for growth traits in a full diallel cross of three strains of common carp, Cyprinus carpio L. Afr. J. Biotechnol. 2013, 12, 3514–3521. [Google Scholar]
- Luo, W.; Zeng, C.; Yi, S.; Robinson, N.; Wang, W.; Gao, Z. Heterosis and combining ability evaluation for growth traits of blunt snout bream (Megalobrama amblycephala) when crossbreeding three strains. China Sci. Bull. 2014, 59, 857–864. [Google Scholar] [CrossRef]
- Drescher, D. Combining Ability of Channel Catfish (Ictalurus punctatus) Females and Blue Catfish (I. furcatus) Males for Tolerance of Low Oxygen. Master’s Thesis, Auburn University, Auburn, AL, USA, 2017. [Google Scholar]
- Johnson, A. Analyses of Texture and Sensory Traits, Carcass Traits, and Fillet Color in Different Genetic Types of Farmed Catfish: Genetic Approaches to Enhance Catfish Fillets. Master’s Thesis, Auburn University, Auburn, AL, USA, 2021. [Google Scholar]
- Falconer, D.S. Introduction to Quantitative Genetics, 3rd ed.; Oliver & Boyd: London, UK, 1989. [Google Scholar]
- Dunham, R.A.; Smitherman, R.O. Ancestry and Breeding of Channel Catfish in the United States; Circular 273; Alabama Agricultural Experiment Station, Auburn University: Auburn, AL, USA, 1984; 100p. [Google Scholar]
- Dunham, R.A.; Masser, M. Production of Hybrid Catfish; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2012; Publication Number, 190. [Google Scholar]
- Su, B.; Perera, D.A.; Zohar, Y.; Abraham, E.; Stubblefield, J.; Fobes, M.; Beam, R.; Argue, B.; Ligeon, C.; Padi, J.; et al. Relative effectiveness of carp pituitary extract, luteinizing hormone releasing hormone analog (LHRHa) injections and LHRHa implants for producing hybrid catfish fry. Aquaculture 2013, 372–375, 133–136. [Google Scholar] [CrossRef]
- Becker, W.A. Manual of Quantitative Genetics; Academic Enterprises: Pullman, WA, USA, 1984. [Google Scholar]
- Jeppsen, T. Comparison of Performance of Channel Catfish, Ictalurus punctatus, Female × Blue Catfish, I. furcatus, Male Hybrids from Kansas Select and Kansas Random Dams. Master’s Thesis, Auburn University, Auburn, AL, USA, 1995. [Google Scholar]
- Makhubu, N.P. Genotype-Environment Interactions for Growth, Survival, Sexual Dimorphism and Seinability for Different Genetic Types of Channel Catfish (Ictalurus punctatus) ♀ × Blue Catfish (I. furcatus) ♂ Cultured in Three Environments. Master’s Thesis, Auburn University, Auburn, AL, USA, 2014. [Google Scholar]
- Alsaqufi, A.S. Approaches to Improve Production and Performance of Channel Catfish (Ictalurus punctatus) Female × Blue Catfish (I. furcatus) Male Hybrid Catfish. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2015. [Google Scholar]
- Chan, M.T. Experience with the determination of realized heritability in the tilapia (Tilapia mossambica). Genetica 1971, 7, 53–59. [Google Scholar]
- Lamkom, T.; Kucuktas, H.; Liu, Z.; Li, P.; Na-Nakorn, U.; Klinbunya, S.; Hutson, A.; Chaimongkol, A.; Ballenger, J.; Umali, G.; et al. Microsatellite variation among domesticated populations of channel (Ictalurus punctatus) and blue catfish (I. furcatus). Kasetsart Univ. Fish. Res. Bull. 2008, 32, 37–47. [Google Scholar]
- Lv, A.-Z.; Zhang, H.; Zhang, Z.-X.; Tao, Y.-S.; Yue, B.; Zheng, Y.-L. Conversion of the statistical combining ability into a genetic concept. J. Integr. Agric. 2012, 11, 43–52. [Google Scholar] [CrossRef]
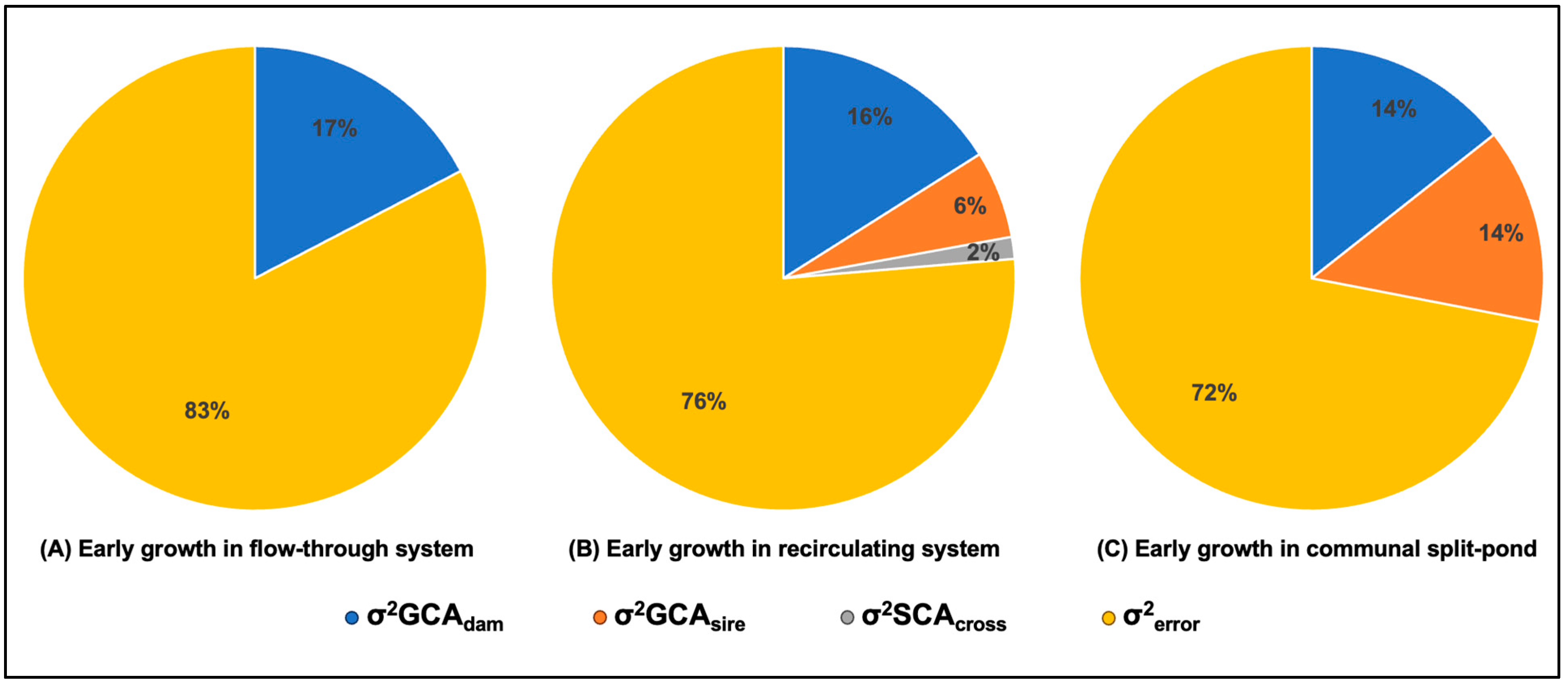
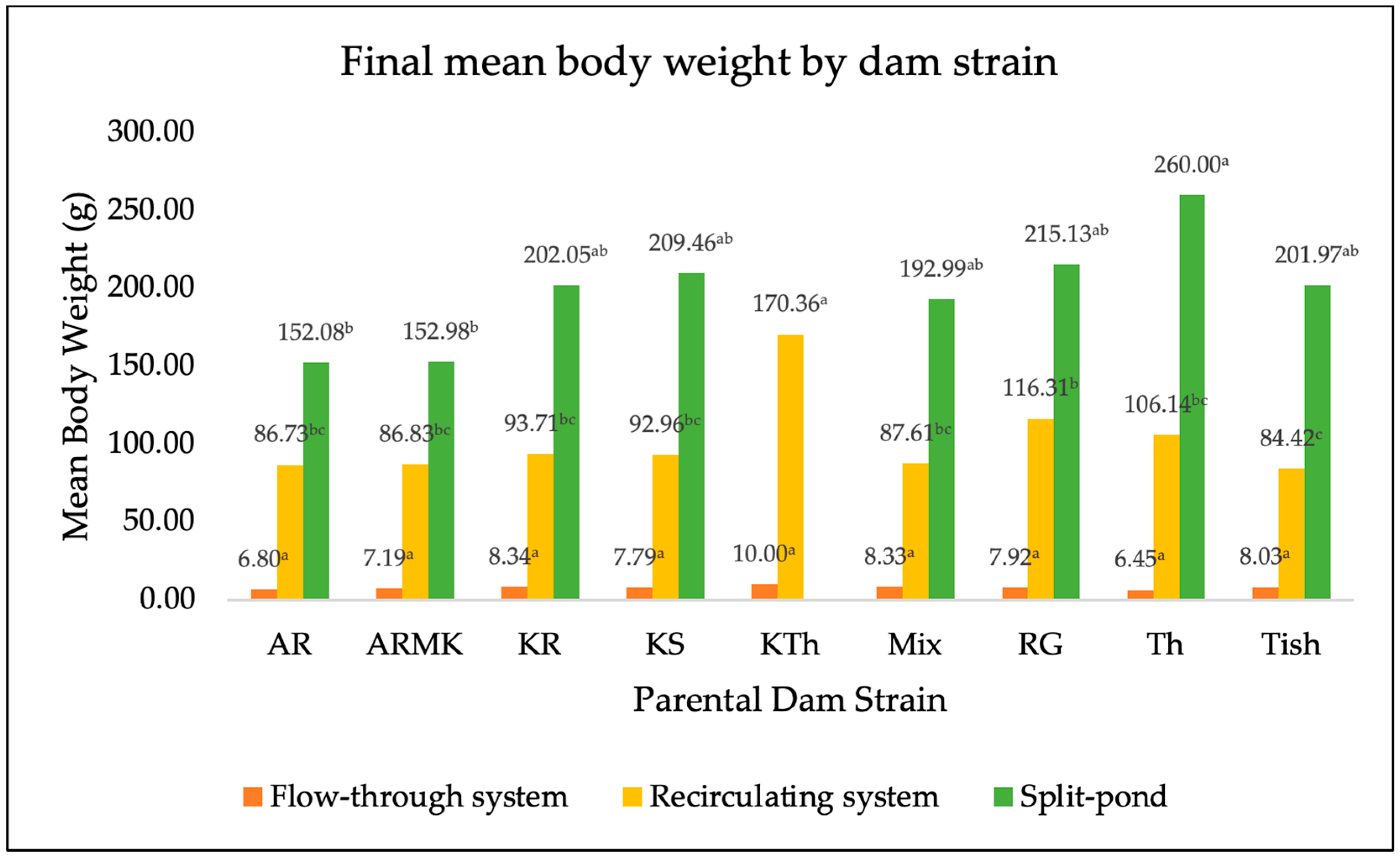
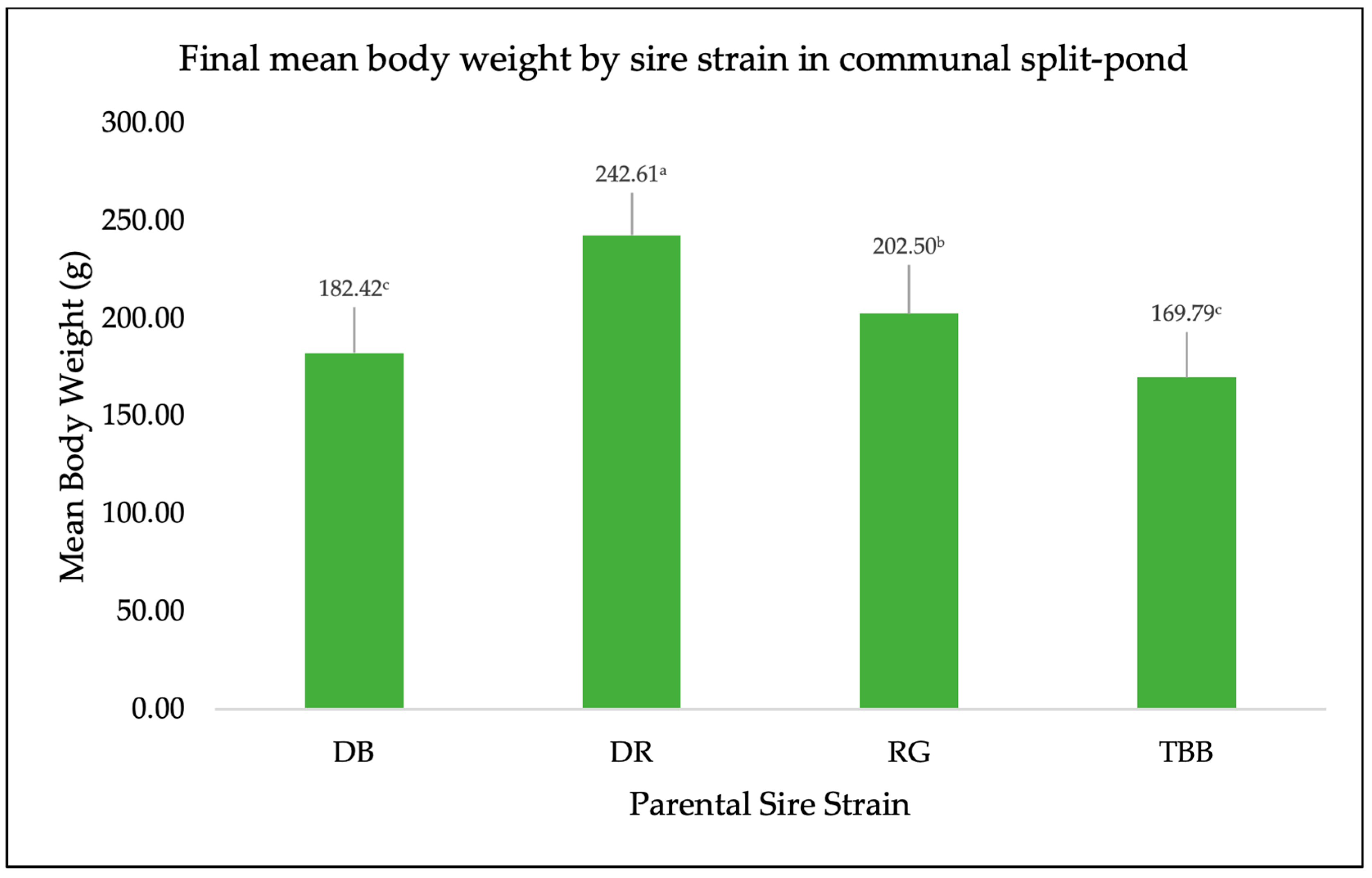
| Rearing System | Age (Days) | Final Mean Body Weight (g) | Phenotypic SD |
|---|---|---|---|
| Flow-through system (initial 311-day separate tank rearing) | 311 | 8.07 g | 1.79 |
| Recirculating system (additional 245-day separate tank rearing) | 556 | 91.25 g | 36.21 |
| Split-pond system (additional 245-day communal rearing) | 556 | 194.21 g | 94.07 |
| Rearing System | Age (Days) | Source of Variation | |||
|---|---|---|---|---|---|
| Dam (d.f.) | Sire (d.f.) | Dam × Sire (d.f.) | Error MS (d.f.) | ||
| Flow-through system (initial 311-day separate tank rearing) | 311 | 4.81 * (19) | 1.72 ns (11) | 3.28 ns (8) | 2.78 (65) |
| Recirculating system (additional 245-day separate tank rearing) | 556 | 5059.56 * (19) | 3028.11 * (11) | 908.11 ns (8) | 1064.29 (677) |
| Split-pond system (additional 245-day communal rearing) | 556 | 66867 * (18) | 85175 * (11) | 7699.88 ns (7) | 7315.98 (1480) |
| Rearing System | Age (Days) | Genetic Parameter Estimates | |||
|---|---|---|---|---|---|
| σ2GCAd (±SE) | σ2GCAs (±SE) | σ2SCA (±SE) | σ2E | ||
| Flow-through system (initial 311-day separate tank rearing) | 311 | 0.57 (0.37) | 0 | 0 | 2.68 |
| Recirculating system (additional 245-day separate tank rearing) | 556 | 222.83 (124.68) | 85.43 (68.21) | 20.7 (56.32) | 1060.16 |
| Split-pond system (additional 245-day communal rearing) | 556 | 1463.21 (587.61) | 1388.25 (663.43) | 0 | 7322.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odin, R.; Elaswad, A.; Khalil, K.; Vo, K.; Backenstose, N.J.C.; Taylor, Z.; Drescher, D.; Bugg, W.S.; Robinson, D.; Gosh, K.; et al. Combining Ability of Female Channel Catfish, Ictalurus punctatus, and Male Blue Catfish, I. furcatus, for Early Growth Performance of Their Progeny. Fishes 2024, 9, 115. https://doi.org/10.3390/fishes9040115
Odin R, Elaswad A, Khalil K, Vo K, Backenstose NJC, Taylor Z, Drescher D, Bugg WS, Robinson D, Gosh K, et al. Combining Ability of Female Channel Catfish, Ictalurus punctatus, and Male Blue Catfish, I. furcatus, for Early Growth Performance of Their Progeny. Fishes. 2024; 9(4):115. https://doi.org/10.3390/fishes9040115
Chicago/Turabian StyleOdin, Ramjie, Ahmed Elaswad, Karim Khalil, Khoi Vo, Nathan J. C. Backenstose, Zachary Taylor, David Drescher, William S. Bugg, Dalton Robinson, Kamal Gosh, and et al. 2024. "Combining Ability of Female Channel Catfish, Ictalurus punctatus, and Male Blue Catfish, I. furcatus, for Early Growth Performance of Their Progeny" Fishes 9, no. 4: 115. https://doi.org/10.3390/fishes9040115
APA StyleOdin, R., Elaswad, A., Khalil, K., Vo, K., Backenstose, N. J. C., Taylor, Z., Drescher, D., Bugg, W. S., Robinson, D., Gosh, K., Ye, Z., Qin, G., Creamer, D., & Dunham, R. (2024). Combining Ability of Female Channel Catfish, Ictalurus punctatus, and Male Blue Catfish, I. furcatus, for Early Growth Performance of Their Progeny. Fishes, 9(4), 115. https://doi.org/10.3390/fishes9040115







