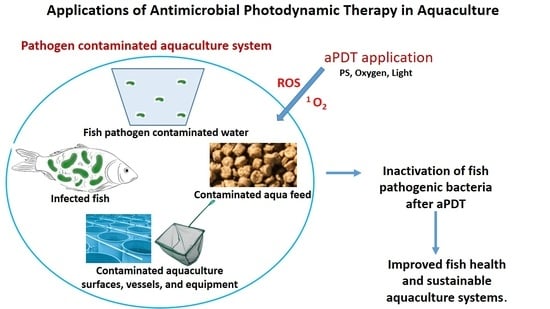
Applications of Antimicrobial Photodynamic Therapy in Aquaculture: Effect on Fish Pathogenic Bacteria
Abstract
1. Introduction
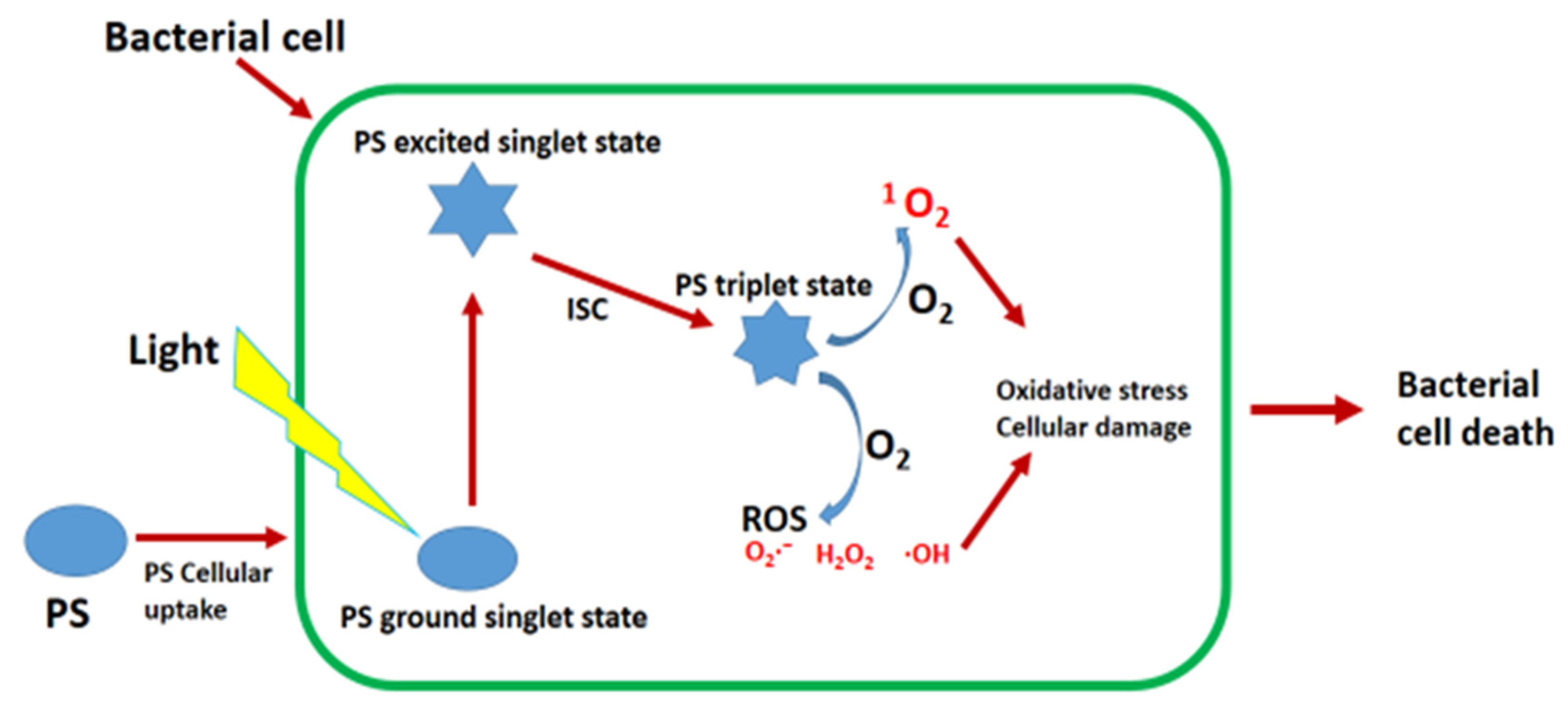
1.1. Antimicrobial Photodynamic Therapy
1.2. Fish Pathogenic Bacteria and the Effect of Their Membrane Structure on PS Uptake and aPDT Activity
2. Photosensitizers Utilized for aPDT against Fish Pathogens
2.1. Use of Porphyrins as PSs against Fish Bacterial Pathogens

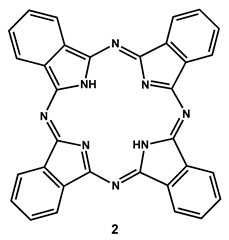
2.2. Use of Phthalocyanines As PSs against Fish Bacterial Pathogens
2.3. Use of Chlorins as PSs against Fish Bacterial Pathogens

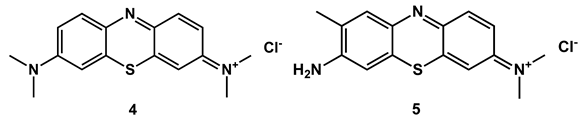
2.4. Use of Methylene Blue and Toluidine Blue as PSs against Fish Bacterial Pathogens
2.5. Use of Rose Bengal and Eosin Y as PSs against Fish Bacterial Pathogens

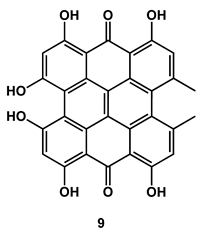
2.6. Natural Occurring PSs Used against Fish Bacterial Pathogens



3. Application of aPDT in Aquaculture
3.1. Treatment of Fish Infections
3.2. Prevention of Fish Infections
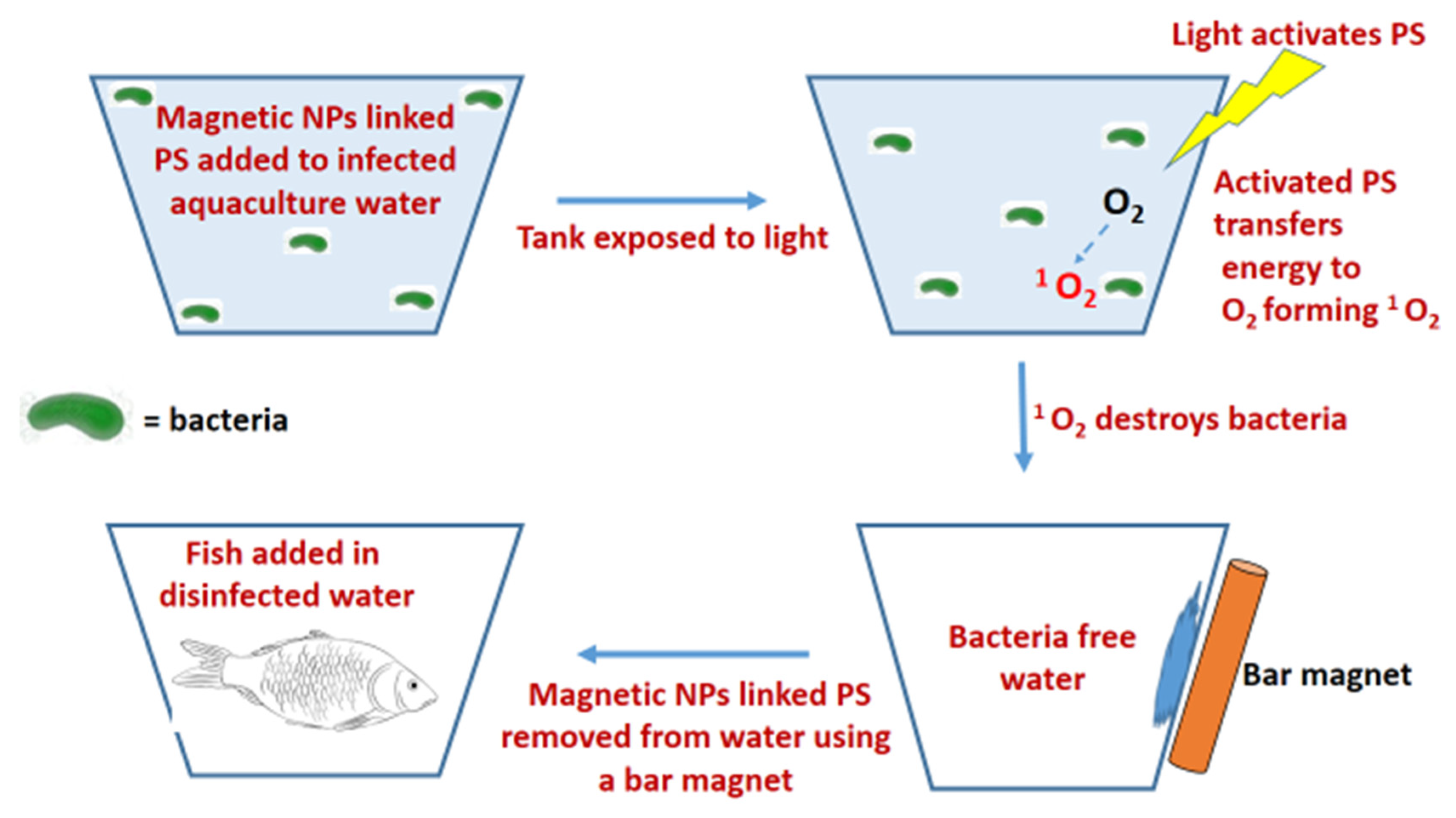
3.2.1. Disinfection of Aquaculture Water
3.2.2. Disinfection of Aquaculture Surfaces, Vessels, and Equipment
3.2.3. Disinfection of Aquaculture Feed
4. Advantages and Limitations of Using aPDT in Aquaculture
4.1. Advantages of aPDT
4.2. Limitations of aPDT
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Froehlich, H.E.; Couture, J.; Falconer, L.; Krause, G.; Morris, J.A.; Perez, M.; Stentiford, G.D.; Vehviläinen, H.; Halpern, B.S. Mind the Gap between ICES Nations’ Future Seafood Consumption and Aquaculture Production. ICES J. Mar. Sci. 2021, 78, 468–477. [Google Scholar] [CrossRef]
- Stephen, J.; Mukherjee, S.; Lekshmi, M.; Kumar, S.H. Diseases and Antimicrobial Use in Aquaculture. In Handbook on Antimicrobial Resistance; Mothadaka, M.P., Vaiyapuri, M., Rao Badireddy, M., Nagarajrao Ravishankar, C., Bhatia, R., Jena, J., Eds.; Springer: Singapore, 2023; pp. 1–23. [Google Scholar]
- Eltholth, M.; Govindaraj, G.; Das, B.; Shanabhoga, M.B.; Swamy, H.M.; Thomas, A.; Cole, J.; Shome, B.R.; Holmes, M.A.; Moran, D. Factors Influencing Antibiotic Prescribing Behavior and Understanding of Antimicrobial Resistance Among Veterinarians in Assam, India. Front. Vet. Sci. 2022, 9, 864813. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Sharma, S.; Sinha, K.K. Evidence of Antibiotic Resistance and Virulence Factors in Environmental Isolates of Vibrio Species. Antibiotics 2023, 12, 1062. [Google Scholar] [CrossRef] [PubMed]
- Adah, D.A.; Saidu, L.; Oniye, S.J.; Adah, A.S.; Daoudu, O.B.; Ola-fadunsin, S.D. Molecular Characterization and Antibiotics Resistance of Aeromonas Species Isolated from Farmed African Catfish Clarias gariepinus Burchell, 1822. BMC Vet. Res. 2024, 20, 16. [Google Scholar] [CrossRef]
- Limbu, S. The Current Status of Antibiotic-Resistant Bacteria and Resistance Genes in African Aquaculture. In Antimicrobial Research and One Health in Africa; Abia, A.L.K., Essack, S., Eds.; Springer: Cham, Switzerland, 2023; pp. 81–106. [Google Scholar]
- March, A.; Failler, P. Small-Scale Fisheries Development in Africa: Lessons Learned and Best Practices for Enhancing Food Security and Livelihoods. Mar. Policy 2022, 136, 104925. [Google Scholar] [CrossRef]
- Hu, X.; Shou, B.; Yang, L.; Li, L.; Ren, H.T.; Lin, J.H.; Lou, C.W.; Li, T.T. Antimicrobial Photodynamic Therapy Encapsulation Technology: Frontier Exploration and Application Prospects of Novel Antimicrobial Technology. Chem. Eng. J. 2023, 477, 146773. [Google Scholar] [CrossRef]
- Dube, E.; Soy, R.; Shumba, M.; Nyokong, T. Photophysicochemical Behaviour of Phenoxy Propanoic Acid Functionalised Zinc Phthalocyanines When Grafted onto Iron Oxide and Silica Nanoparticles: Effects in Photodynamic Antimicrobial Chemotherapy. J. Lumin. 2021, 234, 117939. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The Role of the Light Source in Antimicrobial Photodynamic Therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef]
- Martins Antunes de Melo, W.D.C.; Celiešiūtė-Germanienė, R.; Šimonis, P.; Stirkė, A. Antimicrobial Photodynamic Therapy (APDT) for Biofilm Treatments. Possible Synergy between APDT and Pulsed Electric Fields. Virulence 2021, 12, 2247–2272. [Google Scholar] [CrossRef]
- Alvarez, N.; Sevilla, A. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. Int. J. Mol. Sci 2024, 25, 1023. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, Y.; Li, D.; Pang, Z.; Wang, S.; Chen, H.; Jiang, M.; Yan, H.; Li, T.; Fu, H.; et al. 5-Aminolevulinic Acid-Photodynamic Therapy Ameliorates Cutaneous Granuloma by Killing Drug-Resistant Mycobacterium Marinum. Photodiagn. Photodyn. Ther. 2022, 38, 102839. [Google Scholar] [CrossRef]
- da Silva Canielles Caprara, C.; da Silva Freitas, L.; Iglesias, B.A.; Ferreira, L.B.; Ramos, D.F. Charge Effect of Water-Soluble Porphyrin Derivatives as a Prototype to Fight Infections Caused by Acinetobacter baumannii by APDT Approaches. Biofouling 2022, 38, 605–613. [Google Scholar] [CrossRef]
- Jornada, D.C.; de Queiroz Garcia, R.; da Silveira, C.H.; Misoguti, L.; Mendonça, C.R.; Santos, R.C.V.; De Boni, L.; Iglesias, B.A. Investigation of the Triplet Excited State and Application of Cationic Meso-Tetra(Cisplatin)Porphyrins in Antimicrobial Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2021, 35, 102459. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Xu, Z.P.; Ma, Y.Y.; Ma, J.D.; Hong, G. Photodynamic Antimicrobial Chemotherapy in Mice with Pseudomonas aeruginosa-Infected Wounds. PLoS ONE 2020, 15, e0237851. [Google Scholar] [CrossRef]
- Clifton, L.A.; Skoda, M.W.A.; Le Brun, A.P.; Ciesielski, F.; Kuzmenko, I.; Holt, S.A.; Lakey, J.H. Effect of Divalent Cation Removal on the Structure of Gram-Negative Bacterial Outer Membrane Models. Langmuir 2015, 31, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lin, S.; Zhang, J.; Kong, Z.; Tan, B.K.; Hamzah, S.S.; Hu, J. Enhancement of Photodynamic Bactericidal Activity of Curcumin against Pseudomonas aeruginosa Using Polymyxin B. Photodiagn. Photodyn. Ther. 2022, 37, 102677. [Google Scholar] [CrossRef] [PubMed]
- Fabio, G.B.; Martin, B.A.; Dalmolin, L.F.; Lopez, R.F.V. Antimicrobial Photodynamic Therapy and the Advances Impacted by the Association with Nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 80, 104147. [Google Scholar] [CrossRef]
- Rupel, K.; Zupin, L.; Brich, S.; Mardirossian, M.; Ottaviani, G.; Gobbo, M.; Di Lenarda, R.; Pricl, S.; Crovella, S.; Zacchigna, S.; et al. Antimicrobial Activity of Amphiphilic Nanomicelles Loaded with Curcumin against Pseudomonas aeruginosa Alone and Activated by Blue Laser Light. J. Biophotonics 2021, 14, e202000350. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.W.; Kim, Y.K.; An, J.L.; Kim, J.S.; Kwon, P.S.; Yu, Y.B. The Effect of Photodynamic Therapy Using Radachlorin on Biofilm-Forming Multidrug-Resistant Bacteria. Osong Public Health Res. Perspect. 2022, 13, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Anane, Y.A.; Apalata, T.; Vasaikar, S.; Okuthe, G.E.; Songca, S.P. In Vitro Antimicrobial Photodynamic Inactivation of Multidrug-Resistant Acinetobacter baumannii Biofilm Using Protoporphyrin IX and Methylene Blue. Photodiagn. Photodyn. Ther. 2020, 30, 101752. [Google Scholar] [CrossRef] [PubMed]
- Babaeekhou, L.; Ghane, M.; Mohammad Rafiee, M. Photodynamic Therapy and Its Synergism with Melittin Against Drug-Resistant Acinetobacter baumannii Isolates with High Biofilm Formation Ability. Curr. Microbiol. 2023, 80, 324. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Vyčaitė, E.; Badokas, K.; Sužiedelienė, E.; Bagdonas, S. Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms. Int. J. Mol. Sci. 2023, 24, 722. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Klimkaitė, L.; Sužiedėlienė, E.; Bagdonas, S. Inactivation of Opportunistic Pathogens Acinetobacter baumannii and Stenotrophomonas maltophilia by Antimicrobial Photodynamic Therapy. Microorganisms 2022, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Boluki, E.; Pourhajibagher, M.; Bahador, A. The Combination of Antimicrobial Photocatalysis and Antimicrobial Photodynamic Therapy to Eradicate the Extensively Drug-Resistant Colistin Resistant Acinetobacter baumannii. Photodiagn. Photodyn. Ther. 2020, 31, 101816. [Google Scholar] [CrossRef]
- Liu, W.; Gu, H.; Ran, B.; Liu, W.; Sun, W.; Wang, D.; Du, J.; Fan, J.; Peng, X. Accelerated Antibacterial Red-Carbon Dots with Photodynamic Therapy against Multidrug-Resistant Acinetobacter baumannii. Sci. China Mater. 2022, 65, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Geng, S.; Wang, X.; Cui, Z.; Ma, W.; Yuan, M.; Liu, C.; Ji, Y. Aloe-Emodin-Mediated Antimicrobial Photodynamic Therapy against Multidrug-Resistant Acinetobacter baumannii: An in Vivo Study. Photodiagn. Photodyn. Ther. 2021, 34, 102311. [Google Scholar] [CrossRef]
- Figueiredo-Godoi, L.M.A.; Garcia, M.T.; Pinto, J.G.; Ferreira-Strixino, J.; Faustino, E.G.; Pedroso, L.L.C.; Junqueira, J.C. Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii. Antibiotics 2022, 11, 619. [Google Scholar] [CrossRef]
- Fekrirad, Z.; Darabpour, E.; Kashef, N. Eradication of Acinetobacter baumannii Planktonic and Biofilm Cells Through Erythrosine-Mediated Photodynamic Inactivation Augmented by Acetic Acid and Chitosan. Curr. Microbiol. 2021, 78, 879–886. [Google Scholar] [CrossRef]
- Niu, P.; Dai, J.; Wang, Z.; Wang, Y.; Feng, D.; Li, Y.; Miao, W. Sensitization of Antibiotic-Resistant Gram-Negative Bacteria to Photodynamic Therapy via Perfluorocarbon Nanoemulsion. Pharmaceuticals 2022, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, Z.Q.; Jin, H.; Sun, S.; Liu, T.J.; Wang, X.; Fan, H.J.; Hou, S.K.; Ding, H. Photodynamic Antimicrobial Chemotherapy with the Novel in Vitro and in Vivo Studies. PLoS ONE 2017, 12, e0176529. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Hosseini, N.; Bahador, A. Antimicrobial Activity of D-Amino Acid in Combination with Photo-Sonoactivated Hypericin Nanoparticles against Acinetobacter baumannii. BMC Microbiol. 2023, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Arrojado, C.; Pereira, C.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Calado, R.; Gomes, N.C.M.; et al. Applicability of Photodynamic Antimicrobial Chemotherapy as an Alternative to Inactivate Fish Pathogenic Bacteria in Aquaculture Systems. Photochem. Photobiol. Sci. 2011, 10, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, S.; Shiroodi, S.; Schwarz, M.H.; Nitin, N.; Ovissipour, R. Inactivation of Aeromonas hydrophila and Vibrio parahaemolyticus by Curcumin-Mediated Photosensitization and Nanobubble-Ultrasonication Approaches. Foods 2020, 9, 1306. [Google Scholar] [CrossRef] [PubMed]
- Mantareva, V.N.; Kussovski, V.; Orozova, P.; Dimitrova, L.; Kulu, I.; Angelov, I.; Durmus, M.; Najdenski, H. Photodynamic Inactivation of Antibiotic-Resistant and Sensitive Aeromonas hydrophila with Peripheral Pd(II)- vs. Zn(II)-Phthalocyanines. Biomedicines 2022, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Kulu, I.; Mantareva, V.; Kussovski, V.; Angelov, I.; Durmuş, M. Effects of Metal Ion in Cationic Pd(II) and Ni(II) Phthalocyanines on Physicochemical and Photodynamic Inactivation Properties. J. Mol. Struct. 2022, 1247, 131288. [Google Scholar] [CrossRef]
- Kussovski, V.; Mantareva, V.; Angelov, I.; Orozova, P.; Wöhrle, D.; Schnurpfeil, G.; Borisova, E.; Avramov, L. Photodynamic Inactivation of Aeromonas hydrophila by Cationic Phthalocyanines with Different Hydrophobicity. FEMS Microbiol. Lett. 2009, 294, 133–140. [Google Scholar] [CrossRef]
- Mantareva, V.; Kussovski, V.; Orozova, P.; Angelov, I.; Durmuş, M.; Najdenski, H. Palladium Phthalocyanines Varying in Substituents Position for Photodynamic Inactivation of Flavobacterium hydatis as Sensitive and Resistant Species. Curr. Issues Mol. Biol. 2022, 44, 1950–1959. [Google Scholar] [CrossRef]
- Anju, V.T.; Paramanantham, P.; S.B, S.L.; Sharan, A.; Syed, A.; Bahkali, N.A.; Alsaedi, M.H.; Kaviyarasue, K.; Busi, S. Antimicrobial Photodynamic Activity of Toluidine Blue-Carbon Nanotube Conjugate against Pseudomonas aeruginosa and Staphylococcus aureus—Understanding the Mechanism of Action. Photodiagn. Photodyn. Ther. 2019, 27, 305–316. [Google Scholar] [CrossRef]
- Kim, J.; Lim, H. Effect of Antimicrobial Photodynamic Therapy with Radachlorin and a 660 Nm Diode Laser on Pseudomonas aeruginosa: An in Vitro Study. Photodiagn. Photodyn. Ther. 2020, 31, 101931. [Google Scholar] [CrossRef]
- Motallebi, M.; Khorsandi, K.; Sepahy, A.A.; Chamani, E.; Hosseinzadeh, R. Effect of Rutin as Flavonoid Compound on Photodynamic Inactivation against P. aeruginosa and S. aureus. Photodiagn. Photodyn. Ther. 2020, 32, 102074. [Google Scholar] [CrossRef]
- Hampden-Martin, A.; Fothergill, J.; El Mohtadi, M.; Chambers, L.; Slate, A.J.; Whitehead, K.A.; Shokrollahi, K. Photodynamic Antimicrobial Chemotherapy Coupled with the Use of the Photosensitizers Methylene Blue and Temoporfin as a Potential Novel Treatment for Staphylococcus aureus in Burn Infections. Access Microbiol. 2021, 3, 000273. [Google Scholar] [CrossRef]
- Ucuncu, M.; Mills, B.; Duncan, S.; Staderini, M.; Dhaliwal, K.; Bradley, M. Polymyxin-Based Photosensitizer for the Potent and Selective Killing of Gram-Negative Bacteria. Chem. Commun. 2020, 56, 3757–3760. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, A.; Rastegari, B.; Sharifi, H.; Khajeh-Zadeh, H.; Moayedi, J. The Effectiveness of Antimicrobial Photodynamic Therapy with Prodigiosin against Reference Strains of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Lasers Med. Sci. 2022, 37, 3631–3638. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Khorsandi, K.; Kianmehr, Z. Photodynamic Inactivation with Curcumin and Silver Nanoparticles Hinders Pseudomonas aeruginosa Planktonic and Biofilm Formation: Evaluation of Glutathione Peroxidase Activity and ROS Production. World J. Microbiol. Biotechnol. 2021, 37, 149. [Google Scholar] [CrossRef] [PubMed]
- Ilizirov, Y.; Formanovsky, A.; Mikhura, I.; Paitan, Y.; Nakonechny, F.; Nisnevitch, M. Effect of Photodynamic Antibacterial Chemotherapy Combined with Antibiotics on Gram-Positive and Gram-Negative Bacteria. Molecules 2018, 23, 3152. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, N.; Özdemir, S.; Tollu, G.; Altuntaş Bayır, Z.; Burkut Koçak, M. Biological Properties of Hexadeca-Substituted Metal Phthalocyanines Bearing Different Functional Groups. J. Inorg. Biochem. 2022, 234, 111888. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, I.; Kałas, W.; Wysokińska, E.; Tylus, W.; Pietrzyk, N.; Popko, K.; Palewska, K. Enhancement of Photo-Bactericidal Effect of Tetrasulfonated Hydroxyaluminum Phthalocyanine on Pseudomonas aeruginosa. Lasers Med. Sci. 2018, 33, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bayat, F.; Karimi, A.R. Design of Photodynamic Chitosan Hydrogels Bearing Phthalocyanine-Colistin Conjugate as an Antibacterial Agent. Int. J. Biol. Macromol. 2019, 129, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.S.; Seeger, M.G.; Moreira, K.S.; Burgo, T.A.L.; Iglesias, B.A.; Vogel, F.S.F.; Cargnelutti, J.F. In Vitro Porphyrin-Based Photodynamic Therapy against Mono and Polyculture of Multidrug-Resistant Bacteria Isolated from Integumentary Infections in Animals. Photodiagn. Photodyn. Ther. 2022, 40, 103179. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, J.; Wang, Y.; Xu, Z.; Zhao, L.; Zhao, J.; Hong, G.; Liu, T. Antimicrobial Photodynamic Therapy Combined with Antibiotic in the Treatment of Rats with Third-Degree Burns. Front. Microbiol. 2021, 12, 622410. [Google Scholar] [CrossRef]
- Seeger, M.G.; Ries, A.S.; Gressler, L.T.; Botton, S.A.; Iglesias, B.A.; Cargnelutti, J.F. In Vitro Antimicrobial Photodynamic Therapy Using Tetra-Cationic Porphyrins against Multidrug-Resistant Bacteria Isolated from Canine Otitis. Photodiagn. Photodyn. Ther. 2020, 32, 101982. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, Y.; Meng, S.; Yang, B.; Pang, L.; Wang, C. Mechanism and In Vivo Evaluation: Photodynamic Antibacterial Chemotherapy of Lysine-Porphyrin Conjugate. Front. Microbiol. 2016, 7, 242. [Google Scholar] [CrossRef]
- Malá, Z.; Žárská, L.; Malina, L.; Langová, K.; Večeřová, R.; Kolář, M.; Henke, P.; Mosinger, J.; Kolářová, H. Photodynamic Effect of TPP Encapsulated in Polystyrene Nanoparticles toward Multi-Resistant Pathogenic Bacterial Strains: AFM Evaluation. Sci. Rep. 2021, 11, 6786. [Google Scholar] [CrossRef]
- Liu, C.; Hu, M.; Ma, D.; Lei, J.; Xu, J. Photodynamic Inactivation of Antibiotic-Resistant Bacteria and Biofilms by Hematoporphyrin Monomethyl Ether. Lasers Med. Sci. 2016, 31, 297–304. [Google Scholar] [CrossRef]
- Maldonado-Carmona, N.; Marchand, G.; Villandier, N.; Ouk, T.S.; Pereira, M.M.; Calvete, M.J.F.; Calliste, C.A.; Żak, A.; Piksa, M.; Pawlik, K.J.; et al. Porphyrin-Loaded Lignin Nanoparticles against Bacteria: A Photodynamic Antimicrobial Chemotherapy Application. Front. Microbiol. 2020, 11, 606185. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, K.; Chikama, T.; Latief, M.A.; Ko, J.A.; Kiuchi, Y.; Sakaguchi, T.; Obana, A. Time-Dependent Antimicrobial Effect of Photodynamic Therapy with TONS 504 on Pseudomonas aeruginosa. Lasers Med. Sci. 2018, 33, 1455–1460. [Google Scholar] [CrossRef]
- Tiganova, I.G.; Zhizhimova, Y.S.; Philipova, N.I.; Tolordava, E.R.; Alekseeva, N.V.; Makarova, E.A.; Lukyanets, E.A.; Meerovich, G.A.; Romanova, Y.M.; Gintsburg, A.L. Antibacterial Properties of Synthetic Cationic Bacteriochlorin Derivatives as Photosensitizers. Mol. Genet. Microbiol. Virol. 2020, 35, 248–256. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Caruso, E. Searching for Antimicrobial Photosensitizers among a Panel of BODIPYs. Photochem. Photobiol. Sci. 2022, 21, 1233–1248. [Google Scholar] [CrossRef]
- Kirar, S.; Thakur, N.S.; Laha, J.K.; Banerjee, U.C. Porphyrin Functionalized Gelatin Nanoparticle-Based Biodegradable Phototheranostics: Potential Tools for Antimicrobial Photodynamic Therapy. CS Appl. Bio Mater. 2019, 2, 4202–4212. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.S.; Bhaumik, J.; Kirar, S.; Banerjee, U.C. Development of Gold-Based Phototheranostic Nanoagents through a Bioinspired Route and Their Applications in Photodynamic Therapy. ACS Sustain. Chem. Eng. 2017, 5, 7950–7960. [Google Scholar] [CrossRef]
- Kirar, S.; Thakur, N.S.; Laha, J.K.; Bhaumik, J.; Banerjee, U.C. Development of Gelatin Nanoparticle-Based Biodegradable Phototheranostic Agents: Advanced System to Treat Infectious Diseases Seema. ACS Biomater. Sci. Eng. 2018, 4, 473–482. [Google Scholar] [CrossRef]
- Notaro, D.A.; Culloty, S.C.; Lynch, S.A. A Pilot Study Investigating the Potential of Antimicrobial Photodynamic Therapy (APDT) to Control Vibrio Spp. Development in Microalgae and Seawater. Aquac. Int. 2021, 29, 355–372. [Google Scholar] [CrossRef]
- Dong, D.; Shaoling, L.; Chongzhen, S.; Wenqi, D.; Xiyang, W.; Shuze, T. Inactivation of Curcumin Photodynamic Technology on Grimontia hollisae and Vibrio alginolyticus in Aquatic Food. J. Chin. Inst. Food Sci. Technol. 2022, 22, 40–48. [Google Scholar]
- Malara, D.; Høj, L.; Oelgemöller, M.; Malerba, M.; Citarrella, G.; Heimann, K. Sensitivity of Live Microalgal Aquaculture Feed to Singlet Oxygen-Based Photodynamic Therapy. J. Appl. Phycol. 2019, 31, 3593–3606. [Google Scholar] [CrossRef]
- Malara, D.; Hoj, L.; Heimann, K.; Citarrella, G.; Oelgemöller, M. Capacity of Cationic and Anionic Porphyrins to Inactivate the Potential Aquaculture Pathogen Vibrio campbellii. Aquaculture 2017, 473, 228–236. [Google Scholar] [CrossRef]
- Moideen, S.K.; Anas, A.; Sobhanan, J.; Zhao, H.; Biju, V. Photoeradication of Aquatic Pathogens by Curcumin for Clean and Safe Drinking Water. J. Photochem. Photobiol. A Chem. 2022, 432, 114104. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Photodynamic Antimicrobial Chemotherapy in Aquaculture: Photoinactivation Studies of Vibrio fischeri. PLoS ONE 2011, 6, e20970. [Google Scholar] [CrossRef]
- Asok, A.; Arshad, E.; Jasmin, C.; Somnath Pai, S.; Bright Singh, I.S.; Mohandas, A.; Anas, A. Reducing Vibrio Load in Artemia nauplii Using Antimicrobial Photodynamic Therapy: A Promising Strategy to Reduce Antibiotic Application in Shrimp Larviculture. Microb. Biotechnol. 2012, 5, 59–68. [Google Scholar] [CrossRef]
- Deng, X.; Tang, S.; Wu, Q.; Tian, J.; Riley, W.W.; Chen, Z. Inactivation of Vibrio parahaemolyticus by Antimicrobial Photodynamic Technology Using Methylene Blue. J. Sci. Food Agric. 2016, 96, 1601–1608. [Google Scholar] [CrossRef]
- Chen, B.; Huang, J.; Li, H.; Zeng, Q.H.; Wang, J.J.; Liu, H.; Pan, Y.; Zhao, Y. Eradication of Planktonic Vibrio parahaemolyticus and Its Sessile Biofilm by Curcumin-Mediated Photodynamic Inactivation. Food Control. 2020, 113, 107181. [Google Scholar] [CrossRef]
- Malara, D.; Mielke, C.; Oelgemöller, M.; Senge, M.O.; Heimann, K. Sustainable Water Treatment in Aquaculture—Photolysis and Photodynamic Therapy for the Inactivation of Vibrio Species. Aquac. Res. 2017, 48, 2954–2962. [Google Scholar] [CrossRef]
- Wong, T.W.; Wang, Y.Y.; Sheu, H.M.; Chuang, Y.C. Bactericidal Effects of Toluidine Blue-Mediated Photodynamic Action on Vibrio vulnificus. Antimicrob. Agents Chemother. 2005, 49, 895–902. [Google Scholar] [CrossRef]
- Levine, H.; Sepulveda-Beltran, P.A.; Amescua, G. Rose Bengal Photodynamic Antimicrobial Therapy as Potential Adjuvant Treatment for Serratia marcescens Corneal Ulcer. Am. J. Ophthalmol. 2021, 231, e1–e2. [Google Scholar] [CrossRef]
- Parente, T.M.A.L.; de Lima Rebouças, E.; dos Santos, V.C.V.; Barbosa, F.C.B.; Zanin, I.C.J. Serratia marcescens Resistance Profile and Its Susceptibility to Photodynamic Antimicrobial Chemotherapy. Photodiagn. Photodyn. Ther. 2016, 14, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Fekrirad, Z.; Kashef, N.; Arefian, E. Photodynamic Inactivation Diminishes Quorum Sensing-Mediated Virulence Factor Production and Biofilm Formation of Serratia marcescens. World J. Microbiol. Biotechnol. 2019, 35, 191. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yao, Q.; Cheng, W.; Lu, Y.; Zhang, T.; Ren, H. The Combination of Photodynamic Therapy and Fractional CO2 Laser for Mycobacterium Marinum Infection. Photodiagn. Photodyn. Ther. 2021, 35, 102391. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Buccafurni, L.; Battini, V.; Zazzaron, S.; Barbieri, P.; Orlandi, V. Antibacterial Activity of Tetraaryl-Porphyrin Photosensitizers: An in Vitro Study on Gram Negative and Gram Positive Bacteria. J. Photochem. Photobiol. B Biol. 2006, 85, 28–38. [Google Scholar] [CrossRef]
- Oluwole, D.O.; Báthori, N.B. Multicomponent Crystals of Phthalocyanines–A Possibility of Fine-Tuning Properties. Colorants 2023, 2, 405–425. [Google Scholar] [CrossRef]
- Spesia, M.B.; Durantini, E.N. Evolution of Phthalocyanine Structures as Photodynamic Agents for Bacteria Inactivation. Chem. Rec. 2022, 22, e202100292. [Google Scholar] [CrossRef]
- Günsel, A.; Bilgiçli, A.T.; Kandemir, C.; Sancak, R.; Arabaci, G.; Nilüfer Yarasir, M. Comparison of Novel Tetra-Substituted Phthalocyanines with Their Quaternized Derivatives: Antioxidant and Antibacterial Properties. Synth. Met. 2020, 260, 116288. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Estevão, B.M.; Pellosi, D.S.; Scarminio, I.S.; Caetano, W.; Hioka, N.; Batistela, V.R. Chemical Equilibria of Eosin Y and Its Synthetic Ester Derivatives in Non-Ionic and Ionic Micellar Environments. J. Mol. Liq. 2021, 327, 114794. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a Photosensitizer: From Molecular Structure to Recent Advances in Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Peng, Z.; Lu, J.; Liu, K.; Xie, L.; Wang, Y.; Cai, C.; Yang, D.; Xi, J.; Yan, C.; Li, X.; et al. Hypericin as a Promising Natural Bioactive Naphthodianthrone: A Review of Its Pharmacology, Pharmacokinetics, Toxicity, and Safety. Phyther. Res. 2023, 37, 5639–5656. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Meng, P.S.; Zhang, H.C.; Liu, X.; Wang, M.X.; Cao, W.W.; Hu, Z.; Zhang, Z.G. Inhibitory Effect of Aloe Emodin Mediated Photodynamic Therapy on Human Oral Mucosa Carcinoma In Vitro and In Vivo. Biomed. Pharmacother. 2018, 97, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Insińska-Rak, M.; Sikorski, M.; Wolnicka-Glubisz, A. Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells 2023, 12, 2304. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic Natural Products as Photosensitizers for Antimicrobial Photodynamic Therapy: Recent Advances and Future Prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef] [PubMed]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Dharmaratne, P.; Sapugahawatte, D.N.; Wang, B.; Chan, C.L.; Lau, K.M.; Lau, C.; Fung, K.P.; Ng, D.K.; Ip, M. Contemporary Approaches and Future Perspectives of Antibacterial Photodynamic Therapy (APDT) against Methicillin-Resistant Staphylococcus aureus (MRSA): A Systematic Review. Eur. J. Med. Chem. 2020, 200, 112341. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential Applications of Porphyrins in Photodynamic Inactivation beyond the Medical Scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef]
- Admasu, F.; Wakjira, M. Non-Infectious Diseases and Biosecurity Management Practices of Fishes Health in Aquaculture. J. Fish. Sci. 2021, 15, 10182. [Google Scholar]
- Gnanasekar, S.; Kasi, G.; He, X.; Zhang, K.; Xu, L.; Kang, E.T. Recent Advances in Engineered Polymeric Materials for Efficient Photodynamic Inactivation of Bacterial Pathogens. Bioact. Mater. 2023, 21, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Kaplan, M.; Jensen, G.J.; Scheidt, F.R.; Oliveira, E.M.; Suzuki, S.S. Effects of Antimicrobial Photodynamic Therapy on Antibiotic-Resistant Escherichia coli. Photodiagn. Photodyn. Ther. 2020, 32, 102029. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Rodrigues, J.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Lin, Z.; Cunha, Â.; Nadais, M.H.; Tomé, J.P.C.; Almeida, A. A New Insight on Nanomagnet-Porphyrin Hybrids for Photodynamic Inactivation of Microorganisms. Dyes Pigments 2014, 110, 80–88. [Google Scholar] [CrossRef]
- Powell, C.D.; Atkinson, A.J.; Ma, Y.; Marcos-Hernandez, M.; Villagran, D.; Westerhoff, P.; Wong, M.S. Magnetic Nanoparticle Recovery Device (MagNERD) Enables Application of Iron Oxide Nanoparticles for Water Treatment. J. Nanopart. Res. 2020, 22, 48. [Google Scholar] [CrossRef]
- Dias, L.D.; Mfouo-Tynga, I.S. Learning from Nature: Bioinspired Chlorin-Based Photosensitizers Immobilized on Carbon Materials for Combined Photodynamic and Photothermal Therapy. Biomimetics 2020, 5, 53. [Google Scholar] [CrossRef]
- Majiya, H.; Chowdhury, K.F.; Stonehouse, N.J.; Millner, P. TMPyP Functionalised Chitosan Membrane for Efficient Sunlight Driven Water Disinfection. J. Water Process Eng. 2019, 30, 100475. [Google Scholar] [CrossRef]
- Foggiato, A. Photodynamic Therapy Used to Reduce Microbial Contamination and Disinfection on Solid Surfaces of Diverse Materials in a Sustainable and Ecological Way. J. Ecosyst. Ecography 2019, 9, 18–19. [Google Scholar]
- George, L.; Müller, A.; Röder, B.; Santala, V.; Efimov, A. Photodynamic Self–Disinfecting Surface Using Pyridinium Phthalocyanine. Dyes Pigments 2017, 147, 334–342. [Google Scholar] [CrossRef]
- Harada, N.; Masuda, K.; Nakamura, J.I.; Uyama, H. Fabrication and Evaluation of Durable, Optically Clear, and Self-Disinfecting Films. Polym. J. 2021, 53, 1383–1391. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, L. The Microbial Safety of Fish and Fish Products: Recent Advances in Understanding Its Significance, Contamination Sources, and Control Strategies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 738–786. [Google Scholar] [CrossRef] [PubMed]
- Garapati, C.; Boddu, S.H.; Jacob, S.; Ranch, K.M.; Patel, C.; Babu, R.J.; Tiwari, A.K.; Yasin, H. Photodynamic Therapy: A Special Emphasis on Nanocarrier-Mediated Delivery of Photosensitizers in Antimicrobial Therapy. Arab. J. Chem. 2023, 16, 104583. [Google Scholar] [CrossRef]
- Piksa, M.; Fortuna, W.; Lian, C.; Gacka, M.; Samuel, I.D.W.; Matczyszyn, K.; Pawlik, K.J. Treatment of Antibiotic-Resistant Bacteria Colonizing Diabetic Foot Ulcers by OLED Induced Antimicrobial Photodynamic Therapy. Sci. Rep. 2023, 13, 14087. [Google Scholar] [CrossRef]
- Leanse, L.G.; Marasini, S.; dos Anjos, C.; Dai, T. Antimicrobial Resistance: Is There a ‘Light’ at the End of the Tunnel? Antibiotics 2023, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic Disinfection and Its Role in Controlling Infectious Diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; Truscott, T.G. The Reactive Oxygen Species Singlet Oxygen, Hydroxy Radicals, and the Superoxide Radical Anion—Examples of Their Roles in Biology and Medicine. Oxygen 2021, 1, 77–95. [Google Scholar] [CrossRef]
- Wylie, M.P.; Irwin, N.J.; Howard, D.; Heydon, K.; McCoy, C.P. Hot-Melt Extrusion of Photodynamic Antimicrobial Polymers for Prevention of Microbial Contamination. J. Photochem. Photobiol. B Biol. 2021, 214, 112098. [Google Scholar] [CrossRef]
- Gideskog, M.; Falkeborn, T.; Welander, J.; Melhus, Å. Source Control of Gram-Negative Bacteria Using Self-Disinfecting Sinks in a Swedish Burn Centre. Microorganisms 2023, 11, 965. [Google Scholar] [CrossRef]
- Spagnul, C.; Turner, L.C.; Boyle, R.W. Immobilized Photosensitizers for Antimicrobial Applications. J. Photochem. Photobiol. B Biol. 2015, 150, 11–30. [Google Scholar] [CrossRef]
- Gutberlet, B.; Preis, E.; Roschenko, V.; Bakowsky, U. Photothermally Controlled Drug Release of Poly(d,l-Lactide) Nanofibers Loaded with Indocyanine Green and Curcumin for Efficient Antimicrobial Photodynamic Therapy. Pharmaceutics 2023, 15, 327. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.-C.; Lin, J.-H.; Zhang, S.; Lou, C.-W.; Li, T.-T. Synergistic Antibacterial Strategy Based on Photodynamic Therapy: Progress and Perspectives. Chem. Eng. J. 2022, 450, 138129. [Google Scholar] [CrossRef]
- Mohamad, F.; Alzahrani, R.R.; Alsaadi, A.; Alrfaei, B.M.; Yassin, A.E.B.; Alkhulaifi, M.M.; Halwani, M. An Explorative Review on Advanced Approaches to Overcome Bacterial Resistance by Curbing Bacterial Biofilm Formation. Infect. Drug Resist. 2023, 16, 19–49. [Google Scholar] [CrossRef] [PubMed]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]

| Pathogenic Bacteria | Bacteria Type | PSs Reported against Fish Pathogens | aPDT Disinfected Areas |
|---|---|---|---|
| Acinetobacter baumannii | Gn | Radachlorin [22], protoporphyrin IX [23] and Methylene blue [23,24], riboflavin, chlorophyllin [25,26], Toluidine blue O combined with TiO2 and ZnO nanoparticles [27], red-carbon dots [28], aloe-emodin [29], fotoenticine [30], erythrosine [31], Chlorin e6 and Perfluorodecalin nanoemulsion [32], meso-tetra(4-N-methyl-pyridyl)porphyrin [15], 5, 10, 15, 20-trakis(4-((s)-2, 6-diaminohexanamido)-phenyl) porphyrin [33], hypericin nanoparticles combined with D-Tryptophan [34]. | * |
| Aeromonas salmonicida | Gn | 5,10,15-tris(1-methylpyridinium4-yl)-20-pentafluorophenylporphyrin tri-iodide (Tri-Py+-Me-PF) [35]. | Aquaculture water [35] |
| Aeromonas hydrophila | Gn | Curcumin [36], 2,(3),9(10),16(17),23(24)-Tetrakis-[(2-pyridyloxy) hthalocyaninato] Palladium (II) and 2,(3),9(10),16(17),13(24)-Tetrakis-{[(2-(N-methyl)pyridyloxy]phthalocyaninato} Palladium (II) Sulphate [37], 1(4),8(11),15(18),22(25)-Tetrakis-[(2-pyridyloxy)phthalocyaninato]nickel (II) [38], tetrakis-(3-methylpyridyloxy-phthalocyanine Zn(II) [39]. | * |
| Flavobacterium hydatis | Gn | Palladium phthalocyanines with methylpyridiloxy groups linked peripherally or non-peripherally [40] | * |
| Photobacterium damselae | Gn | 5,10,15-tris(1-methylpyridinium4-yl)-20-pentafluorophenylporphyrin tri-iodide (Tri-Py+-Me-PF) [35] | Aquaculture water [35] |
| Pseudomonas aeruginosa | Gn | Toluidine blue-carbon nanotube conjugate [41], Radachlorin [42], methylene blue [43,44], methylene blue-polymyxin conjugate [45], Temoporfin [44], prodigiosin [46], curcumin [19,47], Rose Bengal alone and when combined with methicillin [48], metal phthalocyanines [M = Zn(II), Cu (II), Co(II), In(III), and Lu (III)] bearing chlorine and dipentylmalonyl groups on peripheral positions and hexyloxy groups on non-peripheral positions [49], tetrasulfonated hydroxyaluminum phthalocyanine [50], zinc phthalocyanine-colistin conjugate [51], meso-tetra(3-N-methylpyridyl)porphyrin and meso-tetra(4-N-methylpyridyl)porphyrin [52], isomeric meso-tetra (pyridyl)-substituted porphyrin (5,10,15,20-Tetra(4-pyridyl)porphyrin (TPyP)) derivatives of cisplatin (3-cis-Pt-TPyP and 4-cis-Pt-TPyP) [16], dimethyl-8,13-divinyl-3,7,12,17-tetramethyl-21H, 23H-porphyrin-2,18-bis[-N-2-(dimethylamine)ethyl] propenamide [53], 5,10,15,20-tetrakis(N-4-methylpyridyl)porphyrin and 5,10,15,20-tetrakis[(N-4-methylpyridyl)porphyrinate]zinc(II) [54], N, N’-bis (2-aminoethyl)-2,7,12,18-tetramethyl-3,8-divinyl-21H, 23H-porphyrin-13,17-bispropanamide porphyrin [17], 5,10,15,20-trakis(4-((S)-2,6-diaminohexanamido)-phenyl) porphyrin [55], sulfonated polystyrene nanoparticles with encapsulated 5,10,15,20-tetraphenylporphyrin [56], 7(12)-(1-methoxyethyl)-12(7)-(1-hydroxyethyl)-3, 8, 13, 17-tetramethyl-21H, 23H-porphyrin-2, 18-dipropionic acid [57], 5,10,15,20-tetrakis(4-hydroxyphenyl)-21H,23H-porphyrin [58], [13,17-bis(1-carboxyethyl)carbamoyl(3-methylpyridine)-3-(1,3-dioxane-2-yl) methylidene-8-ethenyl-2-hydroxy-2,7,12,18-tetramethyl chlorin, diN-methy iodide [59], meso-tetrakis(1-undecyl-3-pyridyl)bacteriochlorin tetrabromide, meso-tetrakis [1-(4′-bromobutyl)-3-pyridyl]bacteriochlorin tetrabromide, meso-tetrakis[1-(4′-pyridiniobutyl)-3-pyridyl]bacteriochlorin octabromide, Meso-tetrakis(1-heptyl-3-pyridyl)-bacteriochlorin tetrabromide, meso-tetrakis[1-(2′-bromoethyl)-3-pyridyl]-bacteriochlorin tetrabromide, meso-tetrakis[1-(2′-pyridinioethyl)-3-pyridyl]bacteriochlorin octabromide [60], boron-dipyrromethenes (BODIPYs) [61]. | * |
| Pseudomonas putida | Gn | trans-AB-porphyrin, trans-AB-porphyrin-gelatin nanoparticle conjugate [62], Rose bengal-gold nanoparticle conjugate, pyridyl porphyrin-gold nanoparticle conjugate [63], Rose Bengal-gelatin nanoparticle conjugate [64] | * |
| Vibrio aestuarianus | Gn | Curcumin, methylene blue and eosin Y [65] | Microalgae feed and tank seawater [65] |
| Vibrio alginolyticus | Gn | Curcumin [66] | * |
| Vibrio anguillarum | Gn | 5,10,15-tris(1-methylpyridinium4-yl)-20-pentafluorophenylporphyrin tri-iodide (Tri-Py+-Me-PF) [35] | Aquaculture water [35] |
| Vibrio campbellii | Gn | Tetra-cationic 5,10,15,20-tetrakis (1-methyl-4-pyridinio) porphyrin tetra (p-toluenesulfonate) [TMPyP] [67,68], curcumin [69] | Microalgae feed [67] water [68] |
| Vibrio cholerae | Gn | Curcumin [69] | Water [69] |
| Vibrio fischeri | Gn | 5,10,15-tris(1-methylpyridinium-4-yl)-20-(pentafluorophenyl)porphyrin tri-iodide (Tri-Py+-Me-PF) [70] | aquaculture water [70] |
| Vibrio harveyi | Gn | Rose Bengal [71], curcumin [69] | larviculture systems [71], water [69] |
| Vibrio parahemolyticus | Gn | methylene blue [72], curcumin [36,73], 5,10,15-tris(1-methylpyridinium4-yl)-20-pentafluorophenylporphyrin tri-iodide (Tri-Py+-Me-PF) [35], 5,10,15,20-Tetrakis[N-methyl-4-pyridyl)porphyrin and 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin [74] | Aquaculture water [35,74] |
| Vibrio splendidus | Gn | Curcumin, methylene blue and eosin Y [65] | Microalgae feed and tank seawater [65] |
| Vibrio vulnificus | Gn | Toluidine blue O [75] | * |
| Serratia marcescens | Gn | Rose Bengal [76], toluidine blue [77], methylene blue [78], meso-tetra(3-N-methylpyridyl)porphyrin and meso-tetra(4-N-methylpyridyl)porphyrin [52], trans-AB-porphyrin, trans-AB-porphyrin- gelatin nanoparticle conjugate [62]. | * |
| Stenotrophomonas maltophilia | Gn | Riboflavin and chlorophyllin [26], | * |
| Mycobacterium marinum | Gp | 5-aminolevulinic acid [14,79] | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dube, E.; Okuthe, G.E. Applications of Antimicrobial Photodynamic Therapy in Aquaculture: Effect on Fish Pathogenic Bacteria. Fishes 2024, 9, 99. https://doi.org/10.3390/fishes9030099
Dube E, Okuthe GE. Applications of Antimicrobial Photodynamic Therapy in Aquaculture: Effect on Fish Pathogenic Bacteria. Fishes. 2024; 9(3):99. https://doi.org/10.3390/fishes9030099
Chicago/Turabian StyleDube, Edith, and Grace Emily Okuthe. 2024. "Applications of Antimicrobial Photodynamic Therapy in Aquaculture: Effect on Fish Pathogenic Bacteria" Fishes 9, no. 3: 99. https://doi.org/10.3390/fishes9030099
APA StyleDube, E., & Okuthe, G. E. (2024). Applications of Antimicrobial Photodynamic Therapy in Aquaculture: Effect on Fish Pathogenic Bacteria. Fishes, 9(3), 99. https://doi.org/10.3390/fishes9030099