Integrative Taxonomy Clarifies the Historical Flaws in the Systematics and Distributions of Two Osteobrama Fishes (Cypriniformes: Cyprinidae) in India
Abstract
1. Introduction
2. Material and Methods
2.1. Material Examined
2.2. Sampling and Morphological Investigation
2.3. Molecular Experiments
2.4. Dataset Preparation and Genetic Analyses
3. Results and Discussion
3.1. Morphological Amendment of O. vigorsii
3.2. Note on O. tikarpadaensis, with Urohyal Features
3.3. Genetic Inferences
3.4. Revised Key to Species of the Genus Osteobrama
| 1. Barbels absent | 2. |
| - Barbels present | 5. |
| 2. Lateral line scales 42–63, pre-dorsal scales 21–30 | 3. |
| - Lateral line scales 71–76, pre-dorsal scales 30–32 | O. belangeri. |
| 3. Branched pectoral fin rays 14–15, lateral line scales 55–63 | 4. |
| - Branched pectoral fin rays 12, lateral line scales 42–53 | O. cunma. |
| 4. Lateral-line scales 55–60 | O. peninsularis. |
| - Lateral-line scales 62–63 | O. cotio. |
| 5. Both rostral and maxillary barbels present | 6. |
| - Only maxillary barbels present, branched anal fin rays 16–18, lateral line scales 68–70 | O. dayi. |
| 6. Barbels prominent | 7. |
| - Barbels minute | 9. |
| 7. Branched anal fin rays 11–18 | 8. |
| - Branched anal fin rays 22–27, pre-dorsal scales 34–38, branched pectoral fin rays 14 | O. feae. |
| 8. Pre-dorsal scales 15, lateral line scales 44, branched anal fin ray 11 | O. bakeri. |
| - Pre-dorsal scales 19–22, lateral line scales 52–57, branched anal fin rays 16–18 | O. nielli. |
| 9. Branched anal fin rays 25–27, branched pectoral fin rays 15–16, presence of oblique black streak on the body immediately posterior to the operculum, lateral line scales 59–71 | O. tikarpadaensis. |
| - Branched anal fin rays 21–23, branched pectoral fin rays 13–14, lateral line scales 74–84, no oblique black streak on the body | O. vigorsii. |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berra, T.M. Freshwater Fish Distribution; The University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Banerjee, D.; Raghunathan, C.; Rizvi, A.N.; Das, D. Animal Discoveries 2022: New Species and New Records; Zoological Survey of India: Kolkata, India, 2023; pp. 1–349. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. Version (08/2022). Available online: www.fishbase.org (accessed on 15 January 2024).
- Hubert, N.; Hanner, R.; Holm, E.; Maandrak, N.E.; Taylor, E.; Burridge, M.; Watkinson, D.; Dumont, P.; Curry, A.; Bentzen, P.; et al. Identifying Canadian freshwater fishes through DNA barcodes. PLoS ONE 2008, 3, e2490. [Google Scholar] [CrossRef] [PubMed]
- Steinke, D.; Zemlak, T.S.; Hebert, P. Barcoding Nemo: DNA-based identifications for the ornamental fish trade. PLoS ONE 2009, 4, e6300. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.A.; Armstrong, K.F.; Meier, R.; Yi, Y.; Brown, S.D.J.; Cruickshank, R.H.; Keeling, S.; Johnston, C. Barcoding and border biosecurity: Identifying cyprinid fishes in the aquarium trade. PLoS ONE 2012, 7, e28381. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, M.J.; Laskar, B.A.; Dhar, B.; Ghosh, S.K. Identification and re-evaluation of freshwater catfishes through DNA barcoding. PLoS ONE 2012, 7, e49950. [Google Scholar] [CrossRef] [PubMed]
- Laskar, B.A.; Bhattacharjee, M.J.; Dhar, B.; Mahadani, P.; Kundu, S.; Ghosh, S.K. The species dilemma of northeast Indian mahseer (Actinopterygii: Cyprinidae): DNA barcoding in clarifying the riddle. PLoS ONE 2013, 8, e53704. [Google Scholar] [CrossRef]
- Khedkar, G.D.; Jamdade, R.; Naik, S.; David, L.; Haymer, D. DNA barcodes for the fishes of the Narmada, one of India’s longest rivers. PLoS ONE 2014, 9, e101460. [Google Scholar] [CrossRef]
- Lakra, W.S.; Singh, M.; Goswami, M.; Gopalakrishnan, A.; Lal, K.K.; Mohindra, V.; Sarkar, U.K.; Punia, P.P.; Singh, K.V.; Bhatt, J.P.; et al. DNA barcoding Indian freshwater fishes. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 4510–4517. [Google Scholar] [CrossRef]
- Barman, A.S.; Singh, M.; Singh, S.K.; Saha, H.; Singh, Y.J.; Laishram, M.; Pandey, P.K. DNA barcoding of freshwater fishes of Indo-Myanmar biodiversity hotspot. Sci. Rep. 2018, 8, 8579. [Google Scholar] [CrossRef]
- Laskar, B.A.; Kumar, V.; Kundu, S.; Tyagi, K.; Chandra, K. Taxonomic quest: Validating two mahseer fishes (Actinopterygii: Cyprinidae) through molecular and morphological data from biodiversity hotspots in India. Hydrobiologia 2018, 815, 113–124. [Google Scholar] [CrossRef]
- Kundu, S.; Chandra, K.; Tyagi, K.; Pakrashi, A.; Kumar, V. DNA barcoding of freshwater fishes from Brahmaputra River in Eastern Himalaya biodiversity hotspot. Mitochondrial DNA B Resour. 2019, 4, 2411–2419. [Google Scholar] [CrossRef]
- Laskar, B.A.; Kumar, V.; Kundu, S.; Darshan, A.; Tyagi, K.; Chandra, K. DNA barcoding of fishes from River Diphlu within Kaziranga National Park in northeast India. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2019, 30, 126–134. [Google Scholar] [CrossRef]
- Ward, R.D.; Hanner, R.; Hebert, P.D. The campaign to DNA barcode all fishes, FISH-BOL. J. Fish Biol. 2009, 74, 329–356. [Google Scholar] [CrossRef]
- Talwar, P.K.; Jhingran, A.G. Inland Fishes of India and Adjacent Countries; Oxford & IBH Publishing Co.: New Delhi/Bombay/Calcutta, India, 1991; Volume 1, 541p. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species, References. 2023. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 15 January 2024).
- Jayaram, K.C. The Freshwater Fishes of the Indian Region, 2nd ed.; Narendra Publishing House: Delhi, India, 2010; 616p. [Google Scholar]
- Rahman, M.M.; Norén, M.; Mollah, A.R.; Kullander, S. The identity of Osteobrama cotio, and the status of “Osteobrama serrata” (Teleostei: Cyprinidae: Cyprininae). Zootaxa 2018, 4504, 105–118. [Google Scholar] [CrossRef]
- Shangningam, B.; Rath, S.; Tudu, A.K.; Kosygin, L. A new species of Osteobrama (Teleostei: Cyprinidae) from the Mahanadi River, India with a note on the validity of O. dayi. Zootaxa 2020, 4722, 68–76. [Google Scholar] [CrossRef]
- Rath, S.; Tudu, A.K.; Shangningam, B. First record of Osteobrama tikarpadaensis (Teleostei: Cyprinidae) from Maharashtra India. Int. J. Fish. Aquat. Stud. 2021, 9, 250–252. [Google Scholar] [CrossRef]
- Hora, S.L.; Misra, K.S. Notes on fishes in the Indian Museum. XL. On fishes of the genus Rohtee Sykes. Rec. Indian Mus. 1940, 42, 155–172. [Google Scholar]
- Jayaram, K.C. The Krishna River System Bioresources Study; Occasional Paper, No. 160; Zoological Survey of India: Kolkata, India, 1995. [Google Scholar]
- Jadhav, S.; Paingankar, M.; Dahanukar, N. Osteobrama bhimensis (Cypriniformes: Cyprinidae): A junior synonym of O. vigorsii. J. Threat. Taxa 2011, 3, 2078–2084. [Google Scholar] [CrossRef]
- Hamilton, F. An Account of the Fishes Found in the River Ganges and Its Branches; A. Constable & Co.: Edinburgh/London, UK, 1822; vii + 405p, 39 pls. [Google Scholar]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Schwartz, T.; Pickett, B.E.; He, S.; Klem, E.B.; Scheuermann, R.H.; Passarotti, M.; Kaufman, S.; O’Leary, M.A. A RESTful API for access to phylogenetic tools via the CIPRES science gateway. Evol. Bioinform. 2015, 11, 43–48. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Sykes, W.H. On the fishes of the Dukhun. Trans. Zool. Soc. Lond. 1841, 2, 349–378. [Google Scholar] [CrossRef]
- Day, F. The Fauna of British India, Including Ceylon and Burma; Taylor and Francis: London, UK, 1889; Volume 2, 509p. [Google Scholar]
- Singh, D.F.; Yazdani, G.M. Osteobrama bhimensis, a new cyprinid fish from Bhima River, Pune District, Maharahtra. J. Bombay Nat. Hist. Soc. 1992, 89, 96–99. [Google Scholar]
- Singh, M.; Verma, R.; Yumnam, R.; Vishwanath, W. Molecular phylogenetic analysis of genus Osteobrama Heckel, 1843 and discovery of Osteobrama serrata sp. nov. from northeast India. Mitochondrial DNA Part A 2018, 29, 361–366. [Google Scholar] [CrossRef]
- Moritz, C.; Cicero, C. DNA barcoding: Promise and pitfalls. PLoS Biol. 2004, 2, e354. [Google Scholar] [CrossRef] [PubMed]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]



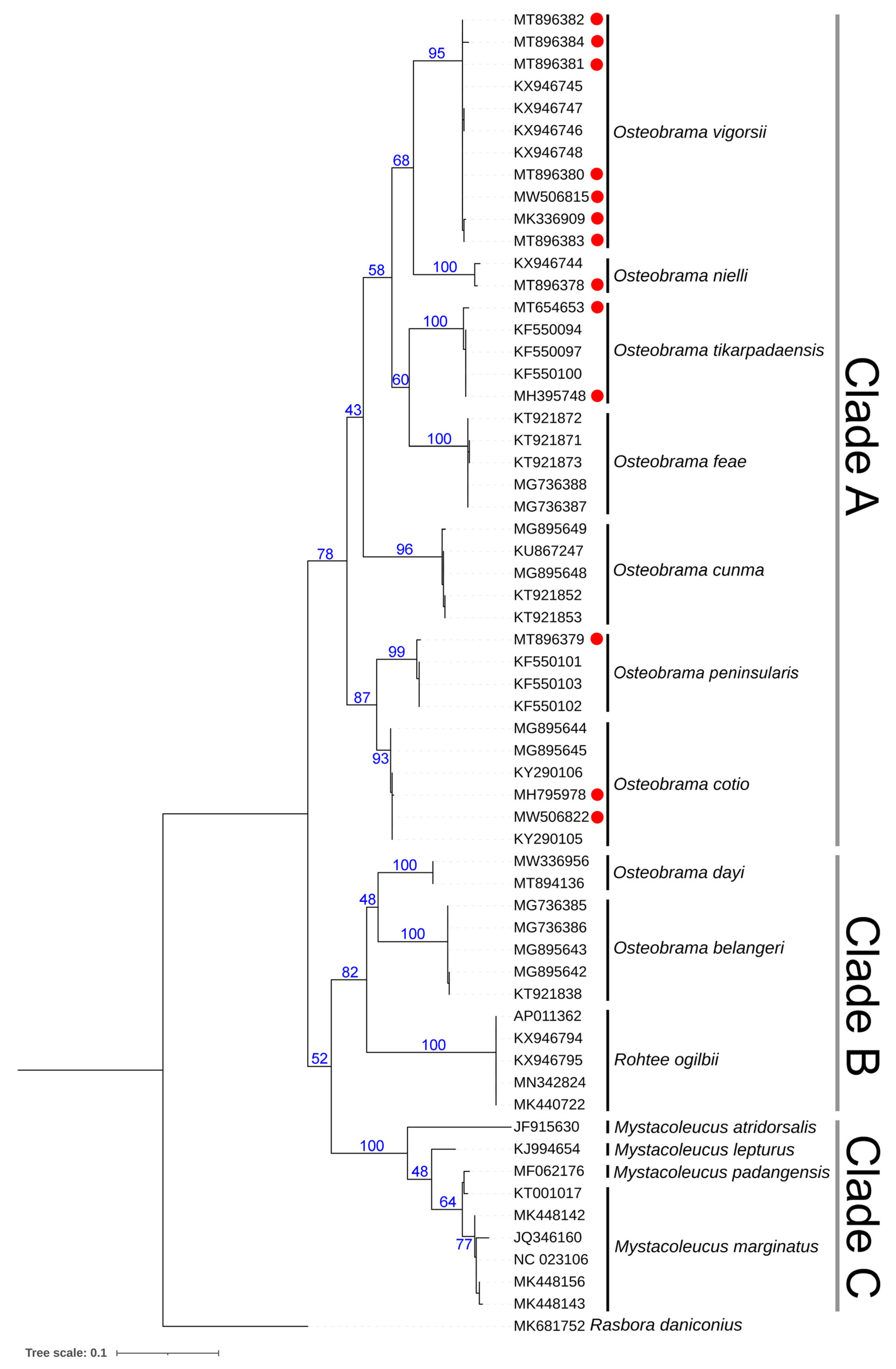
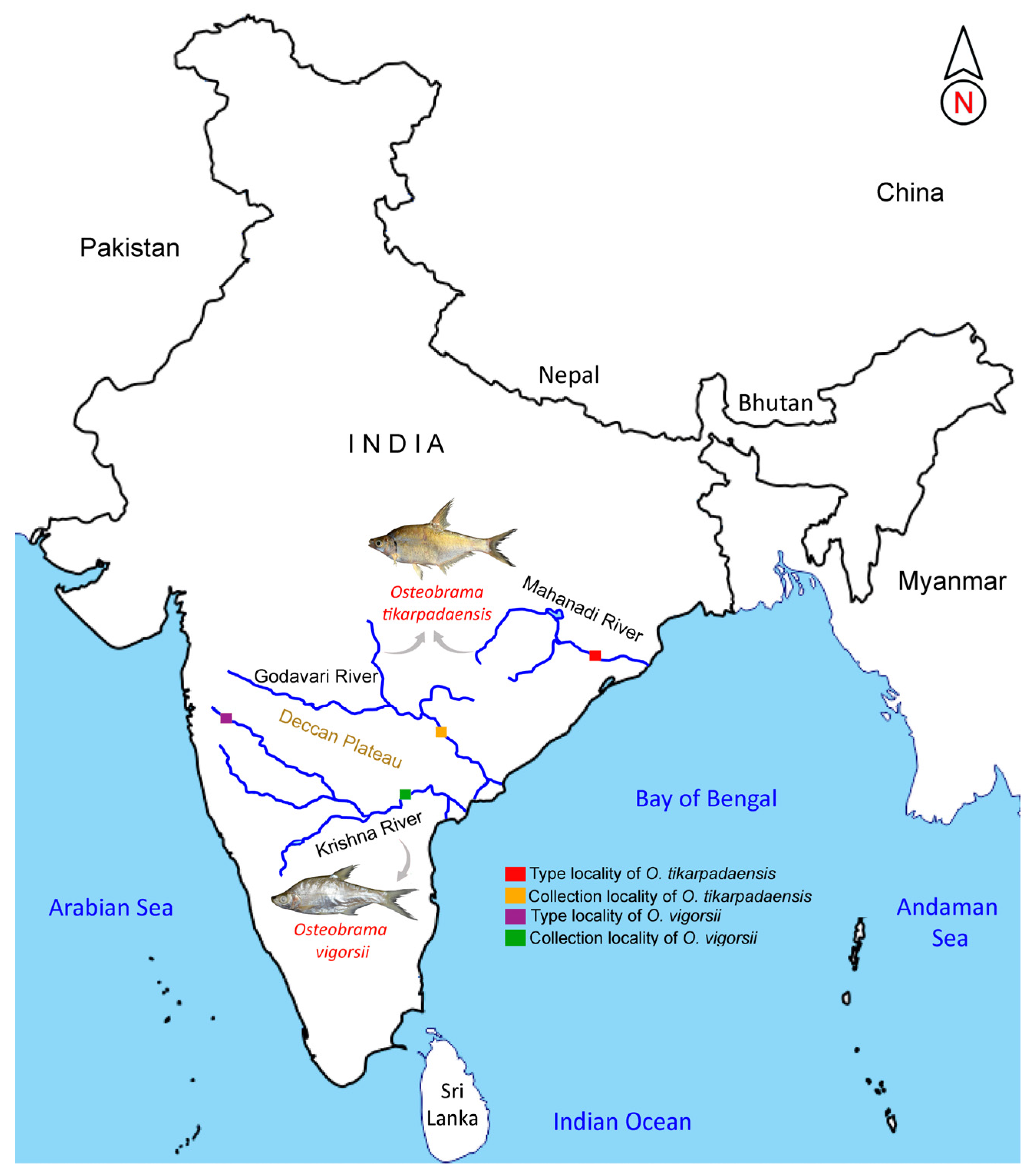
| Species | Museum Registration | Locality | GenBank Accession Number | BOLD-IDs |
|---|---|---|---|---|
| Osteobrama cotio | FBRC_ZSI_F_2707 | Maharashtra, 20.450° N, 74.403° E | MH795978 | BOLD:AAE6868 |
| Osteobrama cotio | FBRC_ZSI_DNA907_F3880 | Jurala project, Kistampally, Telangana, 16.370° N, 77.694° E | MW506822 | - |
| Osteobrama tikarpadaensis | FBRC_ZSI_F_2616 | Godavari River, Telangana, 17.7431° N, 80.8798° E | MH395748 | BOLD:ABY3071 |
| Osteobrama tikarpadaensis | FBRC_ZSI_DNA616_F3416 | Godavari River, Andhra Pradesh, 17.5721° N, 81.2587° E | MT654653 | BOLD:ABY3071 |
| Osteobrama neilli | FBRC_ZSI_DNA833_F3548 | Krishna River at somasila near temple, Telangana, 16.046° N, 78.326° E | MT896378 | BOLD:ACR7173 |
| Osteobrama peninsularis | FBRC_ZSI_DNA864_F3549 | Wyra lake, Telangana, 17.252° N, 80.384° E | MT896379 | BOLD:ACJ3278 |
| Osteobrama vigorsii | FBRC_ZSI_DNA861_F3550 | Krishna River somasila near temple, Telangana, 16.046° N, 78.326° E | MT896380 | BOLD:ACM5411 |
| Osteobrama vigorsii | FBRC_ZSI_F2783_DNA301 | Tungabhadra River, Andhra Pradesh, 16.02° N, 78.327° E | MK336909 | BOLD:ACM5411 |
| Osteobrama vigorsii | FBRC_ZSI_DNA814_F3551 | Krishna River at somasila, Telangana, 16.048° N, 78.334° E | MT896381 | BOLD:ACM5411 |
| Osteobrama vigorsii | FBRC_ZSI_DNA862_F3552 | Krishna River somasila near temple, Telangana, 16.046° N, 78.326° E | MT896382 | BOLD:ACM5411 |
| Osteobrama vigorsii | FBRC_ZSI_DNA863_F3552 | Krishna River somasila near temple, Telangana, 16.046° N, 78.326° E | MT896383 | BOLD:ACM5411 |
| Osteobrama vigorsii | FBRC_ZSI_DNA836_F3552 | Krishna River somasila near temple, Telangana, 16.046° N, 78.326° E | MT896384 | BOLD:ACM5411 |
| Osteobrama vigorsii | FBRC_ZSI_DNA897_F3872 | Jurala project, Kistampally, Telangana, 16.370° N, 77.694° E | MW506815 | - |
| Rasbora daniconius | FBRC_ZSI_DNA326_F3464 | Andhra Pradesh, 18.0733° N, 82.9505° E | MK681752 | - |
| Parameters | Range | Mean ± SE |
|---|---|---|
| Standard Length | 90.5–132.0 mm | |
| In % SL | ||
| Head length | 24.1–28.0 | 25.8 ± 0.95 |
| Head depth | 17.6–22.0 | 18.9 ± 1.03 |
| Head width | 9.9–10.6 | 7.8 ± 2.59 |
| Mouth width | 6.3–7.4 | 5.2 ± 1.73 |
| Body depth | 31.5–35.2 | 33.2 ± 0.84 |
| Body width | 8.6–11.0 | 9.8 ± 0.49 |
| Pre-dorsal length | 51.8–56.9 | 55.0 ± 1.15 |
| Pre-anal length | 59.4–64.3 | 61.3 ± 1.14 |
| Pre-pelvic length | 32.6–42.0 | 38.6 ± 2.12 |
| Pre-pectoral length | 24.8–28.2 | 26.6 ± 0.75 |
| Pelvic–anal distance | 16.5–21.6 | 19.0 ± 1.21 |
| Dorsal fin base length | 11.3–12.4 | 11.8 ± 0.22 |
| Anal fin base length | 22.9–27.5 | 24.9 ± 0.93 |
| Caudal peduncle length | 12.6–16.5 | 13.9 ± 0.87 |
| Caudal peduncle depth | 11.5–13.8 | 12.1 ± 0.56 |
| Snout length | 6.4–8.3 | 7.3 ± 0.40 |
| Eye diameter | 7.0–7.4 | 7.3 ± 0.07 |
| Inter-orbital distance | 6.0–6.4 | 6.3 ± 0.11 |
| Inter-narial space | 4.3–4.9 | 4.6 ± 0.13 |
| Dorsal fin height | 28.3–34.3 | 30.5 ± 1.40 |
| Pectoral fin length | 19.8–20.2 | 20.0 ± 0.08 |
| Anal fin height | 16.5–19.9 | 18.1 ± 0.70 |
| Pelvic fin length | 20.5–23.2 | 21.4 ± 0.61 |
| In % HL | ||
| Eye diameter | 26.3–30.0 | 28.3 ± 0.82 |
| Interorbital width | 23.0–26.7 | 24.6 ± 0.77 |
| Head depth | 62.7–82.8 | 73.8 ± 4.14 |
| Head width | 37.9–40.9 | 39.1 ± 0.73 |
| Mouth width | 24.8–30.5 | 26.6 + 1.34 |
| Parameters | Range | Shangningam et al. [21] |
| Standard Length | 101.0–102.0 mm | |
| In % SL | Specimens from Godavari River | |
| Head length | 26.6–26.6 | 24.5–28.8 |
| Head depth | 11.7–12.7 | 16.4–18.6 |
| Head width | 11.0–12.0 | 13.2–14.4 |
| Mouth width | 5.2–5.7 | 5.6–7.1 |
| Body depth | 32.3–35.1 | 34.5–39.5 |
| Body width | 9.7–10.8 | 9.3–11.7 |
| Pre-dorsal length | 50.0–53.2 | 37.8–40.4 |
| Pre-anal length | 53.2–59.7 | 60.0–61.7 |
| Pre-pelvic length | 40.5–41.6 | 39.9–43.1 |
| Pre-pectoral length | 26.0–26.6 | 24.7–26.6 |
| Pelvic–anal distance | 13.9–15.6 | 19.7–21.3 |
| Dorsal fin base length | 11.7–12.0 | 13–14.2 |
| Anal fin base length | 27.8–28.6 | 29.5–32 |
| Caudal peduncle length | 12.0–15.6 | 14.5–15.6 |
| Caudal peduncle depth | 10.1–11.0 | 10.3–12.2 |
| Snout length | 7.1–7.6 | 7.1–8.3 |
| Eye diameter | 6.5–7.0 | 6.7–8.3 |
| Inter-orbital distance | 7.8–8.2 | 8.7–10.0 |
| Inter-narial space | 5.2–5.7 | 5.0–6.0 |
| Dorsal fin height | 27.2–27.3 | 24.6–29.4 |
| Pectoral fin length | 18.8–19.0 | 19.2–21.2 |
| Anal fin height | 13.0–13.3 | 29.7–31.7 |
| Pelvic fin length | 17.1–18.8 | 17.6–18.9 |
| Species | Genetic Distance (K2P) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Between Species (%) | Within Species (%) | ||||||||
| O. cotio | 0.2 | ||||||||
| O. peninsularis | 5.28–5.68 | 0.3 | |||||||
| O. cunma | 10.64–11.23 | 10.46–11.25 | 0.3 | ||||||
| O. feae | 12.23–13.23 | 12.58–13.88 | 13.34–14.94 | 0.1 | |||||
| O. tikarpadaensis | 12.54–13.43 | 13.90–15.17 | 13.33–14.33 | 9.25–9.77 | 0.3 | ||||
| O. neilli | 12.14–12.75 | 12.50–12.96 | 11.91–12.58 | 11.48–12.23 | 11.53–11.80 | 0.8 | |||
| O. vigorsii | 10.95–11.95 | 12.91–14.47 | 13.88–15.31 | 10.96–11.97 | 10.64–12.35 | 9.31–10.26 | 0.3 | ||
| O. belangeri | 13.50–14.37 | 15.22–16.16 | 15.33–16.24 | 18.92–20.08 | 16.48–17.73 | 16.98–17.60 | 15.32–17.50 | 0.1 | |
| O. dayi | 16.2–16.5 | 16.7–16.9 | 15.5–15.8 | 17.6–17.8 | 16.5–17.3 | 16.3–16.5 | 17.5–18.5 | 11.1–11.4 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskar, B.A.; Banerjee, D.; Chung, S.; Kim, H.-W.; Kim, A.R.; Kundu, S. Integrative Taxonomy Clarifies the Historical Flaws in the Systematics and Distributions of Two Osteobrama Fishes (Cypriniformes: Cyprinidae) in India. Fishes 2024, 9, 87. https://doi.org/10.3390/fishes9030087
Laskar BA, Banerjee D, Chung S, Kim H-W, Kim AR, Kundu S. Integrative Taxonomy Clarifies the Historical Flaws in the Systematics and Distributions of Two Osteobrama Fishes (Cypriniformes: Cyprinidae) in India. Fishes. 2024; 9(3):87. https://doi.org/10.3390/fishes9030087
Chicago/Turabian StyleLaskar, Boni Amin, Dhriti Banerjee, Sangdeok Chung, Hyun-Woo Kim, Ah Ran Kim, and Shantanu Kundu. 2024. "Integrative Taxonomy Clarifies the Historical Flaws in the Systematics and Distributions of Two Osteobrama Fishes (Cypriniformes: Cyprinidae) in India" Fishes 9, no. 3: 87. https://doi.org/10.3390/fishes9030087
APA StyleLaskar, B. A., Banerjee, D., Chung, S., Kim, H.-W., Kim, A. R., & Kundu, S. (2024). Integrative Taxonomy Clarifies the Historical Flaws in the Systematics and Distributions of Two Osteobrama Fishes (Cypriniformes: Cyprinidae) in India. Fishes, 9(3), 87. https://doi.org/10.3390/fishes9030087







