1. Introduction
One of the major global challenges for mankind is how to sustainably meet the demand for food and livelihoods to support the expected growing population of 9.7 billion people in 2030 [
1] while facing the impacts of environmental degradation and climate change [
2]. Meanwhile, the demand for fish consumption has globally increased, and aquaculture production has progressively grown [
3,
4]. Nowadays, aquaculture produces more fish than wild captured fishes; worldwide, aquatic species production available for human consumption constitutes 56% of the market share with an estimation to expand 14% more by 2030 [
5]. It is reasonable, therefore, that aquaculture has been considered as a promising solution to support the increasing world population through the provision of food and nutrition, employment and livelihoods, and incomes from the trade of fish and seafood products [
2,
6,
7]. In the European Union (EU), however, aquaculture production has not followed similar growth as in other parts of the world since it only accounts less than 2% of the world production [
8]. In the EU, particularly in the Mediterranean, mariculture production is of substantial economic importance and has exhibited progressive growth regarding the transitional rearing of marine finfish in net cages in the last few decades. Almost 70% of mariculture production comes from Spain, France, Italy and Greece. The main culture species are the gilthead seabream (
Sparus aurata) and the European sea bass (
Dicentrarchous labrax), which constitutes 95% of the total finfish production in the Mediterranean [
9].
However, long-established mariculture has frequently occurred at the expense of the environment, producing many environmental impacts and socioeconomic concerns that can affect its sustainability [
10,
11]. One of the main recognized impacts is nutrient enrichment of the water column and sediment, near and under cages, which can produce unfavorable biological and geochemical alterations [
12,
13,
14]. These effects originate mainly from uneaten feeds, fish metabolic waste and fish fecal waste [
15]. Furthermore, the quality and quantity of fish fecal waste is altered by the type of the culture system and the fish feed composition [
16,
17]. Hence, these unfavorable ecological consequences have prompted policy, science and industry to search for new, more eco-friendly methods and technologies for a sustainable mariculture. Since 2002, the EU fisheries policy on aquaculture has aimed to increase the production and the diversification of species as well as the product quality in order to improve the competitive position of the sector and to promote environmental, economic and social sustainability [
18]. Notable progress has been made in species selection, feed composition and the development of integrated circular systems. For instance, to mitigate the environmental impacts and achieve the aims of ecosystem services, an ecosystem-responsible aquaculture practice, the integrated multi-trophic aquaculture (IMTA) concept, has been suggested as an innovative method for sustainable aquaculture development and ecosystem services [
19,
20,
21,
22,
23].
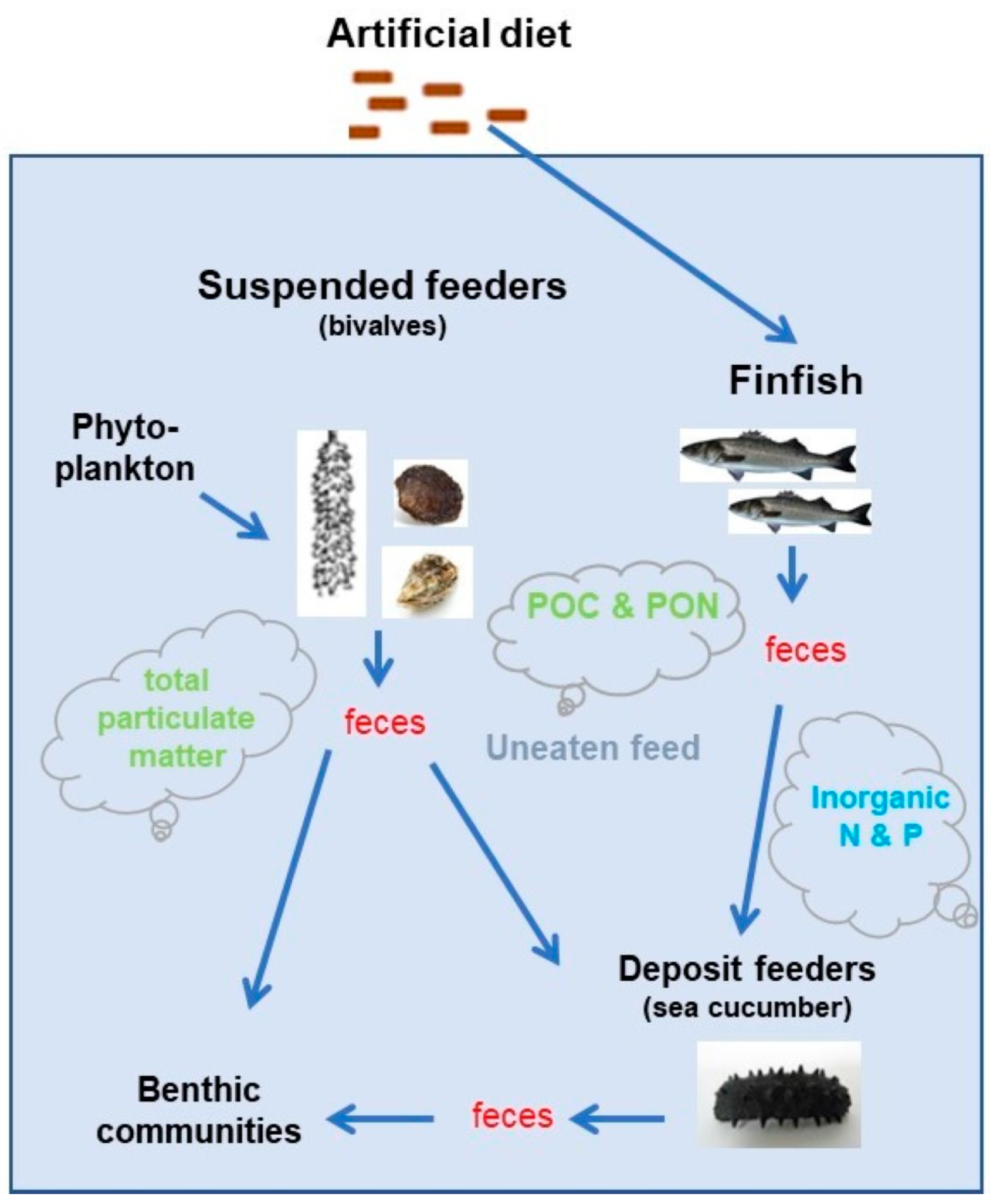
In the broader context of global food security and the growing demand for seafood, IMTA emerges as a promising approach to meet these needs while minimizing the industry’s environmental impact. By adopting IMTA practices, aquaculture can play a more sustainable role in feeding the world while preserving aquatic ecosystems. IMTA integrates the co-cultivation of feeding finfish species with a balanced combination of organic components (such as suspension filter and deposit feeders) and inorganic extractive species (such as seaweed). This harmonious assembly establishes systems that offer a trifecta of benefits, encompassing environmental sustainability through biocontrol and biomitigation, economic stability via product diversification and risk reduction, and improved social acceptance through enhanced management operations [
24].
In most IMTA systems, fish represent the only fed component and the only human-provided input of nutrient energy to the system. Within an IMTA system, in their role, fish provide dissolved and particulate nutrients and oxidation reduction potential, degrading compounds to the other co-cultured organisms and thus improving the income to the farmer. But the quantity and form of these nutrients depend, among other factors, on the fish species, rearing density and feed composition [
16,
17]. Feed composition provides probably the most obvious route of fish waste modification for the extractive organisms; conversely, other trends in the aquafeeds industry may impact the fish waste quality of an IMTA system. Formulated feed that is lost in a culture system has a great effect on water quality through decomposition [
25,
26] and still, fish feed contains starch and proteins that have a first-order decomposition rate of about 0.8/day [
27]. This increases bioavailable nitrogenous and phosphorus compounds and CO
2 concentration, while it decreases the dissolved oxygen levels and pH, resulting in lower alkalinity values of the seawater. Alkalinity is a major component of salinity in seawater as total alkalinity is positively correlated with salinity [
28]. Then, the bioavailable nitrogen and phosphorus compounds along with CO
2 enhance photosynthesis and in turn the production of natural food (phytoplankton). Next, organic extractive species such as the filter-feeding bivalves cultured adjacent to net fish cages reduce nutrient loadings by filtering and assimilating particulate wastes and any phytoplankton production stimulated by introduced dissolved nutrient wastes. Thus, waste nutrients rather than being lost to the environment, as in traditional monoculture, are removed upon harvest of the cultured filter-feeding bivalves.
Suspension filter feeders such as mussels and oysters (bivalves) can be included in IMTA systems. The farming of bivalves is a major activity in Europe [
29]. Largely, the Mediterranean mussel
Mytilus galloprovincialis continues to be the main species farmed in the Mediterranean, while the flat oyster
Ostrea edulis and the Pacific oyster
Crassostrea gigas have undergone a moderate development.
O. edulis is a native species in the Mediterranean, while
C. gigas was introduced in the European aquaculture in the late 1960s. Successful commercial suspension maricultures of those species operate in Spain, France, Greece and Italy. Bivalves’ growth and the effectiveness of the management actions for their production are monitored based on indicators such as their shell growth, weight of soft tissue and condition index (CI) [
30,
31]. The CI, defined as the ratio between the soft tissue dry weight and the shell dry weight, is commonly used to assess the health and the quality of bivalves for scientific and commercial purposes [
30,
32,
33]. CI is particularly important for the quality assessment and marketing value of bivalves because the higher the proportion of tissue, the better the commercial value [
34]. Therefore, farmers widely use CI as an economic indicator of market product [
35]. Yet, CI is mentioned in government and industry datasets providing an ideal and cost-effective ecological indicator of bivalves’ culture performance and the selection of a suitable area for shellfish aquaculture development [
36]. Studies have shown a significant correlation of CI with food availability in the site selection of bivalves farming [
36,
37,
38,
39]. Although the main food for bivalves is phytoplankton followed by bacteria, zooplankton and detritus [
40,
41], laboratory and field studies using stable isotopes and fatty acids as biomarkers have shown that Mediterranean mussels can ingest and assimilate organic waste from fish farms [
42]. Nonetheless, some studies mention that mussels and oysters grow faster when adjusted to fish cages, while others show no or an insignificant increase in growth [
43]. In the past, some researchers had ascribed these differences to the different environmental conditions and culture systems designs, while others had concluded, on the basis of models, that ambient seston concentration was the major reason for these discrepancies [
44]. Later, the effectiveness of bivalves as organic extractive components in open water IMTA systems has been subjected to several constraints including current velocity, ambient seston concentration, and the organic content and concentration of particulate organic fish waste [
45].
On the other hand, organic extractive species such as sea cucumbers cultivated below finfish and shellfish cultures are responsible for a significant removal of the particulate organic carbon loading to the bottom, reducing the gross load by up to 86% for finfish culture and 99% for shellfish culture [
46]. The effectiveness of these extractive species in mitigating organic loadings underscores their importance in the system’s overall sustainability. This removal process, however, introduces a dynamic element to the sediment environment, influencing the fatty acid profile. Any variations in fatty acid profile of sediment between different IMTA systems may reflect differences in the organic matter and detritus from uneaten feed, feces, and decaying biomass, providing insights into nutrient cycling and recycling within an aquatic ecosystem [
47,
48].
Over the past decade, studies have convincingly demonstrated the multifaceted benefits of integrated multi-trophic aquaculture (IMTA), showcasing its positive impact on ecological sustainability, economic viability, and social accessibility [
23,
49,
50]. Recognized as a viable solution to address the global challenge of feeding a growing population sustainably [
51,
52], IMTA has garnered attention, particularly in North America and North/Western Europe. Despite this recognition, effective implementation has been limited to a few farms in Canada and north Europe, where culture densities remain too low for an easy quantification of environmental benefits [
53]. The potential of IMTA in the oligotrophic Mediterranean Sea has been explored in studies related to remediation and mussel production near fish farms or laboratory remediation for European sea bass waste and sea cucumber [
54,
55,
56,
57]. However, commercial-scale IMTA development faces challenges due to factors such as a lack of knowledge and expertise, complexity in system management, insufficient reference data, and the need for optimal species compatibility. Therefore, it becomes evident that the present work is situated within a context where the concept of IMTA requires establishment and confirmation in various system configurations, aligning with the need to address existing challenges and propel IMTA toward broader adoption and understanding.
There are two main objectives of the present work: (i) to evaluate the efficiency of an integrated multi-trophic aquaculture (IMTA) system comprising of three components with species from three trophic levels: a feeding fish, the European sea bass co-cultivated with organic extractive suspension filters feeders (Mytilus galloprovincialis, Ostrea edulis, Crassostrea gigas) and deposit feeders (Holothuria sanctori, Holothuria polii, Holothuria tubulosa) and (ii) to assess the impact and utilization potential, within this IMTA system, of two commercially available fish feeds with high and low fish meal ingredients by using a multi-indicator assessment approach including growth performance and environmental quality indices, biomarkers and toxicity tests.
4. Discussion
IMTA is considered a sustainable and eco-friendly mariculture system [
43,
72]. In an IMTA system, the selection of suitable species and their biomass (population density) are crucial factors. In the overall design of an IMTA system, two kinds of organisms are considered: fed and extractive. Fed organisms produce wastes (uneaten feed, feces etc.), whereas extractive organisms convert these wastes into fertilizer, food and energy [
24]. Yet, the utilization of suitable indicators is a key component in the process to determine the efficiency of an IMTA system.
In this study, a multi-indicator assessment approach was applied to evaluate the efficiency and the environmental impact of an IMTA system consisting of species from three trophic levels (i.e., feeding European sea bass D. labrax with suspension feeders (the mussel M. galloprovincialis, the oysters O. edulis and C. gigas) and deposit feeders (the sea cucumbers H. sanctori, H. polii and H. tubulosa)) co-cultured in concrete tanks at the seacoast. Multiple indicators of species growth performance along with environmental quality indices and specific biomarkers were utilized to evaluate the growth of these co-cultured species and to estimate particular impacts on the quality status of the water and sediment in the system.
European sea bass was selected as the feeding component, because it is one of the most important and profitable marine farmed species in Europe, especially in the Mediterranean Sea [
29]. Its growth, however, is a complex trait that is very important to fish farmers. Generally, the growth of fish farmed in traditional monocultures is influenced by many factors, including the rearing population density, the quality and quantity of food, the type of culture system, rearing conditions and management practice [
73,
74]. In order to evaluate its growth performance, critical indicators such as the survival rate, body weight gain (WG), specific growth rate (SGR), feed conversion ratio (FCR) and feed efficiency ratio (FE) are considered appropriate and used by many stakeholders (e.g., farmers, scientists, policymakers, legislators). The survival rate is a reliable indicator of fish growth performance as it ensues from several other important factors including development stage, stress in a given rearing condition and disease resistance. Yet, survival rate is correlated also with the economic value of the fish biomass produced. Our results indicated a successful and higher survival in the IMTA system compared to the MONOculture and similar survivals in the two other IMTA systems in which fish were fed on the two commercial diets of different composition. FCR is defined as the units of feed needed to yield one unit of fish biomass. It is therefore the conventional measure of fish production efficiency and a well-established crucial indicator commonly used by fish farmers and scientists; the smaller the FCR ratio, the greater the FE ratio. The FE ratio is defined as the amount of body weight gain for a given total feed consumed. Hence, FE is the inverse of the FCR; the larger the FE, the greater the efficiency of feed consumed. A low FCR is crucial for aquaculture systems as it is associated with reduced uneaten fish feeds and fish wastes entering the culture system, leading to lower feed requirements and reduced feeding costs.
Depending upon the feed type and its composition, the species, the rearing density, the feeding practices, the water quality and the culture system, the FCR ratio can diverge from 1.0 to 2.4 [
75]. Lower FCR values denote that feed is efficiently converted into fish body weight gain, while in overfeeding or underfeeding, the ratio can increase. The FCR ratio of European sea bass farmed in net cage monocultures in the Mediterranean can vary from 1.77 to 1.9 [
76,
77]. The results of our first experiment indicated lower FCR values in both the monoculture and IMTA system than those mentioned in the literature, but a significantly lower FCR ratio was displayed in the IMTA system (1.47) compared to that in the monoculture (1.66). However, despite the significantly lower FCR and the higher FE ratios in the IMTA system, the final fish body weight was similar in the two systems, and the WG in the IMTA system was not significantly high. It was concluded thus that perhaps the feed consumed was probably not alike in the systems. Studies mention that the feed consumed is influenced by many factors such as the management practices, environmental conditions, feed quality and physiological condition of the fish [
78]. In our case, we assumed that the different environmental conditions present in the two systems must have influenced the physiological condition of the fish and the amount of the feed they consumed. In contrast, higher FCR ratios were indicated in our second experiment in both IMTA systems (FM- and LFM-IMTA) than those mentioned in the literature, but FCR was significantly lower in the FM-IMTA system (2.16) than in the LFM-IMTA system (2.39). Yet, both the final fish body weight WG and FE were significantly higher in the FM-IMTA than in LFM-IMTA system. Hence, it was concluded that fish in the FM-IMTA system had better growth performance than those in LFM-IMTA, since they had higher FE and lower FCR ratios. These results can be attributed to the composition of FM (Fish Meal), which consisted of higher amounts of fish meal and fish oil compared to LFM (Low Fish Meal). This is in agreement with Kousoulaki and co-authors [
79], who noted that the optimal performance of European sea bass in traditional monocultures was achieved when the fish were fed with feeds consisting of high fish meal and fish oil. In our study, bivalves exhibited growth only in their shells and not in their soft tissues in all the IMTA systems we tested. Initial and final CI ratios were similar in the IMTA system, and final CI ratios in FM-IMTA and LFM-IMTA did not differ. As CI ratios have been linked to the trophic condition of the farming area [
80], the absence of changes in the CI ratios may be indicative of the fact that the seston quality was low in our systems (the incoming sea water in the tanks and the flow rate) and/or that feeds and fish fecal waste levels were insufficient due to the low fish density in our systems (fish biomass at the start of the experiments was 2 kg/m
3 and 0.5 kg/m
3).
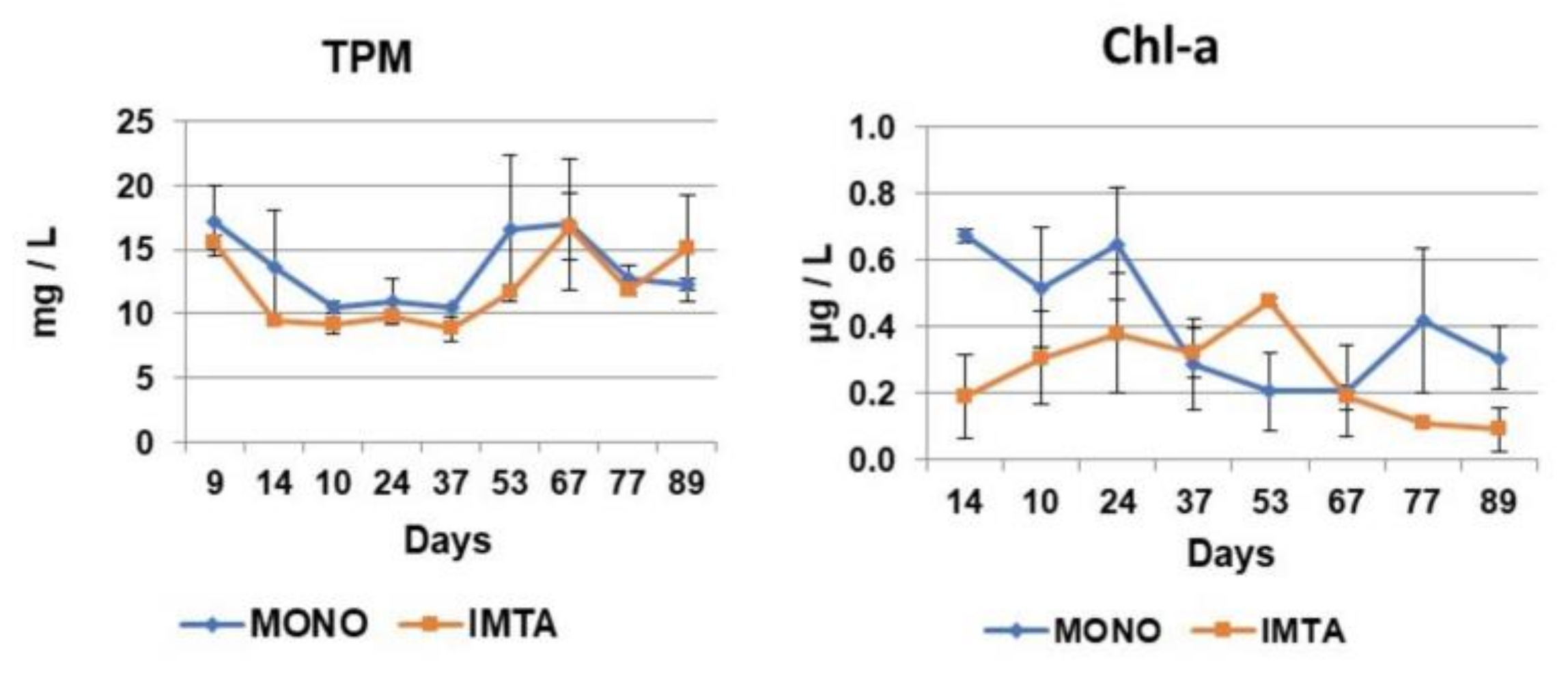
In fact, the aquaculture of bivalves does not thrive in oligotrophic areas, but it does in eutrophic areas where nutrients are in higher levels [
35,
41,
42]. In turn, the level of nutrients can be associated with phytoplankton growth. Chl-a is an indicator of primary production (amount of phytoplankton growing in a water body) in response to nutrients and a key indicator to assess marine water quality and to classify the trophic condition of the water body. Based on trophic classification ranges [
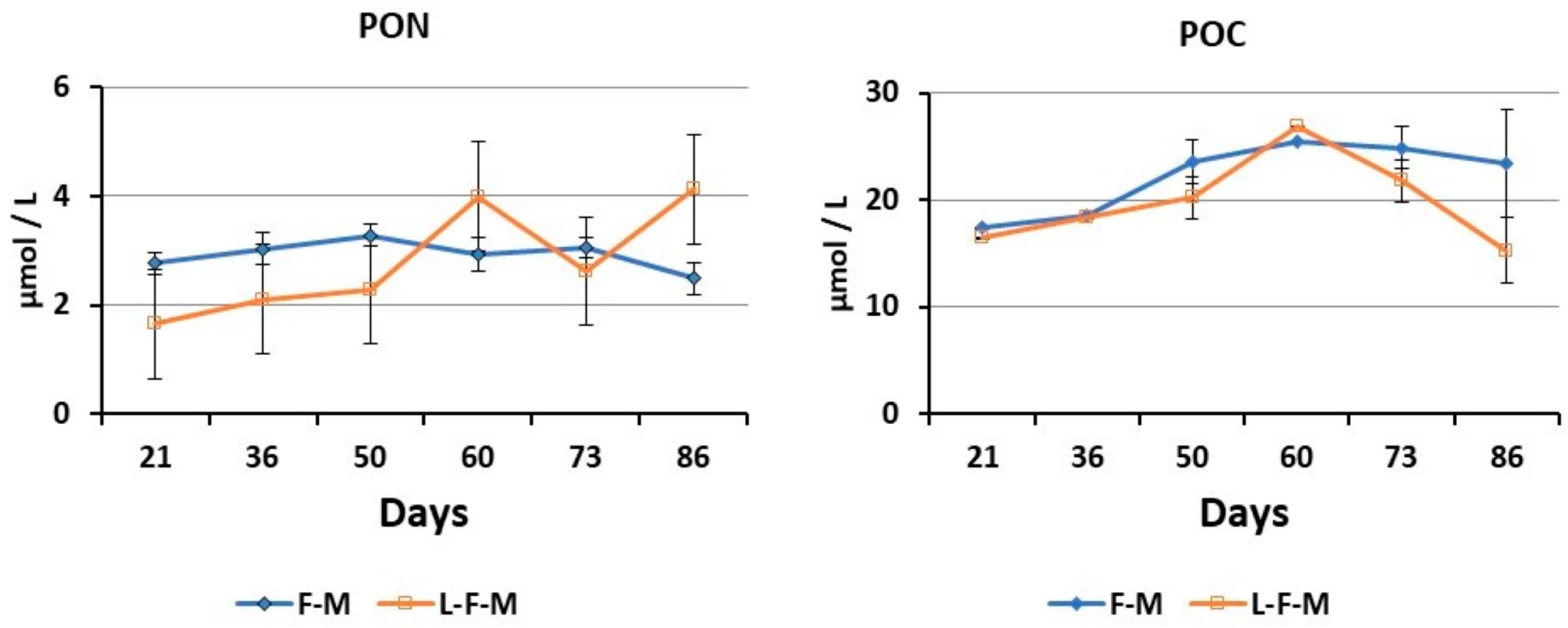
70], levels of Chl-a obtained in our experiments reflected a lower mesotrophic character (0.1 to 0.6 μg/L) in the monoculture and IMTA system and an oligotrophic (<0.1 μg/L) and lower mesotrophic (0.1 to 0.6 μg/L) character in the LFM-IMTA and the FM-IMTA systems, respectively. Furthermore, POC and PON levels were lower in the IMTA system (highest values 71.5 μmol/L for POC, 4.3 μmol/L for PON) compared to the monoculture (highest values 152.2 μmol/L for POC, 13.1 μmol/L PON), whereas their levels in FM-IMTA and LFM-IMTA were very low (<4 μmol/L for PON and <25 μmol/L for POC). These low values of PON and POC together with the oligotrophic-mesotrophic conditions in the water bodies of our systems appeared to affect the growth of the soft tissues of bivalves. Therefore, although filter feeders indicated an increase in their total weight and shell length, the CI ratios showed low values because their soft tissue did not increase either in the IMTA system or in the FM-IMTA and LFM-IMTA. This is another confirmation that bivalves CI are dependent on trophic conditions [
30]. However, it is essential to highlight the notable reduction in PON (particulate organic nitrogen) and POC (particulate organic carbon) concentrations in the IMTA system. This decrease strongly suggests a bio-mitigation of organic nutrients within the water column of the IMTA system.
The settlement of uneaten feed pellets and fish fecal wastes as well as bivalves’ feces can lead to waste particles and organic enrichment of the sediments [
15]. However, nutrients enrichment with organic loads such as proteins, lipids and fatty acids can be reduced with the contribution of deposit feeders like sea cucumbers [
50]. Therefore, we integrated the sea cucumbers
H. sanctori,
H. polii and
H. tubulosa in our IMTA system. Sea cucumbers decrease organic loads and redistribute surface sediment, while the inorganic nutrients (P and N) they excrete enhance the benthic habitat [
30]. Hence, they are considered as excellent bioremediators and ideal candidates for IMTA systems due to their high market value and their ability to feed on the particulate waste generated by other co-culture species [
81]. Usually, sea cucumbers are eaten as a food, medicinal supplements or extracts and tonics in Asian countries, whereas lately, they have also been utilized as nutraceuticals in western markets [
81]. The first attempts to integrate sea cucumbers with fish were made in Canada for fouling mitigation in coastal salmon sea cage systems [
82]. Since then, concerns to integrate them in feeding finfish and filter feeders bivalves farming activities has grown not only for their economic benefits but also for reducing the overall environmental impact of the farming activities. However, there are only a few studies available regarding the co-culture of Mediterranean Sea cucumbers in mussel farms [
83,
84] or in finfish farms [
50,
85,
86,
87].
A positive increase on the growth of sea cucumbers was indicated in this study with a concurrent decrease on the levels of organic loads (proteins, lipids, TOC) and saturated, monounsaturated and polyunsaturated fatty acids concentrations in the IMTA system and the FM-IMTA in comparison to the monoculture and LFM-IMTA, respectively. These showed a higher involvement of feces and uneaten feed in the settled particles in the MONOculture and LFM-IMTA and the contribution of sea cucumbers for bio-mitigating fecal wastes and uneaten feeds in all of our IMTA systems. The fatty acids profile in sediments, particularly, long-chain fatty acids, has been used as a biomarker for the presence of organic waste originated from farmed fish activities [
87]. Moreover, organic carbon, nitrogen and phosphorus loads can alter sediment characteristics beneath and close to the fish cages [
88]. This, in turn, stimulates bacterial activity, which may result in benthic oxygen depletion [
89] causing long-term changes in the structure of the benthic assemblages by reducing density and biodiversity [
90,
91,
92]. In our experiments, bacterial diversity in sediments based on DGGE bands was similar either in MONOculture and IMTA or in FM-IMTA and LFM-IMTA systems compared to the initial stage conditions. In addition, the Sørensen index (Cs) showed very high similarity (100%) between the FM-IMTA and LFM-IMTA systems and high similarity (0.89%) between the IMTA system and MONOculture. However, due to the system design of our IMTA, a direct comparison with results from the literature could not be performed.
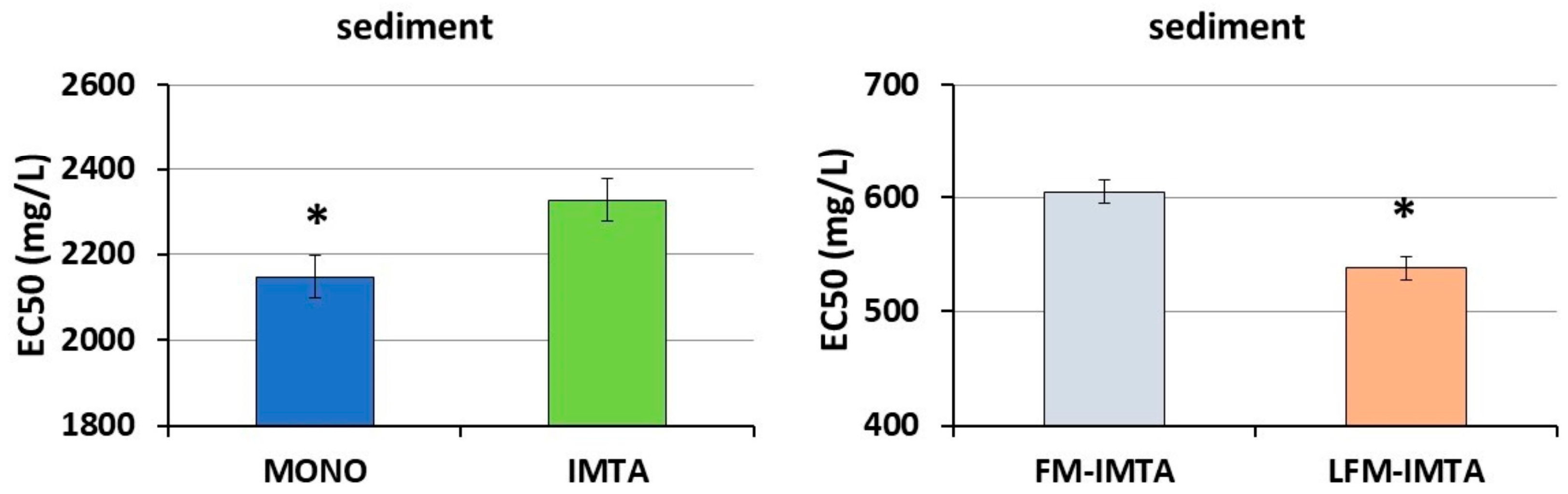
Previous studies have used bioassays with marine luminescent bacteria (
Vibrio fischeri) to characterize fish feeds and fish fecal toxicity [
93] and the toxicity of sediment [
94,
95]. Our results found higher toxicities in the MONOculture and in the LFM-IMTA system than in the IMTA and the FM-IMTA, respectively. Moreover, the toxicity of feeds was higher in the LFM feed compared to the FM feed. Researchers have attributed the toxic effects to the fats and vegetable oils present in feeds and sediments. Campo et al. [
96] indicated that toxic effects are linked to the chemical composition of fat and vegetable triacylglycerols that consist of glycerol molecules esterified with three long-chain fatty acids. The three most abundant fatty acids found in triacylglycerols are the unsaturated 18:1 (oleic), 18:2 (linoleic) and 18:3 (linolenic) oils, particularly corresponding to, prominently and to a lesser extent corresponding to saturated fatty acids 14:0 (myristic), 16:0 (palmitic) and 18:0 (steatic). The fatty acids profiles in the sediments of our first experiment indicated higher values of these fatty acids in the monoculture compared to the IMTA system, while the same fatty acids were lower in the FM-IMTA system than in the LFM-IMTA system. These findings could explain the different EC50s found in our sediment samples among all the systems.