Age- and Size- Based Reproductive Potential of Gray Snapper (Lutjanus griseus) in the Eastern Gulf of Mexico
Abstract
1. Introduction
2. Materials and Methods
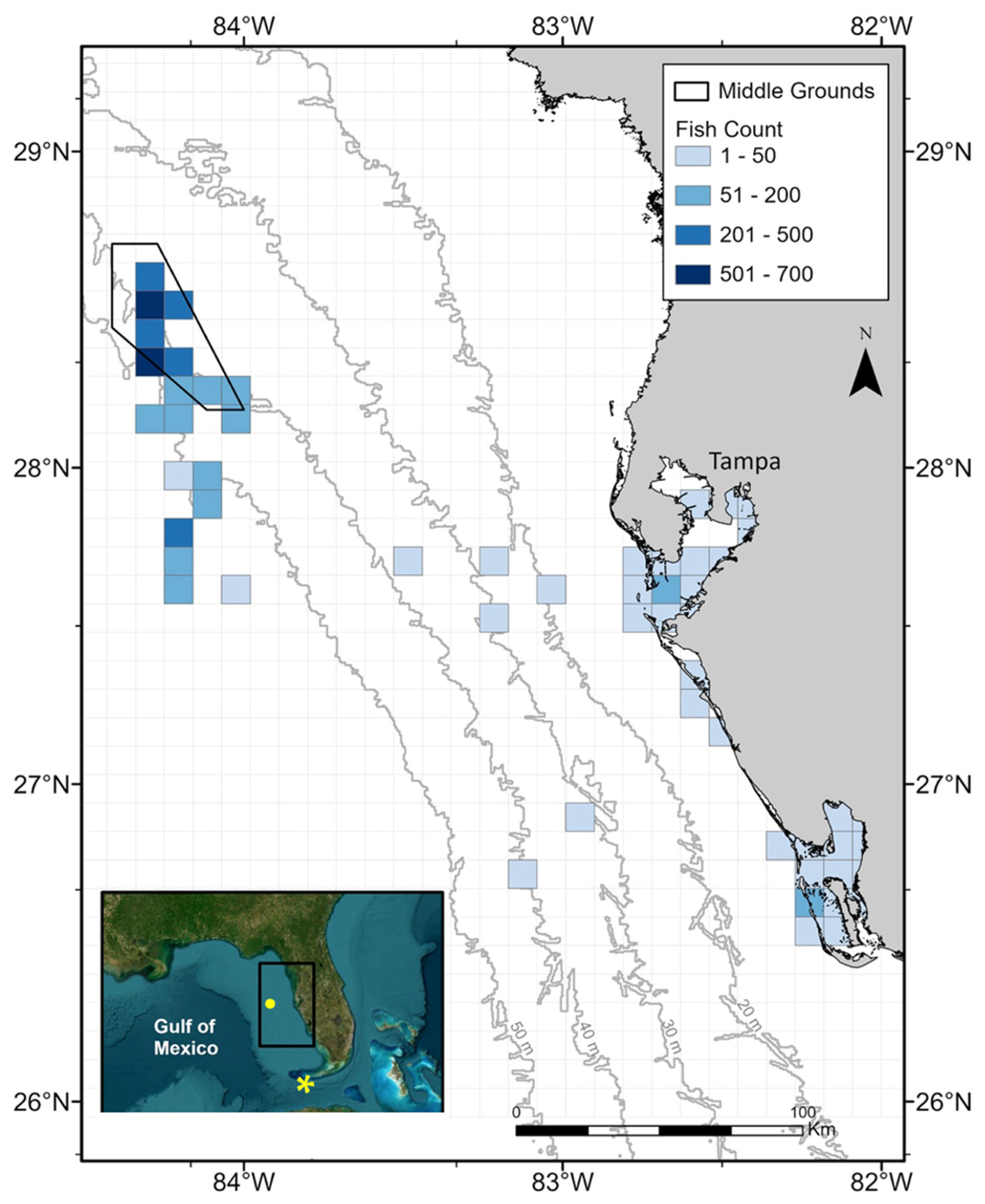
2.1. Sample Collection
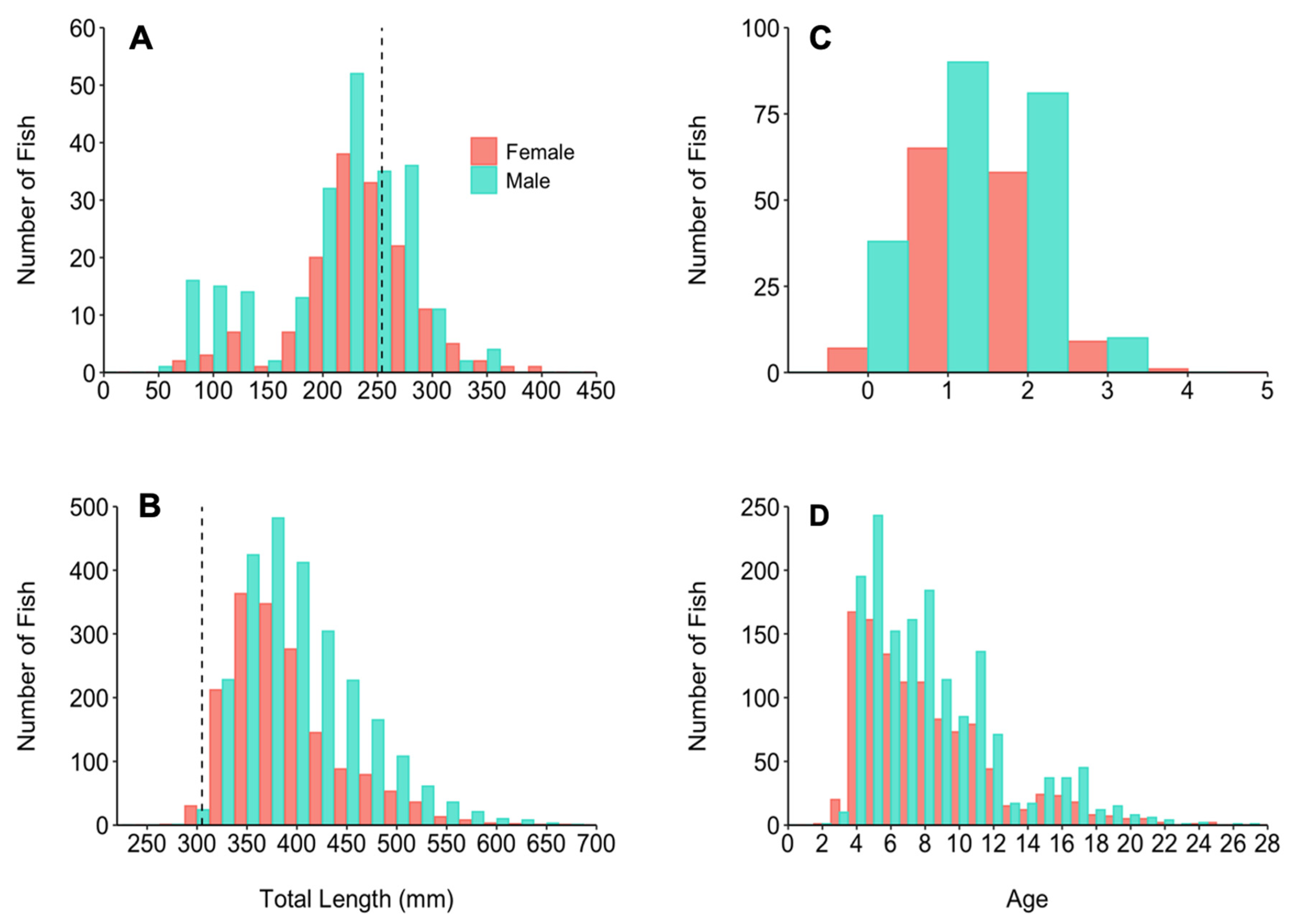
2.2. Otolith Preparation and Aging
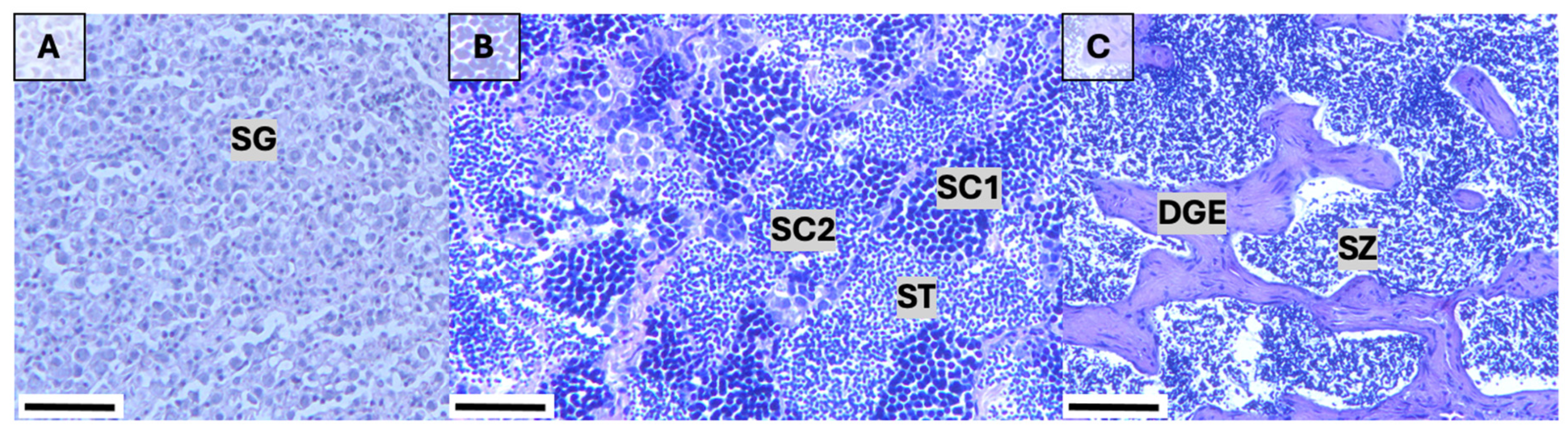
2.3. Histological Preparation and Reproductive Staging
2.4. Spawning Seasonality and Condition in Relation to Spawning
2.5. Spawning Fraction, Interval, and Frequency
2.6. Fecundity
2.7. Lunar Periodicity
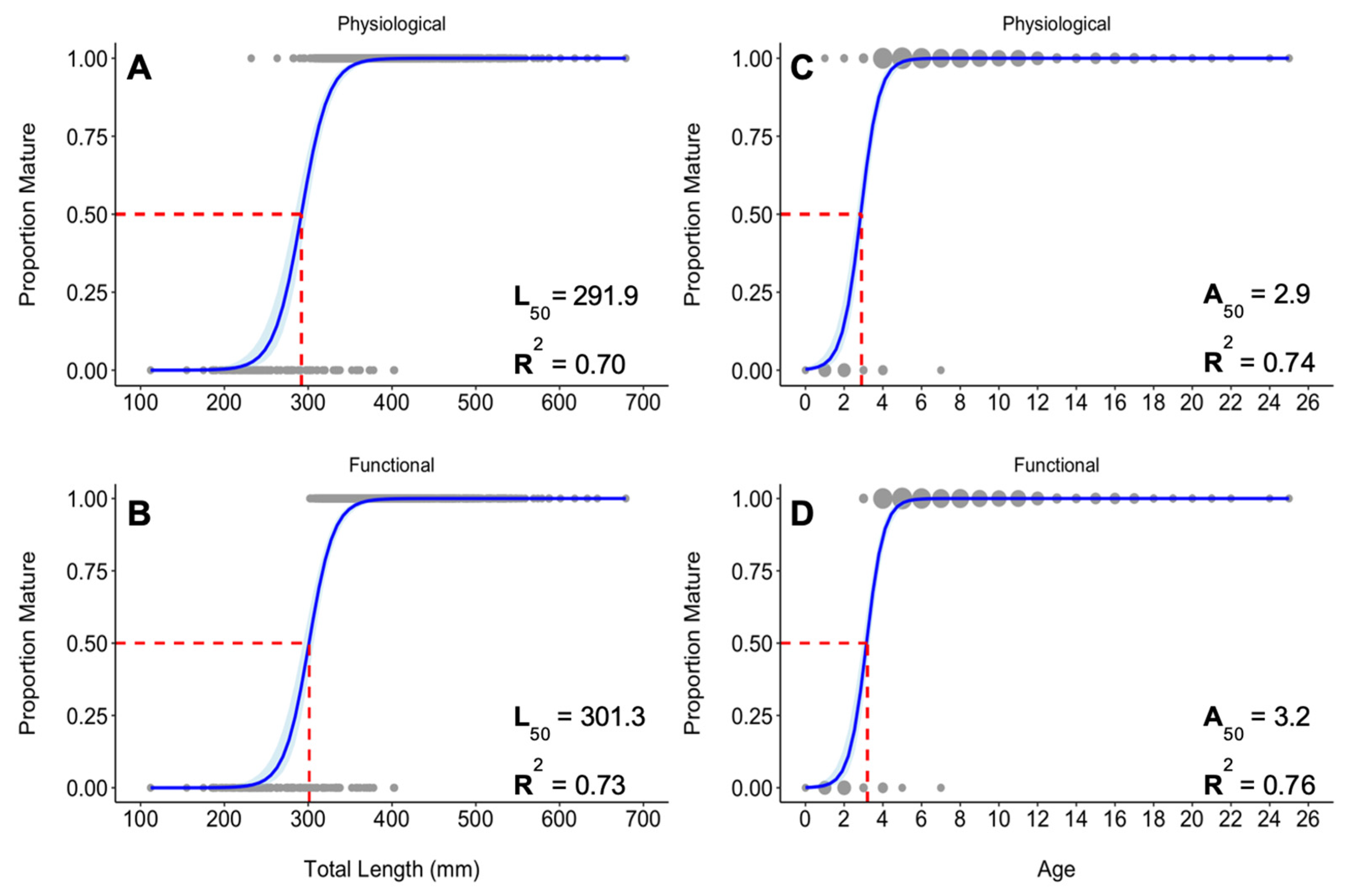
2.8. Sexual Maturity
3. Results
3.1. Sample Collection
3.2. Spawning Seasonality
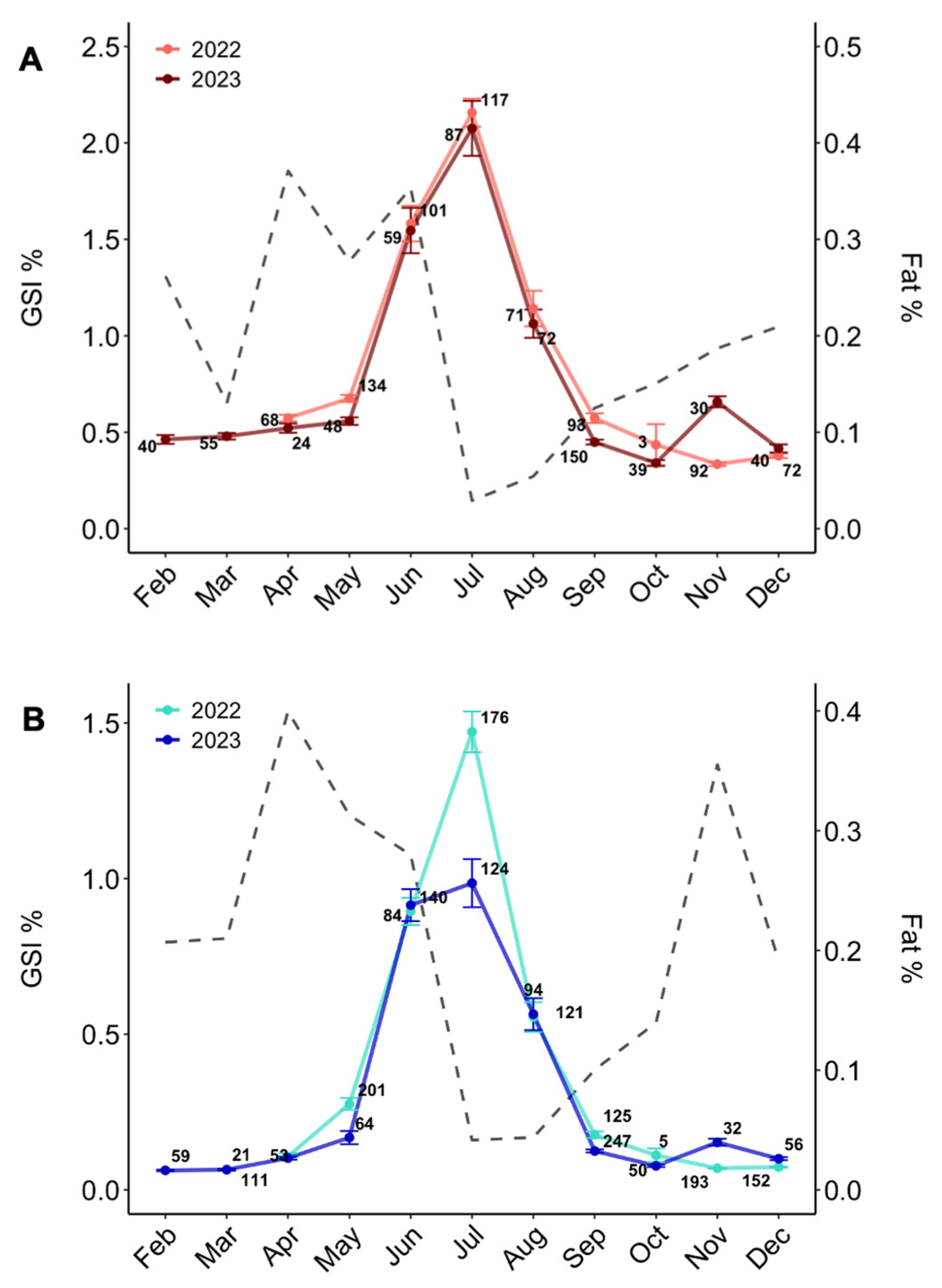
3.2.1. Gonadosomatic Index
3.2.2. Condition in Relation to Spawning
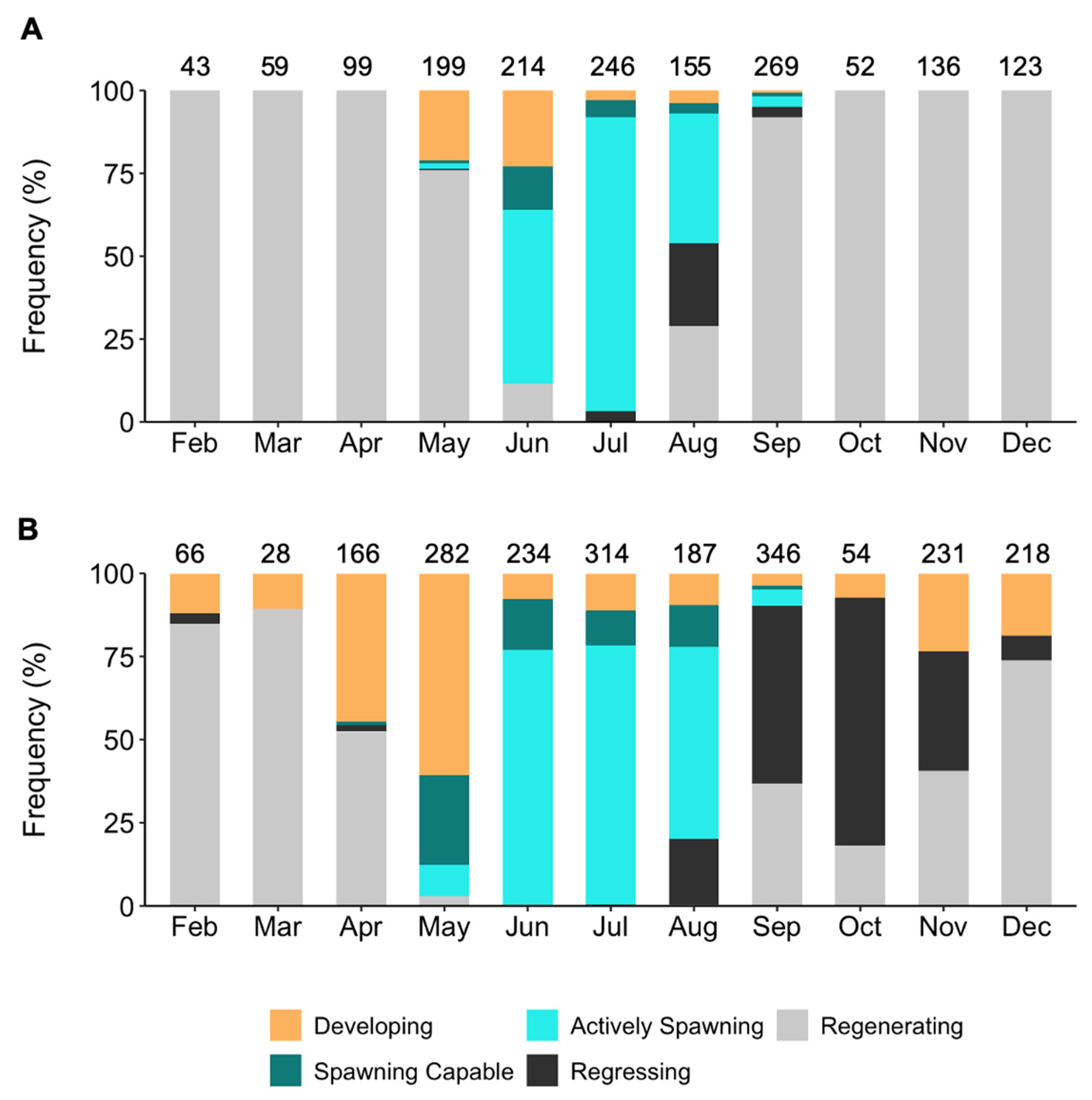
3.2.3. Reproductive Stages and Spawning Duration
3.3. Spawning Fraction, Interval, and Frequency
3.3.1. All Females Pooled
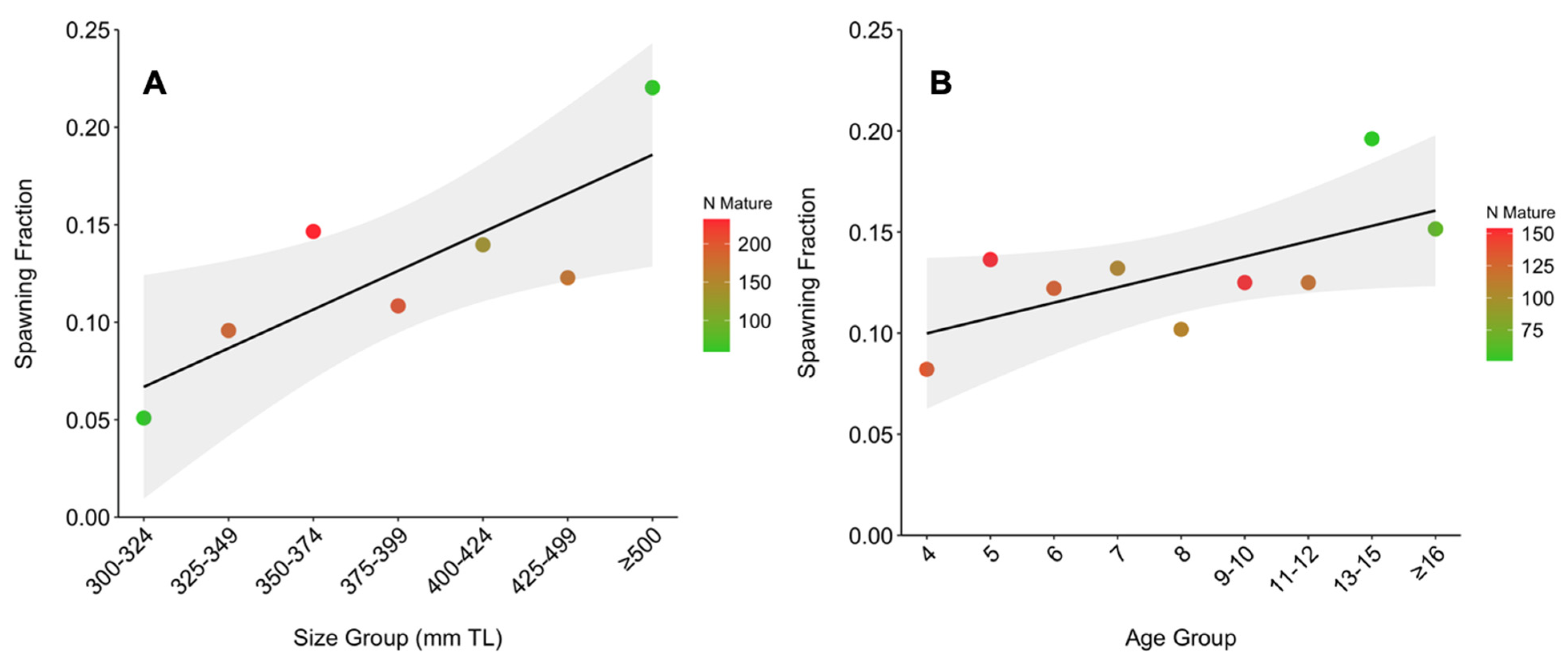
3.3.2. Size-Based
3.3.3. Age-Based
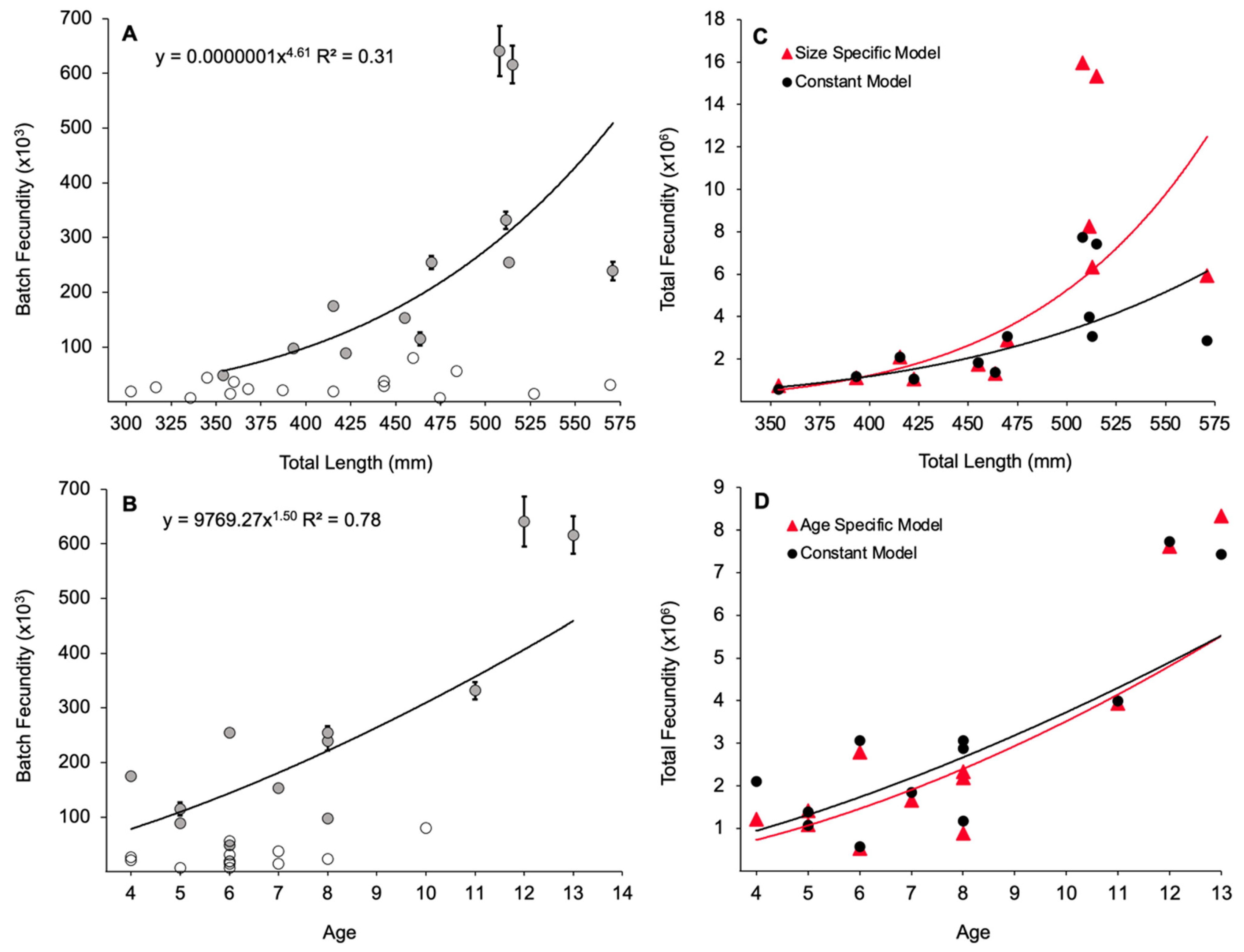
3.4. Batch Fecundity
3.5. Total Annual Fecundity
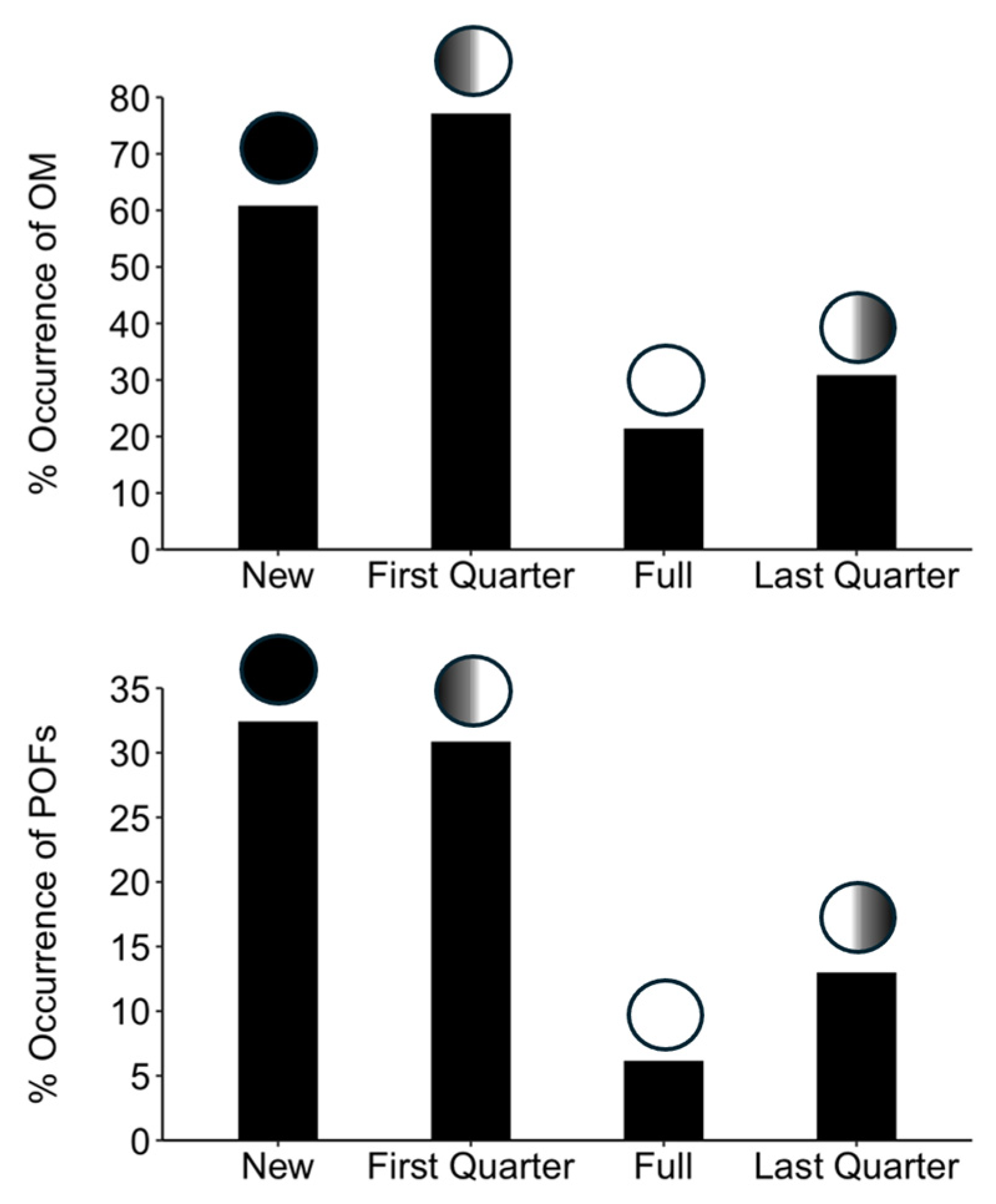
3.6. Lunar Periodicity
3.7. Sexual Maturity
4. Discussion
4.1. Spawning Seasonality
4.2. Spawning Fraction, Interval, and Frequency
4.3. Batch Fecundity
4.4. Total Annual Fecundity
4.5. Lunar Periodicity
4.6. Sexual Maturity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GMFMC (Gulf of Mexico Fishery Management Council). Final Amendment 51 to the Fishery Management Plan for the Reef Fish Resources of the Gulf of Mexico: Establish Gray Snapper Status Determination Criteria and Modify Annual Catch Limits; Gulf of Mexico Fishery Management Council: Tampa, FL, USA, 2019; 122p.
- SEDAR (Southeast Data Assessment and Review). SEDAR 75 Stock Assessment Report: Gulf of Mexico Gray Snapper; SouthEast Data, Assessment, and Review: North Charleston, SC, USA, 2022; 255p. [Google Scholar]
- SEDAR (Southeast Data Assessment and Review). SEDAR 51 Stock Assessment Report: Gulf of Mexico Gray Snapper; SouthEast Data, Assessment, and Review: North Charleston, SC, USA, 2018; 428p. [Google Scholar]
- NOAA. Fisheries of the Caribbean, Gulf of Mexico, and South Atlantic; Reef Fish Fishery of the Gulf of Mexico; Red Snapper Data Calibrations and Gray Snapper Harvest Levels; Federal Register, Vol. 89, No. 94, May 14, 2024; NOAA: Silver Spring, MD, USA, 2024.
- Berkeley, S.A.; Chapman, C.; Sogard, S.M. Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology 2004, 85, 1258–1264. [Google Scholar] [CrossRef]
- Lowerre-Barbieri, S.K.; Brown-Peterson, N.J.; Murua, H.; Tomkiewicz, J.; Wyanski, D.M.; Saborido-Rey, F. Emerging issues and methodological advances in fisheries reproductive biology. Mar. Coast. Fish. 2011, 3, 32–51. [Google Scholar] [CrossRef]
- Macal, K.; Brulé, T.; Torres, R.; Montero-Muñoz, J. Oocyte distribution, ovarian organization, and spawning pattern in Lutjanus griseus. Cybium 2023, 47, 111–114. [Google Scholar] [CrossRef]
- Hunter, J.R.; Lo, N.C.H.; Leong, R.J.H. Batch Fecundity in Multiple Spawning Fishes; NOAA Technical Report National Marine Fisheries Service 36; NOAA: La Jolla, CA, USA, 1985; pp. 67–77.
- Hunter, J.R.; Macewicz, B.J. Measurement of Spawning Frequency in Multiple Spawning Fishes; NOAA Technical Report National Marine Fisheries Service 36; NOAA: La Jolla, CA, USA, 1985; pp. 79–94.
- Fitzhugh, G.R.; Shertzer, K.; Kellison, G.; Wyanski, D. Review of size- and age-dependence in batch spawning: Implications for stock assessment of fish species exhibiting indeterminate fecundity. Fish. Bull. 2012, 110, 413–425. [Google Scholar]
- Barneche, D.R.; Robertson, D.R.; White, C.R.; Marshall, D.J. Fish reproductive-energy output increases disproportionately with body size. Science 2018, 360, 642–645. [Google Scholar] [CrossRef]
- Marshall, D.; Bode, M.; Mangel, M.; Arlinghaus, R.; Dick, E. Reproductive hyperallometry and managing the world’s fisheries. Proc. Natl. Acad. Sci. USA 2021, 118, e2100695118. [Google Scholar] [CrossRef]
- Hixon, M.; Johnson, D.; Sogard, S. BOFFFFs: On the importance of conserving old-growth age structure in fishery populations. ICES J. Mar. Sci. 2014, 71, 2171–2185. [Google Scholar] [CrossRef]
- Marshall, D.J.; Barneche, D.R.; White, C.R. How does spawning frequency scale with body size in marine fishes? Fish Fish. 2022, 23, 316–323. [Google Scholar] [CrossRef]
- Larson, R.J. Seasonal cycles of reserves in relation to reproduction in Sebastes. Environ. Biol. Fish. 1991, 30, 57–70. [Google Scholar] [CrossRef]
- Lowerre-Barbieri, S.K.; Brown-Peterson, N.J.; Wyanski, D.M.; Moncrief-Cox, H.E.; Kolmos, K.J.; Menendez, H.S.; Barnett, B.K.; Friess, C. A unified framework and terminology for reproductive traits integral to understanding fish population productivity. Mar. Coast. Fish. 2023, 15, e10276. [Google Scholar] [CrossRef]
- Lowerre-Barbieri, S.K.; Ganias, K.; Saborido-Rey, F.; Murua, H.; Hunter, J.R. Reproductive timing in marine fishes: Variability, temporal scales, and methods. Mar. Coast. Fish. 2011, 3, 71–91. [Google Scholar] [CrossRef]
- Fitzhugh, G.R.; Beech, V.C.; Lyon, H.M.; Colson, P. Reproductive Parameters for the Gulf of Mexico Gray Snapper, Lutjanus griseus, 1991–2015. SEDAR51-DW-06; SEDAR: North Charleston, SC, USA, 2017; 9p. [Google Scholar]
- Garner, S.; Thornton, L.; Moncrief-Cox, H.; Allman, R. Life History Data for SEDAR 75 Gulf of Mexico Gray Snapper. SEDAR75-WP-05; SEDAR: North Charleston, SC, USA, 2022; 16p. [Google Scholar]
- Kim, E.S.M. Characterizing Gray Snapper (Lutjanus griseus) Life History in the Northcentral Gulf of Mexico: Age and Growth, Mortality, and Reproduction. Master’s Thesis, University of South Alabama, Mobile, AL, USA, 2022; 121p. [Google Scholar]
- Macal-López, K.d.C.; Brulé, T.; Torres-Villegas, J.R.; Colás-Marrufo, T.; Noh-Quiñones, V.E. Reproduction of grey snapper (Teleostei: Lutjanidae) in the southern Gulf of Mexico. Sci. Mar. 2022, 86, e047. [Google Scholar] [CrossRef]
- Pacicco, A.E.; Brown-Peterson, N.J.; Murie, D.J.; Allman, R.J.; Snodgrass, D.; Franks, J.S. Reproductive biology of yellowfin tuna (Thunnus albacares) in the northcentral U.S. Gulf of Mexico. Fish. Res. 2023, 261, 106620. [Google Scholar] [CrossRef]
- Starck, W.A.; Schroeder, R.E. Investigations on the Gray Snapper, Lutjanus griseus; Studies in Tropical Oceanography, University of Miami Press: Coral Gables, FL, USA, 1971; 224p. [Google Scholar]
- Domeier, M.L.; Koenig, C.; Coleman, F. Reproductive biology of the Gray Snapper (Lutjanus griseus), with notes on spawning for other western Atlantic snappers (Lutjanidae). In Biology and Culture of Tropical Groupers and Snappers; Arreguín-Sánchez, F., Munro, J.L., Balgos, M.C., Pauly, D., Eds.; ICLARM: Manila, Philippines, 1996; Volume ICLARM Conference Proceedings 48, pp. 189–201. [Google Scholar]
- Luo, J.; Serafy, J.E.; Sponaugle, S.; Teare, P.B.; Kieckbusch, D. Movement of gray snapper Lutjanus griseus among subtropical seagrass, mangrove, and coral reef habitats. Mar. Ecol. Prog. Ser. 2009, 380, 255–269. [Google Scholar] [CrossRef]
- Anderson, J.; McDonald, D.; Getz, E.; Weixelman, R.; Grubbs, F.; Ferguson, J. Distribution, maturity, age and growth of Gray Snapper (Lutjanus griseus) in the northwestern Gulf of Mexico. Gulf Caribb. Res. 2022, 33, 14–37. [Google Scholar] [CrossRef]
- Arnold, C.R.; Wakeman, J.M.; Williams, T.D.; Treece, G.D. Spawning of red snapper (Lutjanus campechanus) in captivity. Aquaculture 1978, 15, 301–302. [Google Scholar] [CrossRef]
- Feeley, M.; Morley, D.; Acosta, A.; Barbera, P.; Hunt, J.; Switzer, T.; Burton, M. Spawning migration movements of mutton snapper in Tortugas, Florida: Spatial dynamics within a marine reserve network. Fish. Res. 2018, 204, 209–223. [Google Scholar] [CrossRef]
- Heyman, W.D.; Kjerfve, B.; Graham, R.T.; Rhodes, K.L.; Garbutt, L. Spawning aggregations of Lutjanus cyanopterus (Cuvier) on the Belize Barrier Reef over a 6 year period. J. Fish Biol. 2005, 67, 83–101. [Google Scholar] [CrossRef]
- FWC (Florida Fish Wildlife Conservation Commission). FWC Protects Multi-Species Fish Spawning Aggregations Near Western Dry Rocks; FWC: Tallahassee, FL, USA, 26 February 2021.
- Claro, R.; Lindeman, K.C. Spawning aggregation sites of snapper and grouper species (Lutjanidae and Serranidae) on the insular shelf of Cuba. Gulf Caribb. Res. 2003, 14, 91–106. [Google Scholar] [CrossRef]
- Denit, K.; Sponaugle, S. Growth variation, settlement, and spawning of gray snapper across a latitudinal gradient. Trans. Am. Fish. Soc. 2004, 133, 1339–1355. [Google Scholar] [CrossRef]
- Allman, R.; Grimes, C. Temporal and spatial dynamics of spawning, settlement, and growth of gray snapper (Lutjanus griseus) from the West Florida shelf as determined from otolith microstructures. Fish. Bull. 2002, 100, 391–403. [Google Scholar]
- Sadovy, Y.; Domeier, M. Are aggregation-fisheries sustainable? Reef fish fisheries as a case study. Coral Reefs 2005, 24, 254–262. [Google Scholar] [CrossRef]
- Tzioumis, V.; Kingsford, M.J. Reproductive biology and growth of the temperate damselfish Parma microlepis. Copeia 1999, 1999, 348–361. Available online: https://www.jstor.org/stable/1447480. [CrossRef]
- Montgomery, W.L.; Galzin, R. Seasonality in gonads, fat deposits and condition of tropical surgeonfishes (Teleostei: Acanthuridae). Mar. Biol. 1993, 115, 529–536. [Google Scholar] [CrossRef]
- DeMartini, E.E.; Langston, R.C.; Eble, J.A. Spawning seasonality and body sizes at sexual maturity in the bluespine unicornfish, Naso unicornis (Acanthuridae). Ichthyol. Res. 2014, 61, 243–251. [Google Scholar] [CrossRef]
- Dobrzynski, T.; Johnson, K. Regional Council Approaches to the Identification and Protection of Habitat Areas of Particular Concern; NOAA/National Marine Fisheries Service, Office of Habitat Conservation: Silver Spring, MD, USA, 2001; p. 17.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Vanderkooy, S.; Carroll, J.; Elzey, S.; Branscome, J.; Kipp, J. A Practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes, 3rd ed.; Gulf States Marine Fisheries Commission and Atlantic States Marine Fisheries Commission: Ocean Springs, MS, USA, 2020; GSMFC No. 300; 294p. [Google Scholar]
- Fischer, A.; Baker, M.S.; Wilson, C.; Nieland, D. Age, growth, mortality, and radiometric age validation of gray snapper (Lutjanus griseus) from Louisiana. Fish. Bull. 2005, 103, 307–319. [Google Scholar]
- Beamish, R.J.; Fournier, D.A. A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. Available online: https://cdnsciencepub.com/doi/abs/10.1139/f81-132. [CrossRef]
- Hinton, D.E. Histological techniques. In Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society Special Publications Series; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 191–209. [Google Scholar]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Jackson, M.; Nieland, D.; Cowan, J. Diel spawning periodicity of red snapper Lutjanus campechanus in the northern Gulf of Mexico. J. Fish Biol. 2006, 68, 695–706. [Google Scholar] [CrossRef]
- Length, R.V.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; Riebel, H.; et al. Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.10.2. 2024. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 10 June 2024).
- Murua, H.; Kraus, G.; Saborido-Rey, F.; Wittames, P.R.; Thorsen, A.T.; Junquera, S. Procedures to estimate fecundity of wild collected marine fish in relation to fish reproductive strategy. J. Northwest Atl. Fish. Sci. 2003, 33, 33–54. [Google Scholar] [CrossRef]
- Bobko, S.; Berkeley, S. Maturity, ovarian cycle, fecundity, and age-specific parturition of black rockfish (Sebastes melanops). Fish. Bull. 2004, 102, 418–429. [Google Scholar]
- Dick, E.J.; Beyer, S.; Mangel, M.; Ralston, S. A meta-analysis of fecundity in rockfishes (genus Sebastes). Fish. Res. 2017, 187, 73–85. [Google Scholar] [CrossRef]
- Koenig, C.; Bueno, L.; Coleman, F.; Cusick, J.; Ellis, R.; Kingon, K.; Locascio, J.; Malinowski, C.; Murie, D.; Stallings, C. Diel, lunar, and seasonal spawning patterns of the Atlantic goliath grouper, Epinephelus itajara, off Florida, United States. Bull. Mar. Sci. 2017, 92, 391–406. [Google Scholar] [CrossRef]
- Torrejón-Magallanes, J. ‘sizeMat’: An R Package to Estimate Size at Sexual Maturity. 2020. Available online: https://cran.r-project.org/web/packages/sizeMat/vignettes/sizeMat.html (accessed on 22 March 2024).
- Ashton, W.D. The Logit Transformation with Special Reference to Its Uses in Bioassay; Hafner Publishing Company: New York, NY, USA, 1972. [Google Scholar]
- MacFarlane, R.B.; Norton, E.C.; Bowers, M.J. Lipid dynamics in relation to the annual reproductive cyle in yellowtail rockfish (Sebastes flavidus). Can. J. Fish. Aquat. Sci. 1993, 50, 391–401. [Google Scholar] [CrossRef]
- Grieshaber, C.A.; Midway, S.R.; Scharf, F.S.; Koopman, H.; Luckenbach, J.A.; Middleton, M.A. Seasonal physiological dynamics of maturing female southern flounder (Paralichthys lethostigma). Conserv. Physiol. 2016, 4, cow043. [Google Scholar] [CrossRef]
- LaPlante, L.H.; Schultz, E.T. Annual fecundity of tautog in Long Island Sound: Size effects and long-term changes in a harvested population. Trans. Am. Fish. Soc. 2007, 136, 1520–1533. [Google Scholar] [CrossRef]
- Lowerre-Barbieri, S.; Henderson, N.; Llopiz, J.; Walters Burnsed, S.; Bickford, J.; Muller, R. Defining a spawning population (spotted seatrout Cynoscion nebulosus) over temporal, spatial, and demographic scales. Mar. Ecol. Prog. Ser. 2009, 394, 231–245. [Google Scholar] [CrossRef]
- McBride, R.; Thurman, P. Reproductive biology of Hemiramphus brasiliensis and H. balao (Hemiramphidae): Maturation, spawning frequency, and fecundity. Biol. Bull. 2003, 204, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Porch, C.E.; Fitzhugh, G.R.; Lang, E.T.; Lyon, H.M.; Linton, B.C. Estimating the dependence of spawning frequency on size and age in Gulf of Mexico red snapper. Mar. Coast. Fish. 2015, 7, 233–245. [Google Scholar] [CrossRef]
- Gonçalves, P.; Costa, A.M.; Murta, A. Estimates of batch fecundity and spawning fraction for the southern stock of horse mackerel (Trachurus trachurus) in ICES Division IXa. ICES J. Mar. Sci. 2009, 66, 617–622. [Google Scholar] [CrossRef]
- Taylor, M.H. Lunar synchronization of fish reproduction. Trans. Am. Fish. Soc. 1984, 113, 484–493. [Google Scholar] [CrossRef]
- Wright, P.J. Understanding the maturation process for field investigations of fisheries-induced evolution. Mar. Ecol. Prog. Ser. 2007, 335, 279–284. Available online: https://www.jstor.org/stable/24870967. [CrossRef]
- Junquera, S.; Román, E.; Morgan, M.; Sainza, M.; Ramilo, G. Time scale of ovarian maturation in Greenland halibut (Reinhardtius hippoglossoides, Walbaum). ICES J. Mar. Sci. 2003, 60, 767–773. [Google Scholar] [CrossRef]
- Rideout, R.M.; Rose, G.A.; Burton, M.P.M. Skipped spawning in female iteroparous fishes. Fish Fish. 2005, 6, 50–72. [Google Scholar] [CrossRef]
- Reed, E.; Brown-Peterson, N.; DeMartini, E.; Andrews, A. Reproductive Characteristics of Longtailed Red Snapper (Onaga, Etelis Coruscans) in the Main Hawaiian Islands; Pacific Islands Fisheries Science Center Administrative Report H-21-02; NOAA: Honolulu, HI, USA, 2021; 29p.


| Month | n | npof | Spawning Fraction | Spawning Interval (Days) |
|---|---|---|---|---|
| May | 197 | 1 | 0.005 | 197.00 |
| June | 194 | 12 | 0.062 | 16.17 |
| July | 240 | 88 | 0.367 | 2.73 |
| August | 153 | 26 | 0.170 | 5.88 |
| September | 268 | 3 | 0.011 | 89.33 |
| Pooled (May–September) | 1052 | 130 | 0.124 | 8.09 |
| Peak spawning (June–August) | 587 | 126 | 0.215 | 4.66 |
| Size Group (TL, mm) | n | npof | Spawning Fraction | Spawning Interval (Days) | Spawning Frequency |
|---|---|---|---|---|---|
| 300–324 | 59 | 3 | 0.051 | 19.67 | 4.17 |
| 325–349 | 188 | 18 | 0.096 | 10.44 | 8.91 |
| 350–374 | 232 | 34 | 0.147 | 6.82 | 15.54 |
| 375–399 | 203 | 22 | 0.108 | 9.23 | 11.48 |
| 400–424 | 136 | 19 | 0.140 | 7.16 | 12.01 |
| 425–499 | 171 | 21 | 0.123 | 8.14 | 11.43 |
| ≥500 | 59 | 13 | 0.220 | 4.54 | 24.89 |
| Age Group (Years) | n | npof | Spawning Fraction | Spawning Interval (Days) | Spawning Frequency |
|---|---|---|---|---|---|
| 3 | 12 | 0 | 0.00 | 0.00 | 0 |
| 4 | 134 | 11 | 0.082 | 12.18 | 6.98 |
| 5 | 154 | 21 | 0.136 | 7.33 | 12.28 |
| 6 | 131 | 16 | 0.122 | 8.19 | 10.99 |
| 7 | 106 | 14 | 0.132 | 7.57 | 10.83 |
| 8 | 108 | 11 | 0.102 | 9.82 | 9.16 |
| 9–10 | 152 | 19 | 0.125 | 8.00 | 12.75 |
| 11–12 | 120 | 15 | 0.125 | 8.00 | 11.88 |
| 13–15 | 51 | 10 | 0.196 | 5.10 | 13.53 |
| ≥16 | 66 | 10 | 0.152 | 6.60 | 13.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wechsler, A.; Murie, D.J.; Bovee, E. Age- and Size- Based Reproductive Potential of Gray Snapper (Lutjanus griseus) in the Eastern Gulf of Mexico. Fishes 2024, 9, 513. https://doi.org/10.3390/fishes9120513
Wechsler A, Murie DJ, Bovee E. Age- and Size- Based Reproductive Potential of Gray Snapper (Lutjanus griseus) in the Eastern Gulf of Mexico. Fishes. 2024; 9(12):513. https://doi.org/10.3390/fishes9120513
Chicago/Turabian StyleWechsler, Ashley, Debra J. Murie, and Eric Bovee. 2024. "Age- and Size- Based Reproductive Potential of Gray Snapper (Lutjanus griseus) in the Eastern Gulf of Mexico" Fishes 9, no. 12: 513. https://doi.org/10.3390/fishes9120513
APA StyleWechsler, A., Murie, D. J., & Bovee, E. (2024). Age- and Size- Based Reproductive Potential of Gray Snapper (Lutjanus griseus) in the Eastern Gulf of Mexico. Fishes, 9(12), 513. https://doi.org/10.3390/fishes9120513






