Toward Classification of Fish Meat Using Fluorescence Excitation–Emission Matrix and Multivariate Statistics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- Experiment 1. Differentiation between fresh and frozen–thawed fish fillets:
- Experiment 2. Classification of fish species:
2.2. EEM Acquisition
2.3. Preparation of Muscle Extract and Measurement of ATP-Related Compounds
2.4. pH Measurement of Fresh and Frozen–Thawed Fish Fillets
2.5. Data Analysis of EEM Spectra
3. Results
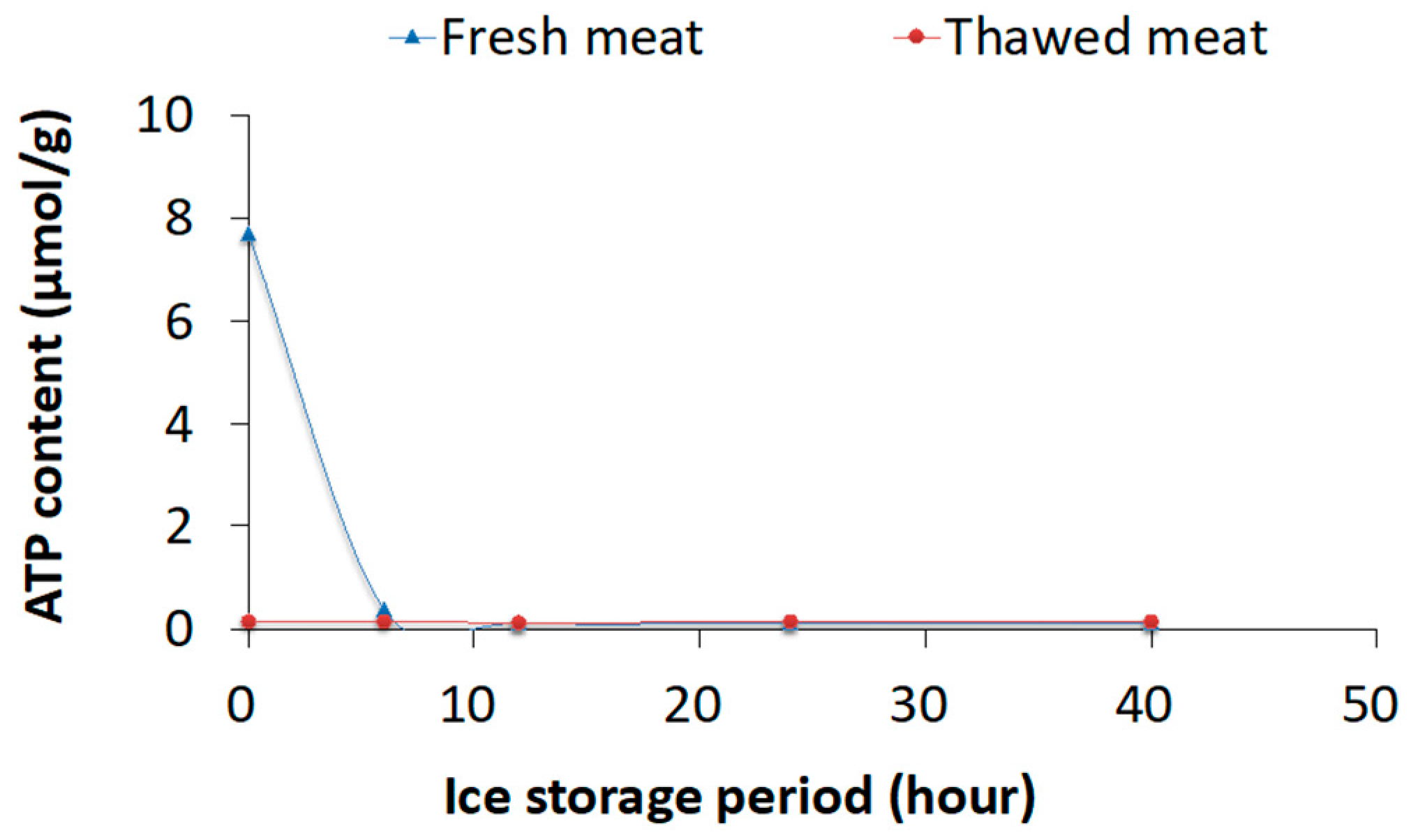
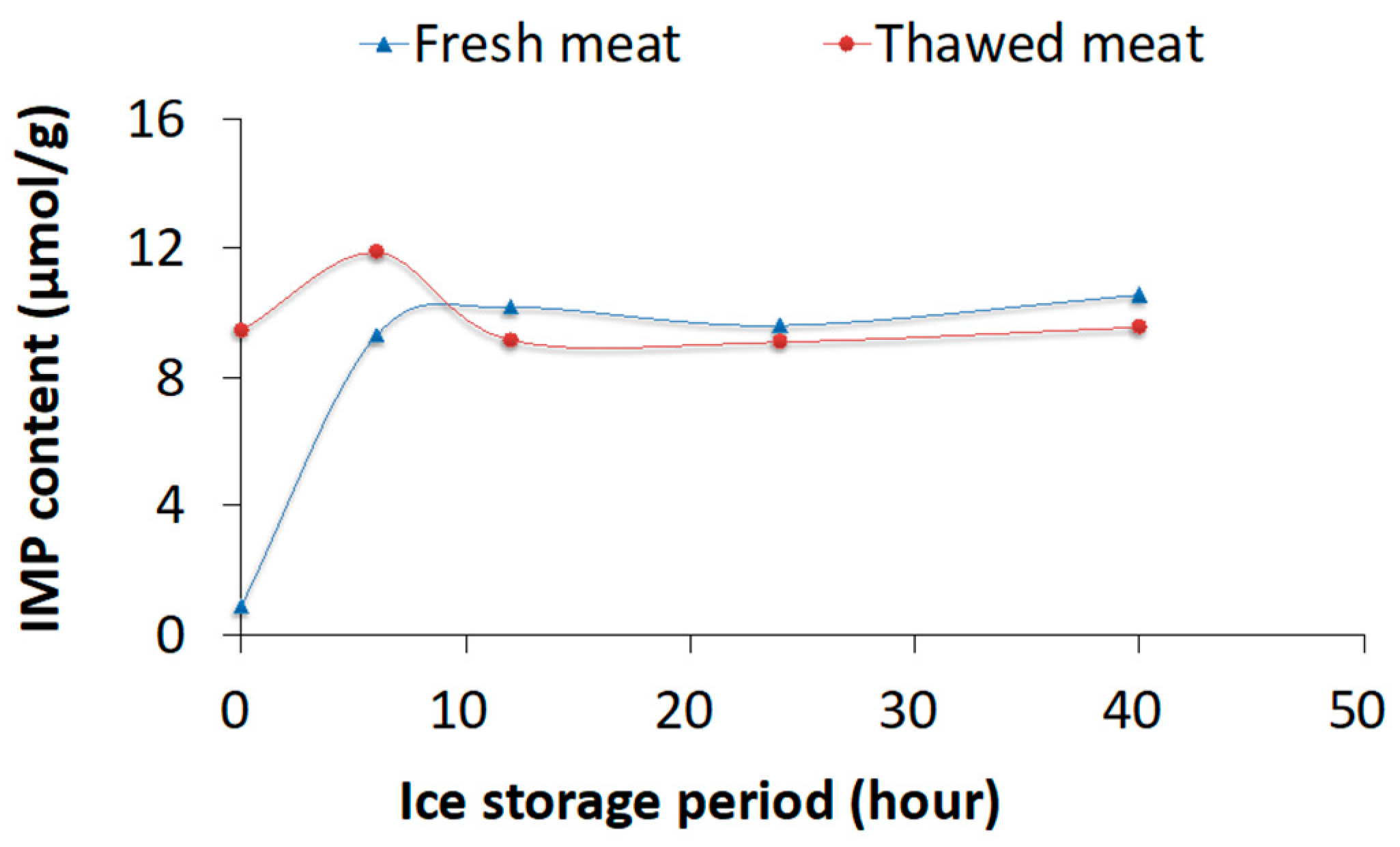
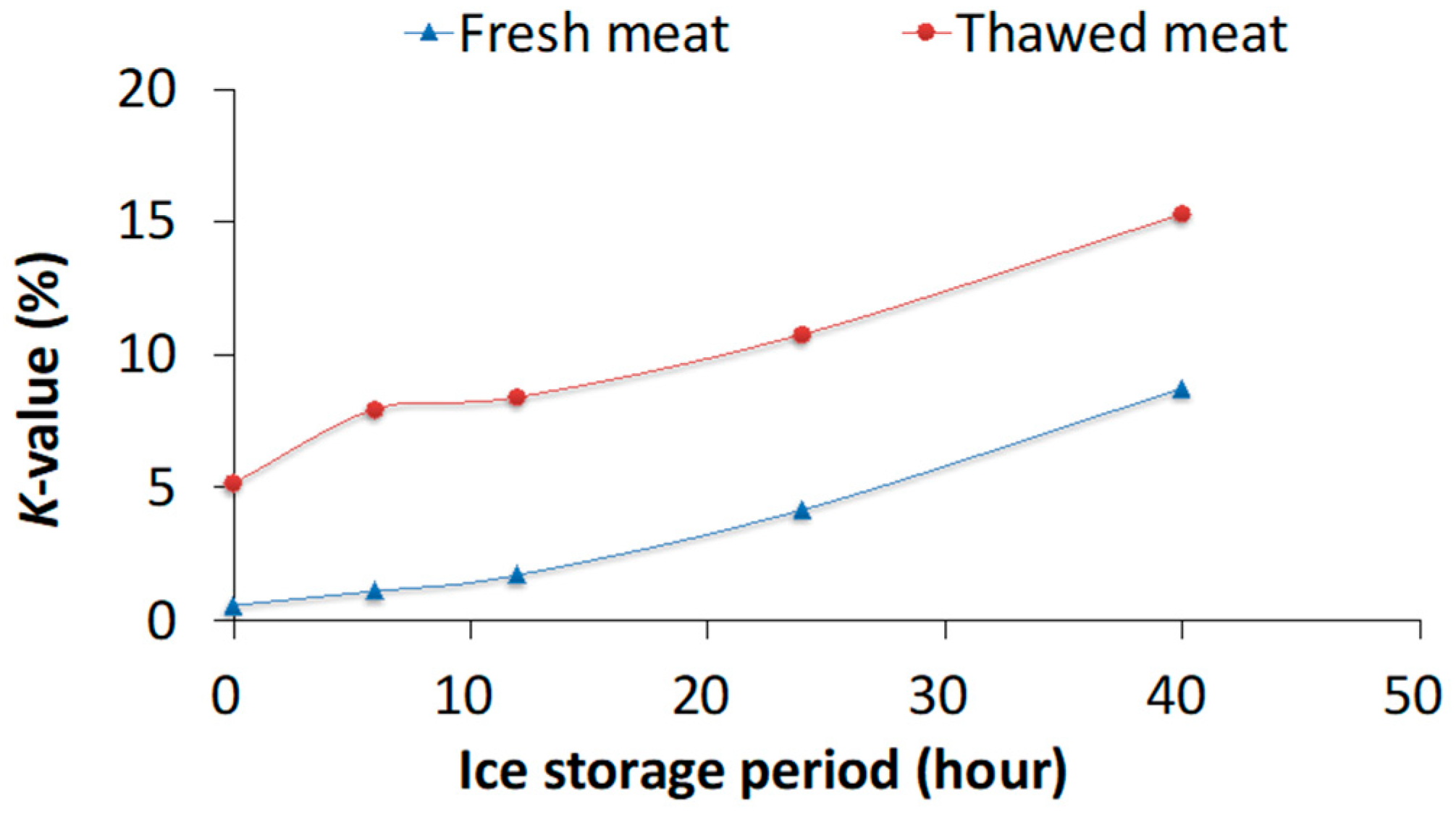
3.1. Experiment 1: Thawing Effects on Freshness of Fish Meat Determined Using Chemical Methods
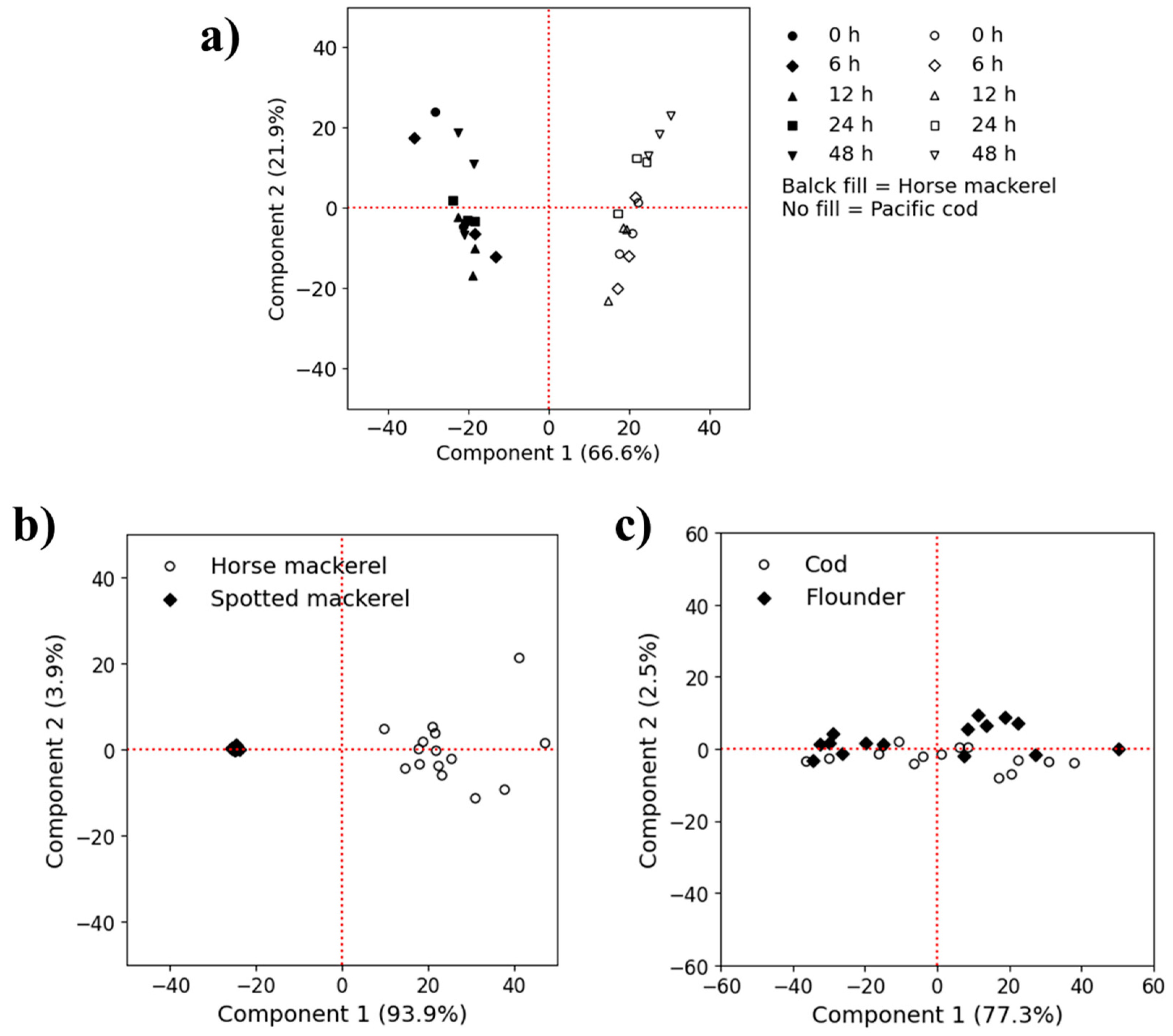
3.2. Discrimination Between Fresh and Frozen–Thawed Fillets by FFs Coupled with PCA
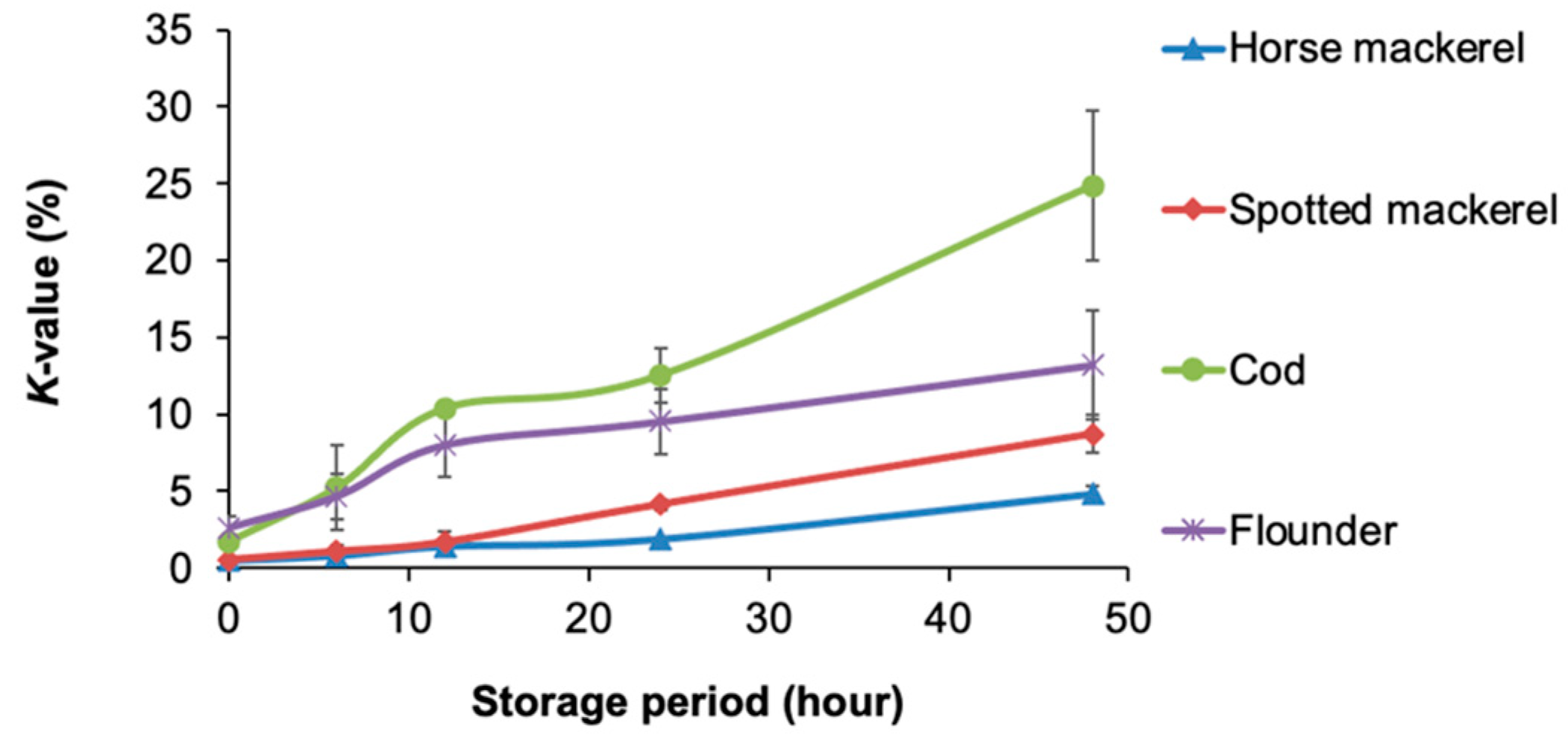
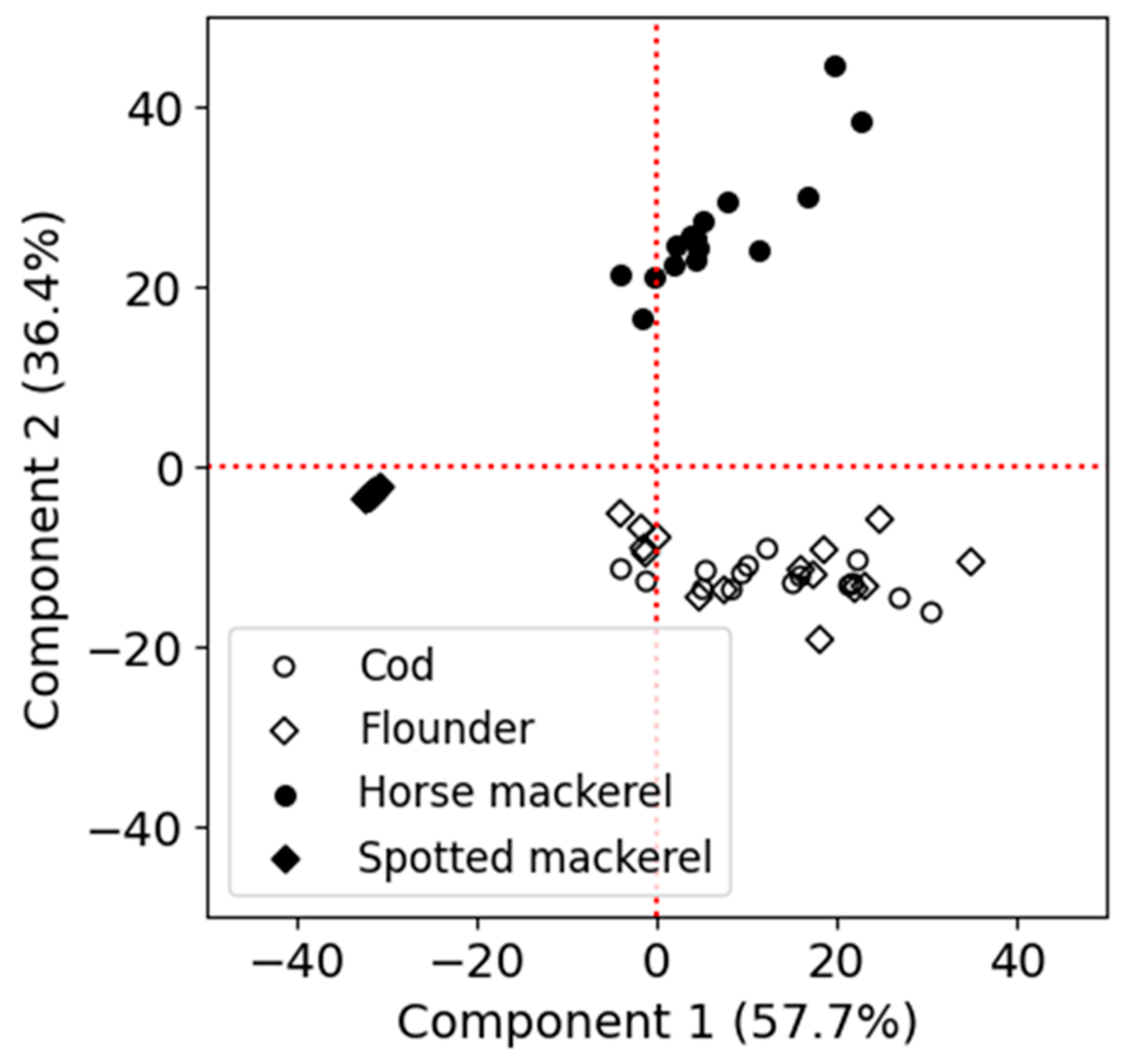
3.3. Experiment 2: Freshness Variations Among Fish Species
3.4. Classification of Frozen Fish Meat Based on EEM Data
3.5. Discrimination of Frozen Fish Meat Based on Muscle Properties Using EEM Data
4. Discussion
4.1. Experiment 1: Chemical Analysis and PCA for Differentiating Fresh and Frozen–Thawed Spotted Mackerel Meat
4.2. Experiment 2: Differentiation of Four Species by K-Value and PCA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alamprese, C.; Casiraghi, E. Application of FT-NIR and FT-IR spectroscopy to fish fillet authentication. LWT 2015, 63, 720–725. [Google Scholar] [CrossRef]
- Ottavian, M.; Fasolato, L.; Serva, L.; Facco, P.; Barolo, M. Data Fusion for Food Authentication: Fresh/Frozen–Thawed Discrimination in West African Goatfish (Pseudupeneus prayensis) Fillets. Food Bioprocess Technol. 2014, 7, 1025–1036. [Google Scholar] [CrossRef]
- Ganapathiraju, P.; Pitcher, T.J.; Mantha, G. Estimates of illegal and unreported seafood imports to Japan. Mar. Policy 2019, 108, 103439. [Google Scholar] [CrossRef]
- Ethuin, P.; Marlard, S.; Delosière, M.; Carapito, C.; Delalande, F.; Van Dorsselaer, A.; Dehaut, A.; Lencel, V.; Duflos, G.; Grard, T. Differentiation between fresh and frozen–thawed sea bass (Dicentrarchus labrax) fillets using two-dimensional gel electrophoresis. Food Chem. 2015, 176, 294–301. [Google Scholar] [CrossRef]
- Hong, H.; Luo, Y.; Zhou, Z.; Bao, Y.; Lu, H.; Shen, H. Effects of different freezing treatments on the biogenic amine and quality changes of bighead carp (Aristichthys nobilis) heads during ice storage. Food Chem. 2013, 138, 1476–1482. [Google Scholar] [CrossRef]
- Kashikura, H.; Yagi, M.; Nakamura, Y.; Sakai, A.; Takahashi, K.; Hiratsuka, S.; Goto, K. Correlation Between Sensory Characteristics and Physicochemical Properties of Wild and Farmed Frozen Southern Bluefin Tuna (Thunnus maccoyii). Fishes 2024, 9, 473. [Google Scholar] [CrossRef]
- Shokralla, S.; Hellberg, R.S.; Handy, S.M.; King, I.; Hajibabaei, M. A DNA Mini-Barcoding System for Authentication of Processed Fish Products. Sci. Rep. 2015, 5, 15894. [Google Scholar] [CrossRef]
- Shehata, H.R.; Naaum, A.M.; Garduño, R.A.; Hanner, R. DNA barcoding as a regulatory tool for seafood authentication in Canada. Food Control 2018, 92, 147–153. [Google Scholar] [CrossRef]
- Adibah, A.B.; Syazwan, S.; Haniza Hanim, M.Z.; Badrul Munir, M.Z.; Intan Faraha, A.G.; Siti Azizah, M.N. Evaluation of DNA barcoding to facilitate the authentication of processed fish products in the seafood industry. LWT 2020, 129, 109585. [Google Scholar] [CrossRef]
- Carrera, E.; Terni, M.; Montero, A.; García, T.; González, I.; Martín, R. ELISA-based detection of mislabeled albacore (Thunnus alalunga) fresh and frozen fish fillets. Food Agric. Immunol. 2014, 25, 569–577. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shibata, M.; Nakazawa, N.; Rithu, M.N.A.; Nakauchi, S.; Hagiwara, T.; Osako, K.; Okazaki, E. Non-destructive Approach for the Prediction of pH in Frozen Fish Meat Using Fluorescence Fingerprints in Tandem with Chemometrics. Fishes 2022, 7, 364. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nakazawa, N.; Shibata, M.; Nakauchi, S.; Okazaki, E. Prospect of fluorescence fingerprints for the noninvasive sensing of seafood freshness: A review. Fish. Sci. 2024, 90, 339–356. [Google Scholar] [CrossRef]
- Liao, Q.; Suzuki, T.; Yasushi, K.; Al Riza, D.F.; Kuramoto, M.; Kondo, N. Monitoring Red Sea Bream Scale Fluorescence as a Freshness Indicator. Fishes 2017, 2, 10. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shibata, M.; Nakazawa, N.; Rithu, M.N.A.; Okazaki, E.; Nakauchi, S. Potential of fluorescence fingerprints for fish meat authentication: Differences in freshness evaluation in white and dark meat at frozen state. J. Food Sci. 2023, 88, 5339–5354. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.K.; Alcivar-Warren, A.; Kenny, J.E. Multidimensional Fluorescence Fingerprinting for Classification of Shrimp by Location and Species. Environ. Sci. Technol. 2012, 46, 2276–2282. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Front-face fluorescence spectroscopy coupled with chemometric tools for monitoring fish freshness stored under different refrigerated conditions. Food Control 2015, 54, 240–249. [Google Scholar] [CrossRef]
- Ehira, S.; Uchiyama, H. Determination of fish freshness using K value and comments on some other biochemical changes in relation to freshness. In Sea Food Quality Determination; Kramer, D.E., Liston, J.C., Eds.; Elesevier Science Publisher: Amsterdam, The Netherlands, 1987; pp. 185–207. [Google Scholar]
- Maeda, T.; Yuki, A.; Sakurai, H.; Watanabe, K.; Itoh, N.; Inui, E.; Seike, K.; Mizukami, Y.; Fukuda, Y.; Harada, K. Alcohol Brine Freezing of Japanese Horse Mackerel (Trachurus japonicus) for Raw Consumption. Trans. Jpn. Soc. Refrig. Air Cond. Eng. 2007, 24, 323–330. [Google Scholar] [CrossRef]
- Saito, T.; Arai, K.; Matsuyoshi, M. A New Method for Estimating the Freshness of Fish. Bull. Jpn. Soc. Sci. Fish. 1959, 24, 749–750. [Google Scholar] [CrossRef]
- Howgate, P. Kinetics of degradation of adenosine triphosphate in chill-stored rainbow trout (Oncorhynchus mykiss). Int. J. Food Sci. Technol. 2005, 40, 579–588. [Google Scholar] [CrossRef]
- Tomioka, K.; Endo, K. K Value-Increasing Rates and IMP-Degrading Activities in Various Fish Muscles. Nippon. Suisan Gakaishi 1984, 50, 889–892. [Google Scholar] [CrossRef]
- Ushio, H.; Watabe, S.; Iwamoto, M.; Hashimoto, K. Ultrastructural Evidence for Temperature-Dependent Ca2+ Release from Fish Sarcoplasmic Reticulum During Rigor Mortis. Food Struct. 1991, 10, 267–275. [Google Scholar]
- Korhonen, R.W.; Lanier, T.C.; Giesbrecht, F. An Evaluation of Simple Methods for Following Rigor Development in Fish. J. Food Sci. 1990, 55, 346–348. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish Spoilage Mechanisms and Preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Ogata, Y.; Koike, H.; Kimura, I.; Yuan, C. Delaying Post-mortem Changes in the Muscle of Spotted Mackerel Killed by an Instantaneous Way of Neck-breaking and Bleeding. J. FisheriesSciences.com 2016, 10, 83–88. [Google Scholar]
- Cai, L.; Wu, X.; Li, X.; Zhong, K.; Li, Y.; Li, J. Effects of different freezing treatments on physicochemical responses and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) fillets during refrigerated storage. LWT 2014, 59, 122–129. [Google Scholar] [CrossRef]
- Lougovois, V.P.; Kyranas, E.R.; Kyrana, V.R. Comparison of selected methods of assessing freshness quality and remaining storage life of iced gilthead sea bream (Sparus aurata). Food Res. Int. 2003, 36, 551–560. [Google Scholar] [CrossRef]
- Karoui, R.; Hassoun, A.; Ethuin, P. Front face fluorescence spectroscopy enables rapid differentiation of fresh and frozen-thawed sea bass (Dicentrarchus labrax) fillets. J. Food Eng. 2017, 202, 89–98. [Google Scholar] [CrossRef]
- Karoui, R.; Thomas, E.; Dufour, E. Utilisation of a rapid technique based on front-face fluorescence spectroscopy for differentiating between fresh and frozen–thawed fish fillets. Food Res. Int. 2006, 39, 349–355. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Shibata, M.; Rithu, M.N.A.; Okazaki, E. Toward Classification of Fish Meat Using Fluorescence Excitation–Emission Matrix and Multivariate Statistics. Fishes 2024, 9, 500. https://doi.org/10.3390/fishes9120500
Rahman MM, Shibata M, Rithu MNA, Okazaki E. Toward Classification of Fish Meat Using Fluorescence Excitation–Emission Matrix and Multivariate Statistics. Fishes. 2024; 9(12):500. https://doi.org/10.3390/fishes9120500
Chicago/Turabian StyleRahman, Md. Mizanur, Mario Shibata, Mst. Nazira Akhter Rithu, and Emiko Okazaki. 2024. "Toward Classification of Fish Meat Using Fluorescence Excitation–Emission Matrix and Multivariate Statistics" Fishes 9, no. 12: 500. https://doi.org/10.3390/fishes9120500
APA StyleRahman, M. M., Shibata, M., Rithu, M. N. A., & Okazaki, E. (2024). Toward Classification of Fish Meat Using Fluorescence Excitation–Emission Matrix and Multivariate Statistics. Fishes, 9(12), 500. https://doi.org/10.3390/fishes9120500





